| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Review
Volume 16, Number 9, September 2024, pages 385-397
Tamsulosin and Dutasteride Combination Therapy for Asian Men With Moderate-to-Severe Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: A Systematic Review of Clinical Considerations That Influence the Prescription
Fan Yanga, b, c, Rahab Hashimb, Julia Philippoub
aDepartment of Urology, Singapore General Hospital, Singapore, Singapore
bFlorence Nightingale Faculty of Nursing, Midwifery and Palliative Care, King’s College London, London, England, UK
cCorresponding Author: Fan Yang, Department of Urology, Singapore General Hospital, Singapore 169608, Singapore
Manuscript submitted July 9, 2024, accepted August 23, 2024, published online September 12, 2024
Short title: Tamsulosin + Dutasteride for LUTS/BPH
doi: https://doi.org/10.14740/jocmr5255
| Abstract | ▴Top |
The goal of combination therapy for moderate-to-severe lower urinary tract symptoms secondary to benign prostatic hyperplasia (LUTS/BPH) is to ease both the dynamic and static symptoms by using agents that have complementary mechanisms of action. Similar to prescribing other drugs, LUTS/BPH combination therapy has been affected by multiple factors. Previous qualitative research discussed the individual perspectives that influenced combination therapy administration. Yet, until recently, there has been limited interest in clinical reasons that physicians have to consider before prescribing LUTS/BPH combination treatment. This systematic review aimed to identify the clinical considerations that influence the decision to prescribe combination therapy of tamsulosin 0.4 mg + dutasteride 0.5 mg for Asian men with LUTS/BPH. This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A systematic search was performed in databases Medline, CINAHL, the Cochrane Library, and Embase from inception until January 2024 using Medical Subject Headings (MeSH) terms and keywords with truncation for alternative acronyms. A citation search was performed to gather works of literature on LUTS/BPH combination treatment in addition to the “PICO” framework for search terms. Five English-language primary randomized controlled trials (RCTs) were included in the narrative analysis using the Critical Appraisal Skills Program (CASP) checklist after critical appraisal. Several dosages of tamsulosin (0.2 mg and 0.4 mg) have been administered in LUTS/BPH combination treatment over the last few decades despite 0.2 mg tamsulosin being standardized as an effective regime in Asian countries. A remarkable correlation between prostate volume (PV) and prostate-specific antigen (PSA) was found in Asian men, which requires higher PSA secretion to enlarge each prostate unit and causes an increased risk of moderate-to-severe LUTS. Additionally, BPH baseline variables may lead to a different response to combination therapy, especially the PV and PSA differences. In conclusion, compared with Caucasian men, a significantly higher risk of moderate-to-severe LUTS was found in Asian men. Initiation of combination therapy, especially dutasteride, depends on a larger PV (≥ 30 mL); it is possible, therefore, that earlier PV and PSA examinations and baseline variables assessments ought to be performed by physicians before the combination therapy prescription. Alternative treatment options may be considered for a patient who prefers an active pattern of sexual activity during their BPH combined pharmacotherapy. These clinical considerations may influence the prescription of tamsulosin 0.4 mg + dutasteride 0.5 mg combination therapy for Asian men with moderate-to-severe LUTS/BPH. This study was registered on PROSPERO (CRD42024575528).
Keywords: Clinical consideration; Combination therapy; Prescription; Lower urinary tract symptoms; Benign prostatic hyperplasia; Systematic review
| Introduction | ▴Top |
The presence of lower urinary tract symptoms secondary to benign prostatic hyperplasia (LUTS/BPH) is a classic clinical problem in males aged 45 years and above worldwide [1]. LUTS/BPH is indicated by a range of lower urinary tract dysfunction characterized by storage, voiding, and post-urination symptoms, which usually result in poor quality of life (QoL) [1, 2]. Prevalence of significant LUTS is often associated with aging, and typically owing to BPH begins around the 60s, while periurethral prostate enlargement could be as early as age 45 [3]. Some fundamental research mentions that approximately one in five men over the age of 45 suffer from a certain degree of BPH with an international prostate symptom score (IPSS) greater than or equal to 7 [4-6].
Investigating LUTS/BPH treatment is a continuing concern of its clinical management, which varies from watchful waiting to conservative therapy, minor procedures, and endoscopic or open surgery [7]. Men with unpleasant LUTS without complications from BPH, such as urinary retention, impaired kidney function, etc., are generally ideal candidates for medication treatment. Nevertheless, the clinical decisions for medication prescribing have been affected by multiple factors. Globally, there are few European countries’ practice guidelines, and the urological clinical handbook of Asian countries recommends that highly urological selective alpha-1a adrenergic receptor antagonists (alfuzosin 5 mg, doxazosin 4 or 8 mg, terazosin 10 mg, tamsulosin 0.2 mg or 0.4 mg) and dual 5-alpha-reductase inhibitors (dutasteride 0.5 mg, finasteride 5 mg) are most commonly prescribed for men with moderate-to-severe LUTS/BPH in monotherapy and combination therapy [8-11].
However, in recent years, novel combination therapy of tamsulosin 0.4 mg + dutasteride 0.5 mg has been emphasized for its efficacy and safety in several randomized controlled trials (RCTs). Yet, its extensive administration was reported in those studies [12-16]. Previous qualitative studies have indicated that individual factors such as healthcare-seeking behaviors, previous experience with treatment, personal preferences and perspectives are all different. Those personal circumstances could influence the prescription of varied dosages of combination therapy [17-21]. In contrast, clinical considerations that affected the prescription of combination therapy for Asian men were not previously described [22].
| Background | ▴Top |
Benign prostatic hyperplasia (BPH), an exceptionally prevalent condition in older adults, is caused by the prostate smooth muscle and epithelial cells’ abnormal proliferation in the transition zone (TZ) of the gland [7, 11, 23, 24]. On histopathology, the unnatural anatomy change of BPH has been described as prostate adenoma. By measuring the intravesical prostatic protrusion (IPP) and prostate volume (PV) under a transabdominal ultrasound, prostate adenoma can be diagnosed [11, 25-27]. When the prostate weights less than 20 g and the maximum urinary flow rate (Qmax) is greater than 20 mL/s in a healthy man, the bladder neck is inverted; whereas in a man with clinical BPH, the bladder neck is deformed by prostate adenoma, and location of adenoma determines the shape of the bladder neck. In early detection of prostate enlargement, the physician may perform a digital rectal examination. Still, a more accurate measurement of the prostate is scanned by ultrasound. In transabdominal ultrasound, the PV is measured as an irregular round shape, which represents the prostate’s clinical size, and abnormal enlargement can be detected here. Meanwhile, the prostatic protrusion is graded based on the millimeter IPP scores [11, 27]. Grade 3 IPP is always present with prostatic obstruction and less void when compared with grade 1; it can, therefore, be assumed that IPP is correlated to Qmax, which is measured through urination into an electronic uroflowmetry detector [11, 27, 28].
In clinical BPH, prostate gland enlargement usually first appears at age 45, but symptoms typically do not present until about 20 years later [3]. Over time, as the progression of BPH manifests, there are varied degrees of LUTS [3]. Inappropriate urination is the emblematic symptom of LUTS, which is not considered a disease but may cause the following symptoms: storage (nocturia, frequency, urgency), voiding (poor urinary stream, hesitancy), and post-micturition (incomplete voiding) [10, 29-31]. Symptoms frequency and prostate hyperplasia worsen with age, leading to a decreased urine flow, an increased urinary tract infection (UTI), acute urinary retention (AUR), and the need for surgical treatment. These have negatively impacted male healthcare and QoL [20, 32].
Prevalence and management approaches of LUTS/BPH
In Asia, studies claim that the prevalence of moderate-to-severe LUTS/BPH has constantly risen over the past decade in males aged 45 years and above. A multiracial society with a large aging population has challenged the healthcare system [1]. It is paramount to address the suitability of LUTS/BPH treatment for Asian men. Thus, it gained massive attention in public health and urged the management of LUTS/BPH to become increasingly concretized, specific, and integrated. Based on the BPH progression and LUTS severity, management approaches range from observation to conservative treatment and endoscopic and invasive surgery. IPSS assessment has been used in primary care when general practitioners investigate the severity of a patient’s BPH and QoL. In secondary care, a specialist consultation with urologists is needed if the condition changes rapidly and severely [7]. The three leading causes of LUTS/BPH have been proven to be dynamic, static, and compensatory. Consequently, the prevalent option is relieving the condition’s dynamic and static components in combination therapy [2].
Rationale of alpha-1 blockers and 5-alpha reductase inhibitors (5ARIs)
Current evidence supports that alpha-1 adrenergic receptor antagonists or alpha-1 blockers block the alpha-1 adrenergic receptors (A1ARs: A1aAR, A1bAR and A1dAR), resulting in a relaxing of the smooth muscle tone, then alleviate the dynamic component of BPH. In addition, A1aAR comprises approximately 70% of A1ARs within the fibromuscular stromal cells of the prostate [3, 33]. Historically, urology selective and non-selective alpha-1a blockers were commonly prescribed for males with BPH [7, 11, 34, 35]. It has been noted that long-term studies state those agents have shown rapid onset of symptom relief and few severe adverse events [36, 37]. A slow-release alpha-1a blocker, tamsulosin, is now mainly prescribed for the treatment of BPH, and it has a relatively higher receptor selectivity to alpha-1a receptors, clinically demonstrating its efficiency and safety. Alpha-1a blockers work as a symptom reliever. However, they cannot inhibit the prostate’s progressive growth while considering a long-term treatment.
Regarding the static symptoms of BPH, 5ARIs (finasteride and dutasteride) prevent the conversion of testosterone to dihydrotestosterone (DHT), and DHT is the essential hormone that promotes prostatic gland proliferation [38-40]. Thus, urological association guidelines in different countries highly recommend dutasteride and finasteride. They are both effective in inhibiting DHT, with dutasteride inhibiting type I and II enzymes over 90% of serum and intraprostatic DHT, and finasteride inhibiting type I enzyme around 70% of serum DHT and 85% of intraprostatic DHT [11, 41]. Additionally, dutasteride has a longer serum half-life of 5 weeks than finasteride’s half-life of less than 1 day [38].
Tamsulosin 0.4 mg plus dutasteride 0.5 mg combination therapy
The goal of combination therapy for LUTS/BPH is to ease both the dynamic and static symptoms by using agents that have complementary mechanisms of action. Tamsulosin and dutasteride combination therapy is often prescribed to males with moderate-to-severe LUTS/BPH, who are at risk of disease progression, with dutasteride specifically recommended for individuals with larger prostates (PV ≥ 30 mL) in a long-term treatment due to its slow onset of action [11, 35, 42-45]. Several necessary clinical trials have recently provided evidence that using tamsulosin 0.4 mg + dutasteride 0.5 mg combination therapy is effective and safe for males with moderate-to-severe LUTS/BPH at risk of disease progression [46-48]. Moreover, combination therapy is more efficient than using either agent alone in long-term treatment [10, 46-48].
In the scope of evidence-based practice, clinical considerations refer to the essential attention which physicians must be aware of or cautious about before making the critical decisions in their daily practice. A previous systematic review by Emberton [12] investigated patient factors that affect general medical treatment for LUTS/BPH. Besides, relevant Asian clinical guidelines have emphasized clinical considerations for LUTS/BPH monotherapy, but there has been no detailed analysis of prescribing combination therapy (A1aARAs and 5ARIs). Thus, this systematic review intends to explore further the clinical considerations that influence the prescription of tamsulosin 0.4 mg + dutasteride 0.5 mg combination therapy for treating moderate-to-severe LUTS/BPH in the background of the Asian population.
| Methods | ▴Top |
Study design
RCTs are prospective studies designed to evaluate the effectiveness of a new intervention or treatment. Randomization reduces bias and provides a rigorous method for examining cause-and-effect relationships between an intervention and its outcome [49]. This systematic review of clinical considerations for combination therapy prescription primarily reveals those utmost important considerations from available RCTs. Primarily, it allows a comprehensive analysis of the effectiveness of tamsulosin 0.4 mg + dutasteride 0.5 mg combination therapy in comparison with varied doses of tamsulosin and dutasteride monotherapy. Secondarily, within the Asian region, whether there are specific clinical reasons that physicians have to be aware of when this combination therapy is prescribed for Asian men needs to be addressed. Then, a systematic review of RCTs was performed using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [50-52]. This systematic review has been registered on PROSPERO (CRD42024575528).
Search strategy
Following the PRISMA guidelines, a systematic search was conducted in databases Medline (OVID), CINAHL, the Cochrane Library, and Embase (OVID) to identify eligible studies that were published up to January 2024. Overall, Medical Subject Headings (MeSH) and keywords related to lower urinary tract symptoms, BPH, combination therapy, alpha-1 blockers, 5ARIs, monotherapy with truncation for alternative acronyms were utilized in the search strategy (Supplementary Material 1, www.jocmr.org). Firstly, the search formula followed the “PICO” framework by indicating the “population” as Asian men who were diagnosed with moderate-to-severe LUTS/BPH, “intervention” as tamsulosin 0.4 mg + dutasteride 0.5 mg combination therapy, “comparator” as tamsulosin and dutasteride monotherapy, and “outcome” as clinical considerations [53]. Many studies regarding various combination therapies were retrieved while using terms “alpha-1 blockers” and “5-alpha reductase inhibitors” during the initial search process. Consequently, additional terms “tamsulosin” and “dutasteride” were added separately, as the current review focused on tamsulosin 0.4 mg + dutasteride 0.5 mg combination therapy. Secondly, the Boolean operators “OR” and “AND” were applied to combine each facet of search terms. Lastly, a citation search was performed with other sources to gather additional literature on LUTS/BPH combination treatment. The retrieval process was conducted by two reviewers independently, and disagreements were resolved by discussion among two reviewers.
Study selection criteria
Inclusion criteria were: 1) The research population is strictly restricted to Asian men aged 45 years and above with diagnosed moderate-to-severe LUTS/BPH [54-56]; 2) Additional criteria for IPSS scores of 8 and greater were included because of the extensive use of IPSS in assessing BPH globally; 3) In order to have a broad spectrum retrieve on relevant research, there were no limitation of the publication year of the study; 4) Conversely, the duration of the study in terms of the treatment cycle was restricted to at least 1 year because medication trials required an extended period for generating evidence regarding efficacy and safety [57]; 5) Most recent (≤ 12 years) RCTs were expected and preferrable for review.
Exclusion criteria were: 1) Eliminated all other combination pharmacotherapy and monotherapy except tamsulosin 0.4 mg + dutasteride 0.5 mg, tamsulosin 0.2 mg + dutasteride 0.4 mg and either tamsulosin 0.2/0.4 mg or dutasteride 0.5 mg monotherapy; 2) Presenting with other prostate issues (prostatitis, prostate cancer, UTI) and conditions that may lead to dysuria (neurogenic bladder, urethral stricture) simultaneously in the study were not considered; 3) History of any current or previous relevant administration of either monotherapy or combination therapy was excluded as this would interrupt the combination therapy cycle, validity of outcome, and rigorousness of study; 4) Studies that were published in non-English language were not included. The above selection criteria were summarized in Table 1 [54-57].
 Click to view | Table 1. Selection Criteria |
Screening and data collection
The electronic search yielded 84 studies, considering the inclusion and exclusion criteria. Two independent reviewers carefully screened the retrieved articles for initial eligibility for inclusion. The article selection and elimination process are illustrated in Figure 1, a mapping of the PRISMA flow diagram [50-52]. Data extraction was performed by two reviewers using standardized data collection sheet: 1) Author and publication year; 2) Study design; 3) Study intervention and control group; 4) Sample size and age profile; 5) Sample inclusion criteria; 6) Clinical outcomes; 7) Statistical analysis used; and 8) Clinical considerations summarized from study.
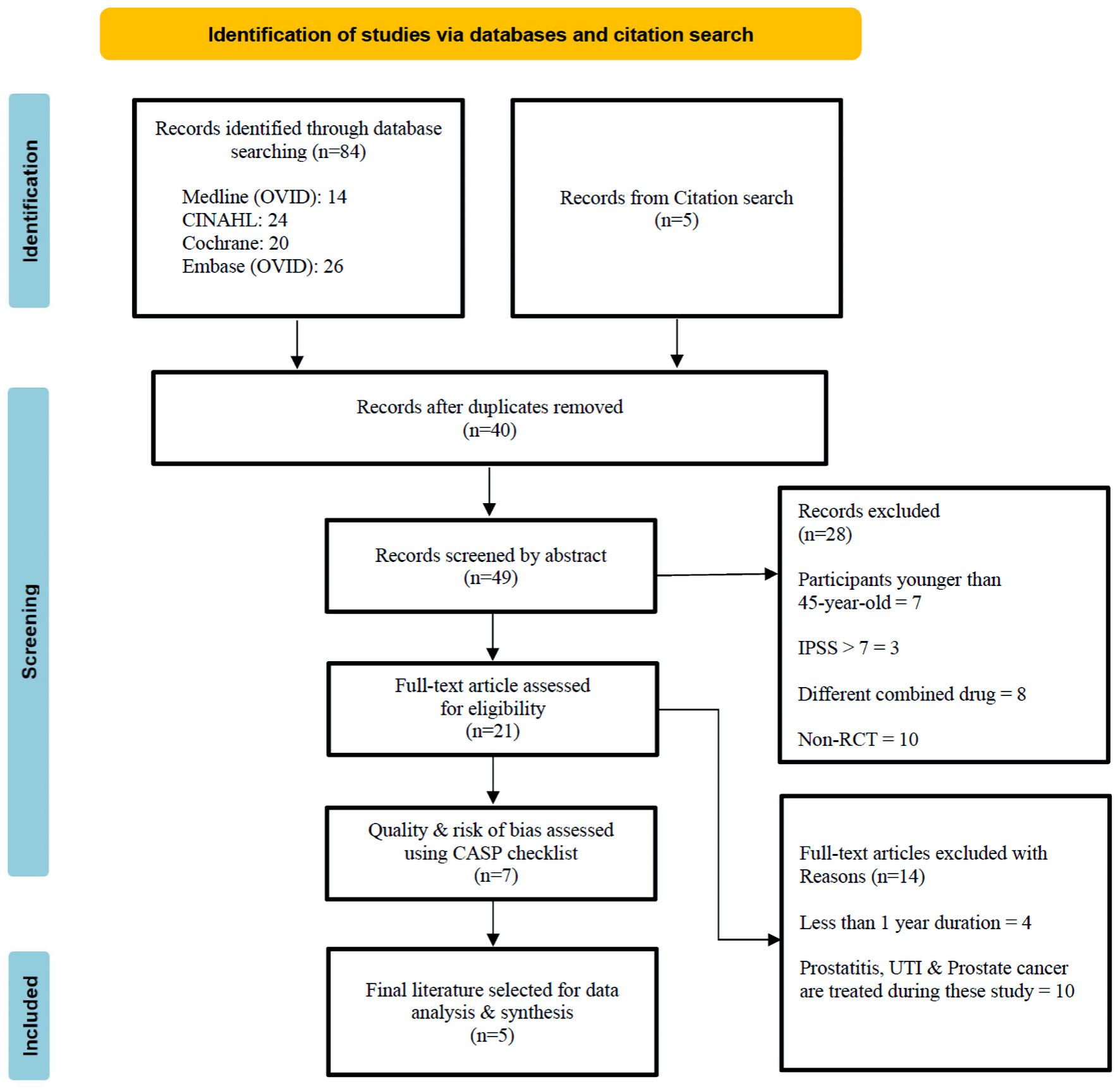 Click for large image | Figure 1. PRSMA flow diagram. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses. IPSS: international prostate symptom score; CASP: Critical Appraisal Skills Program; UTI: urinary tract infection; RCT: randomized controlled trial. |
Quality and bias assessment
The quality of included seven RCTs has been appraised by two independent reviewers using the CASP checklist (Supplementary Material 2, www.jocmr.org) for RCTs that covers 11 questions, and the appraisal results are summarized in Table 2 [58, 59, 64-68]. Disagreements were solved with assistance from the third reviewer. Each question is answered with “yes”, “no”, or “cannot tell”. Two out of seven studies were excluded in the final data synthesis because of high bias, with a total score of “4” and “5”, respectively. A high attrition rate of 20.4% was noted in the RCT by Lee et al [58], which negatively impacted the study’s validity. In contrast, Ngu et al [59] conducted an RCT with a lack of validity in the measurement of study intervention and a loss of comprehensive comparison between the intervention and control group, which caused an insignificant clinical meaning [60].
 Click to view | Table 2. Quality Assessment of RCTs Using CASP Checklist |
Data synthesis and analysis
Five studies were enrolled in the data analysis, which involved a narrative summary of the extracted data based on the effectiveness of relevant pharmacotherapy, and its specific clinical reasons. Attrition bias was assessed by two independent reviewers, who found only a lower drop-out rate of less than 5% in these five studies, as evidenced by CASP question 3 scoring “yes” for them [61]. Randomized allocation and fair treatment were strongly emphasized in all five studies. However, blinding is not always possible, as double-blinded, single-blinded, and open-label were designed in different studies, but they were fit in each study. They had no performance, selection, or detection bias [62]. Also, all five studies’ outcomes or findings were reported verily without bias [63]. Discussion and consultation were carried out with a third researcher to solve the discrepancies.
| Results | ▴Top |
This systematic review aims to explore the clinical considerations that impact the prescription of combination therapy for LUTS/BPH. Eventually, five RCTs are included in the data extraction, as consolidated in Supplementary Material 3 (www.jocmr.org). From the selected five RCTs, clinical considerations seek to conclude those specific precautions by evaluating the prevalence and tendency of prescribing tamsulosin 0.4 mg + dutasteride 0.5 mg combination therapy, and discovering its adverse events and side effects via comparing it with various dosages of available drug formulations worldwide. Besides, baseline variables that affect the change of IPSS in combination therapy were found in one of those RCTs. Hence, a narrative analysis approach was subsequently used in the data synthesis process.
Comparison of clinical response to tamsulosin 0.4 mg + dutasteride 0.5 mg combination therapy between Asian and Caucasian population
Clinical findings suggested that BPH risk may vary by race and ethnicity. A post-hoc analysis of the combination of Avodart and tamsulosin (CombAT) study by Chung et al [64] compared the response to free combination (i.e., medications administered concomitantly) of tamsulosin 0.4 mg + dutasteride 0.5 mg between 325 Asian and 4,259 Caucasian men. Primary outcomes measured the incident percentage of AUR and BPH-related surgery in the second year. The results revealed that differences were not statistically significant between combination therapy (6.5%) and either monotherapy (tamsulosin 0.4 mg, P = 0.27, 10.7% and dutasteride 0.5 mg, P = 0.092, 4.9%) in the Asian subgroup. However, in the Caucasian subgroup, there was significantly lower incidence rate in combination therapy (4.1%) versus tamsulosin 0.4 mg monotherapy (P < 0.00, 12%); there was no statistically significant difference when compared with dutasteride 0.5 mg (P = 0.074, 5.5%). In the fourth year, secondary outcomes measured the clinical progression incident, IPSS change, Qmax increase, PV decrease, and QoL improvement. Overall, there was a significantly lower incidence rate of BPH clinical progression in combination therapy versus tamsulosin 0.4 mg monotherapy, which was observed in Asian (18.7% vs. 33%, P < 0.05) and Caucasian men (12.1% vs. 20.4%, P < 0.05). Also, a lower incidence rate of BPH clinical progression was noted in combination therapy versus dutasteride 0.5 mg monotherapy in Caucasian men (12.1% vs. 17.7%, P < 0.05), but not in Asian men (18.7% vs. 17.9%, P = 0.072). Besides, more remarkable improvements in IPSS, Qmax, and QoL and a more significant reduction in PV were found in combination therapy compared with tamsulosin 0.4 mg monotherapy in Asian and Caucasian men (P < 0.05). Similarly, greater improvements in IPSS, Qmax, and QoL were noted in combination therapy versus dutasteride 0.5 mg in Caucasian men, but there was no significant difference in Asian men. However, reduction of PV did not differ significantly between combination therapy and dutasteride 0.5 mg monotherapy in Asian and Caucasian men.
Comparison of clinical response to tamsulosin 0.4 mg + dutasteride 0.5 mg combination therapy among subgroups with varied baselines
The 4-year results of the CombAT study were examined by Roehrborn et al [65]. They attempted to examine the influence of baseline variables on changes in IPSS, Qmax, and QoL in patients with moderate-to-severe LUTS/BPH after free combination of tamsulosin 0.4 mg + dutasteride 0.5 mg or monotherapy of tamsulosin 0.4 mg and dutasteride 0.5 mg, respectively. Totally, 4,844 patients were enrolled in this RCT. Eight baseline subgroups were developed by authors based on baseline variables of age, body mass index (BMI), BPH impact index (BII), PV, PSA, QoL, Qmax, and IPSS. Combination therapy reported a more remarkable constant improvement in IPSS, Qmax, and QoL than tamsulosin 0.4 mg monotherapy among all eight baseline subgroups at 48 months. In contrast, differences between combination therapy and dutasteride 0.5 mg monotherapy were varied in the PV and PSA subgroups compared with the other six subgroups, which observed that combination therapy has a better benefit over dutasteride 0.5 mg monotherapy in lower baseline of PV (< 60 mL) and PSA (< 4 ng/mL), but combination therapy and dutasteride 0.5 mg monotherapy provided similar benefits in higher baseline of PV (≥ 60 mL) and PSA (≥ 4 ng/mL) concerning improvements in IPSS, Qmax, and QoL.
At 48 months, combination therapy demonstrated a significant improvement in QoL. It was higher than the improvement seen in dutasteride 0.5 mg monotherapy with a lower baseline of PV (< 60 mL) and PSA (< 4 ng/mL), as well as higher than tamsulosin 0.4 mg monotherapy with PV baseline subgroup ≥ 40 mL and all PSA subgroups (P ≤ 0.01). Overall, IPSS, Qmax, and QoL improved synchronously in combination therapy, whereas Qmax was significantly greater compared to tamsulosin 0.4 mg monotherapy, but not compared to dutasteride 0.5 mg monotherapy.
Evaluate the effectiveness of fixed-dose combination of tamsulosin 0.4 mg + dutasteride 0.5 mg
Differently, fixed-dose combination therapy was used by Roehrborn et al [66]. Two years of follow-up with 742 participants allowed researchers to evaluate the effectiveness of the fixed-dose combination of tamsulosin 0.4 mg + dutasteride 0.5 mg along with lifestyle advice in the intervention group, whereas watchful waiting with same lifestyle advice was applied in the control group. Symptomatic change of IPSS (73%, P < 0.001), reduction of BPH progression (43.1%), and overall improvement of QoL (P < 0.001) were statistically more significant in combination therapy. The safety of fixed-dose combination is comparable to known profiles of either monotherapy. This study also revealed that lifestyle advice about caffeine and alcohol avoidance, fluid management, and bladder retraining may be considered part of managing BPH.
Comparison of clinical effectiveness between tamsulosin 0.2 mg and 0.4 mg in monotherapy and combination therapy
Two studies by Chung et al [67] and Haque et al [68] focused on the comparison of efficacy and safety between different dosages of tamsulosin (0.2 vs. 0.4 mg) in monotherapy and combination therapy for Asian men. The former study by Chung et al [67] prescribed tamsulosin 0.4 mg monotherapy (n = 162) and 0.2 mg monotherapy (n = 165) in two intervention groups, while a placebo treatment was given to (n = 167) the control group for Asian men. Among the three groups, baseline characteristics were similar in the beginning. At 12 weeks of study, primary efficacy analyzed the change in total IPSS. The outcome stated that the reduction in IPSS was greater in tamsulosin 0.4 mg monotherapy group than in both tamsulosin 0.2 mg monotherapy and placebo groups (P < 0.0001). Secondary efficacy measured the change in IPSS voiding and storage symptoms sub-score, Qmax, post-void residual (PVR), and QoL. The uroflowmetry test measured Qmax and PVR, which showed improvement in the tamsulosin 0.2 mg and 0.4 mg monotherapy groups. However, the two intervention groups had no statistical difference in the change in IPSS sub-scores, Qmax, PVR, and QoL (P = 0.001, P = 0.9423, P = 0.6137, and P = 0.0009). The safety of three treatment groups was investigated through the adverse events and treatment-emergent adverse events (TEAEs) rate. A total TEAE rate of 15.35% was calculated among three groups, but no statistical difference was observed (P = 0.411).
The latter study by Haque et al [68] divided 607 Asian men into two groups. A free combination of tamsulosin 0.2 mg + dutasteride 0.5 mg was given in the intervention group (n = 305) and tamsulosin 0.2 mg + placebo in the control group (n = 302). IPSS was significantly reduced at 24 months (P < 0.05), and greater improvements in Qmax were found at every measurement (P ≤ 0.006), along with a statistically significant reduction in PV at 12 and 24 months (P < 0.001) in the combination group. In conclusion, the risk of AUR and BPH-related surgery was significantly reduced in the combination group (P = 0.012), with a primary reduction in the AUR risk (P = 0.005). Safety and tolerability reported in the present study data showed that sexual adverse events of ejaculation, libido, and impotence problems were more frequently seen in the combination therapy (7.2%) versus tamsulosin 0.2 mg monotherapy (3.3%). Likewise, these problems were started at the early stage of therapy and remained unsolved by the end of the study. Interestingly, the incidence rate of sexual adverse events in this study was lower than that observed in the CombAT study by Roehrborn et al [65], but cardiovascular adverse events were similar to that observed in the CombAT study. However, tamsulosin 0.4 mg was administered in the CombAT study [64, 65].
Overall, these five RCTs demonstrated that drug therapy accounted for the majority of LUTS/BPH treatments (98.77%), and the number of patients followed an upward trend in the use of combination therapy worldwide [64-68].
| Discussion | ▴Top |
This systematic review was designed to determine the clinical considerations influencing the prescription of tamsulosin 0.4 mg + dutasteride 0.5 mg combination therapy for treating moderate-to-severe LUTS/BPH in an Asian population. Five RCTs were reviewed for the data analysis, which reported statistically significant clinical meaning in LUTS/BPH combination therapy.
Clinical considerations from selected RCTs
In reviewing the literature, data were found on the association between drug dosage and ethnicity on the dimension of clinical considerations that require physicians to be aware of. Asian men have been found to have decreased 5-alpha reductase (5AR) enzyme activity and variable 5AR type 2 gene expression, according to Chung et al [64]. Meanwhile, Asian ethnicity has lower levels of PSA and PV. Still, the amount of PSA released in each volume unit of the prostate gland is greater, which demonstrates a different relationship between PSA and PV compared with Caucasian men [64, 69, 70]. This unique relationship between PV and PSA in Asian men results in a higher risk of experiencing moderate-to-severe LUTS than Caucasian men [71, 72]. Another interesting finding is that Asian and Caucasian men have similar responses to tamsulosin 0.4 mg + dutasteride 0.5 mg combination therapy despite the dissimilarity of the correlation between PV and PSA [64].
In contrast to Western countries, tamsulosin 0.2 mg is recommended as the standard administration regimen in Asia [73]. Therefore, with regards to the efficacy and safety of various doses of tamsulosin (0.2 mg vs. 0.4 mg) in monotherapy and different combination formulations (tamsulosin 0.2 mg + dutasteride 0.5 mg vs. tamsulosin 0.4 mg + dutasteride 0.5 mg), another four RCTs had been reviewed. Tamsulosin 0.2 and 0.4 mg were used in double-blind RCT by Chung et al [67]. Results showed that tamsulosin 0.4 mg is safe to prescribe for Asian men as a greater improvement in IPSS, Qmax, and QoL over tamsulosin 0.2 mg was found and without significant adverse events [67]. Additionally, numerous clinical patients do not respond well to tamsulosin 0.2 mg; the dosage is often raised to 0.4 mg; and tamsulosin 0.4 mg is safe and more efficacious than 0.2 mg dose in Asian men [74]. It was surprising that tamsulosin 0.8 mg was used a decade ago, and study concluded that tamsulosin 0.4 and 0.8 mg were safe and efficient in treating LUTS/BPH. However, tamsulosin 0.8 mg was associated with a significantly higher incidence of adverse events [75].
Tamsulosin 0.4 mg + dutasteride 0.5 mg combination therapy vs. tamsulosin 0.4 mg monotherapy and dutasteride 0.5 mg monotherapy and tamsulosin 0.2 mg + dutasteride 0.5 mg combination therapy vs. tamsulosin 0.2 mg monotherapy were conducted in a double-blind RCT by Roehrborn et al [65] and a single-blind RCT by Haque et al [68], respectively. Former study investigated the baseline variables on change in IPSS, Qmax, and QoL among eight subgroups. Outcomes reported that the combination of tamsulosin 0.4 mg + dutasteride 0.5 mg was significantly superior in improving LUTS. Patients with high PV value ≥ 30 mL and high PSA level ≥ 1.5 ng/mL should considered long-term combination therapy. Patients with higher PV ≥ 60 mL and PSA ≥ 4 ng/mL showed similar responses to combination therapy and dutasteride 0.5 mg monotherapy, whereas limited benefit was determined in the PV < 60 mL or PSA < 4 ng/mL subgroups compared with dutasteride 0.5 mg monotherapy [65]. The latter RCT took Korean and Japanese urological association guideline recommendations into account, and tamsulosin 0.2 mg was used in combination therapy with dutasteride 0.5 mg. A persistent improvement in Qmax and PV was observed from 6 months onwards and throughout the 2 years in the combination therapy group. Overall, drug-related adverse events were mild and occurred within the first 6 to 12 months of the study; more than half of adverse events were reported in combination treatment. Sexual-related problems more frequently happened in combination therapy. However, the incident rate of sexual adverse events was lower than that in the previous CombAT study, and cardiovascular adverse events were similar to those in the CombAT study [64, 65].
Relevant correlations from additional literatures
Tamsulosin 0.4 mg + dutasteride 0.5 mg combination therapy is often administered to males with moderate-to-severe LUTS/BPH who are at risk of the condition worsening, with dutasteride specifically recommended for individuals with larger prostates (PV ≥ 30 mL) in a long-term treatment [11, 35, 42-45]. However, over 50% of males with moderate-to-severe LUTS have a prostate size that is rather modest, measuring 30 mL or less [76, 77]. Furthermore, the possibility of experiencing sexual dysfunction while using dutasteride in combination therapy may limit its use in clinical settings, despite reported satisfaction with the treatment [77] and demonstrated effectiveness in reducing symptoms, disease progression, and the need for surgery [78, 79].
Numerous studies provide evidence for a negative correlation between the size of BPH and the occurrence of prostate cancer (PCa) [80-82]. The prostate consists of three different zones: the central zone (CZ), TZ, and peripheral zone (PZ) [82]. Research confirms that approximately 80% of PCa initiates in the prostate’s PZ [82, 83], while the enlargement of the TZ is widely recognized as the primary cause of aging and enlarging BPH prostate [81, 84]. The growth of the TZ in BPH leads to significant alterations in both the volume and glandular density of the PZ, as well as changes in the prostate capsule. The disease mechanisms described in recent studies revealed that the expanding TZ adds pressure on the PZ, leading to glandular tissue atrophy and fibrosis in the PZ [81, 83, 85].
Recommendation
An open-label, prospective, randomized pilot study conducted in Korea by Lee et al [58] and an RCT conducted by Barkin et al [86] in Europe examined the effect of discontinuing tamsulosin in patients with LUTS/BPH, who had been receiving combination therapy of tamsulosin 0.4 mg + dutasteride 0.5 mg. Still, there has been a vacancy of research that examines the broader Asia population: Can tamsulosin be discontinued from long-term combination therapy for Asian men? Whether an earlier initiation of BPH treatment for Asian men is advisable due to the high risk of moderate-to-severe LUTS? Can fixed-dose combination therapy result in better compliance compared with co-administer of tamsulosin and dutasteride?
Research proves that using shared-decision making (SDM) results in individuals making high-quality decisions, experiencing greater satisfaction, adhering better to medication regimens, and achieving improved clinical outcomes [87-79]. Discussing the options with the patient and acknowledging the optimized and individualized treatment is necessary. It enables healthcare professionals to make informed decisions based on the patient’s circumstances and context [90, 91]. Thus, SDM is highly recommended when the physician is prescribing combination therapy for Asian men with moderate-to-severe LUTS/BPH [92, 93].
Strengths and limitations
This systematic review comprehensively searched RCTs relevant to tamsulosin 0.4 mg + dutasteride 0.5 mg combination therapy. Its efficacy and safety had been reported in several previous published meta-analysis, which supported the benefits of the administration of combination therapy. Including relevant RCTs provides a significant clinical view and research validation on the recommended clinical considerations that physicians must be cautious with while prescribing combination treatment.
Although five RCTs were involved in narrative data analysis, the quality of those studies was high. Different tolerances and preferences for combination therapy, varied durations, and the subjectiveness of each RCT may introduce potential biases that affect the results of the present systematic review. Many other factors, such as personal belief, BPH progression, and delayed treatment, may still reduce the long-term benefits of combination therapy. Unfortunately, these factors are difficult to interpret in this review because much research needs to be involved.
| Conclusions | ▴Top |
Tamsulosin 0.4 mg + dutasteride 0.5 mg combination therapy has been extensively prescribed for men with moderate-to-severe LUTS/BPH in a long-term treatment. Asian ethnicities have similar responses to tamsulosin 0.4 mg but different sensitivity to dutasteride 0.5 mg in combination therapy compared with European races. A relatively higher risk of moderate-to-severe LUTS was found in Asian men. Besides, initiation of medical treatment and consideration of dutasteride relies on a larger PV (≥ 30 mL); it is possible, therefore, that earlier PV and PSA examinations and baseline variables assessments should be launched before combination therapy prescription. Alternative treatments may be considered to minimize the potential sexual adverse events of dutasteride during combination therapy if the patient prefers to maintain active sexual activity. These are factors correlated with clinical considerations that may influence the prescription of tamsulosin 0.4 mg + dutasteride 0.5 mg combination therapy for moderate-to-severe LUTS/BPH in Asian men.
| Supplementary Material | ▴Top |
Suppl 1. Search strategy for Medline (OVID).
Suppl 2. CASP checklist.
Suppl 3. Data extraction of selected RCTs for synthesis and analysis.
Acknowledgments
None to declare.
Financial Disclosure
No financial assistance was received to support this study.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Informed Consent
Not applicable.
Author Contributions
FY contributed to study conception and design, literature search, screening, risk of bias assessment, data extraction, analysis and interpretation of data, manuscript preparation and revision. RH contributed to conception and design of study, literature search, screening, risk of bias assessment, data extraction, analysis and interpretation of data. JP contributed to risk of bias assessment, analysis and interpretation of data. Each author participated sufficiently in review. All authors have been involved in preparation of the article prior to submission. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
AUR: acute urinary retention; A1AR: alpha 1 adrenergic receptor; A1aAR: alpha 1a adrenergic receptor; A1bAR: alpha 1b adrenergic receptor; A1dAR: alpha 1d adrenergic receptor; A1aARA: alpha 1a adrenergic receptor antagonist; AEs: adverse events; ANOVA: analysis of variance; ANCOVA: analysis of covariance; 5AR: 5-alpha reductase; 5ARI: 5-alpha reductase inhibitor; BPH: benign prostatic hyperplasia; BMI: body mass index; BII: benign prostatic hyperplasia impact index; CASP: Critical Appraisal Skills Program; CombAT: Combination of Avodart and Tamsulosin; CZ: central zone; DHT: dihydrotestosterone; MeSH: Medical Subject Heading; IPP: intravesical prostatic protrusion; IPSS: international prostate symptom score; LUTS: lower urinary tract symptoms; LOCF: last observation carried forward; PV: prostate volume; PVR: post-void residual; PVRU: post-void residual urine; PSA: prostate specific antigen; PROSPERO: International Prospective Register of Systematic Review; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PCa: prostate cancer; PZ: peripheral zone; QoL: quality of life; Qmax: maximum urinary flow rate; RCTs: randomized controlled trials; SD: standard deviation; SAS: statistical analysis system; SDM: shared decision-making; TEAEs: treatment emergent adverse events; TZ: transition zone; UTI: urinary tract infection
| References | ▴Top |
- Chong C, Fong L, Lai R, Lau WK, Hartmann M, Chia SE. Erratum: The prevalence of lower urinary tract symptoms and treatment-seeking behavior in males over 40 years in Singapore: A community-based study. Prostate Cancer and Prostatic Diseases. 2012;15(4):402.
doi - Jiwrajka M, Yaxley W, Ranasinghe S, Perera M, Roberts MJ, Yaxley J. Drugs for benign prostatic hypertrophy. Aust Prescr. 2018;41(5):150-153.
doi pubmed pmc - Sherman JJ, Welch RW, Hill TM, McEwen C. Prescriber monitoring for benign prostatic hyperplasia within a family medicine clinic: a comparison of medication classes. J Pharm Pract. 2012;25(2):164-168.
doi pubmed - Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in america project: benign prostatic hyperplasia. J Urol. 2008;179(5 Suppl):S75-80.
doi pubmed pmc - Stranne J, Damber JE, Fall M, Hammarsten J, Knutson T, Peeker R. One-third of the Swedish male population over 50 years of age suffers from lower urinary tract symptoms. Scand J Urol Nephrol. 2009;43(3):199-205.
doi pubmed - Parsons JK. Benign prostatic hyperplasia and male lower urinary tract symptoms: epidemiology and risk factors. Curr Bladder Dysfunct Rep. 2010;5(4):212-218.
doi pubmed pmc - Vaughan ED, Jr. Medical management of benign prostatic hyperplasia—are two drugs better than one? N Engl J Med. 2003;349(25):2449-2451.
doi pubmed - Roehrborn C, Barkin J, Siami P, Tubaro A, Damiao R, Gagnier R, Montorsi F. Effects of combination therapy with dutasteride and Tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: Four-year results from the combat study. Journal of Men's Health. 2009;6(3):268.
doi - Haillot O, Fraga A, Maciukiewicz P, Pushkar D, Tammela T, Hofner K, Chantada V, et al. The effects of combination therapy with dutasteride plus tamsulosin on clinical outcomes in men with symptomatic BPH: 4-year post hoc analysis of European men in the CombAT study. Prostate Cancer Prostatic Dis. 2011;14(4):302-306.
doi pubmed - Joo KJ, Sung WS, Park SH, Yang WJ, Kim TH. Comparison of alpha-blocker monotherapy and alpha-blocker plus 5alpha-reductase inhibitor combination therapy based on prostate volume for treatment of benign prostatic hyperplasia. J Int Med Res. 2012;40(3):899-908.
doi pubmed - Singapore Urological Association Male Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia Guidelines Committee. Singapore Urological Association Clinical Guidelines for male lower urinary tract symptoms/benign prostatic hyperplasia. Singapore Med J. 2017;58(8):473-480.
doi pubmed pmc - Emberton M. Medical treatment of benign prostatic hyperplasia: physician and patient preferences and satisfaction. Int J Clin Pract. 2010;64(10):1425-1435.
doi pubmed - Djavan B, Dianat SS, Kazzazi A. Effect of combination treatment on patient-related outcome measures in benign prostatic hyperplasia: clinical utility of dutasteride and tamsulosin. Patient Relat Outcome Meas. 2011;2:71-79.
doi pubmed pmc - Kang D, Hu C, Fu Y, Wang D. Combination of alpha-blocker and 5alpha-reductase inhibitor for treatment of benign prostatic hyperplasia. Clin Invest Med. 2017;40(5):E200-E210.
doi pubmed - Zhou Z, Cui Y, Wu J, Ding R, Cai T, Gao Z. Meta-analysis of the efficacy and safety of combination of tamsulosin plus dutasteride compared with tamsulosin monotherapy in treating benign prostatic hyperplasia. BMC Urol. 2019;19(1):17.
doi pubmed pmc - Mansbart F, Kienberger G, Sonnichsen A, Mann E. Efficacy and safety of adrenergic alpha-1 receptor antagonists in older adults: a systematic review and meta-analysis supporting the development of recommendations to reduce potentially inappropriate prescribing. BMC Geriatr. 2022;22(1):771.
doi pubmed pmc - Emberton M, Marberger M, de la Rosette J. Understanding patient and physician perceptions of benign prostatic hyperplasia in Europe: The Prostate Research on Behaviour and Education (PROBE) Survey. Int J Clin Pract. 2008;62(1):18-26.
doi pubmed pmc - Morlock R, Goodwin B, Gomez Rey G, Eaddy M. Clinical progression, acute urinary retention, prostate-related surgeries, and costs in patients with benign prostatic hyperplasia taking early versus delayed combination 5alpha-reductase inhibitor therapy and alpha-blocker therapy: a retrospective analysis. Clin Ther. 2013;35(5):624-633.
doi pubmed - Cindolo L, Pirozzi L, Sountoulides P, Fanizza C, Romero M, Castellan P, Antonelli A, et al. Patient's adherence on pharmacological therapy for benign prostatic hyperplasia (BPH)-associated lower urinary tract symptoms (LUTS) is different: is combination therapy better than monotherapy? BMC Urol. 2015;15:96.
doi pubmed pmc - Ababneh M, Shamieh D, Al Demour S, Rababa'h A. Evaluation of the clinical pharmacist role in improving clinical outcomes in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. Int J Clin Pharm. 2019;41(5):1373-1378.
doi pubmed - Zou H, Jiang DX, Zhao WY, Yang JH, Jia HH, Zhang LL. Factors associated with patient delay for older adults with benign prostatic hyperplasia: A descriptive qualitative study. Geriatr Nurs. 2022;46:178-183.
doi pubmed - D'Agate S, Wilson T, Adalig B, Manyak M, Palacios-Moreno JM, Chavan C, Oelke M, et al. Impact of disease progression on individual IPSS trajectories and consequences of immediate versus delayed start of treatment in patients with moderate or severe LUTS associated with BPH. World J Urol. 2020;38(2):463-472.
doi pubmed pmc - Randall A. Surgical pathology of prostatic obstruction. The American Journal of Surgery. 1932;16(1):134–135.
doi - McNeal JE. Normal histology of the prostate. Am J Surg Pathol. 1988;12(8):619-633.
doi pubmed - Yuen JS, Ngiap JT, Cheng CW, Foo KT. Effects of bladder volume on transabdominal ultrasound measurements of intravesical prostatic protrusion and volume. Int J Urol. 2002;9(4):225-229.
doi pubmed - Tan YH, Foo KT. Intravesical prostatic protrusion predicts the outcome of a trial without catheter following acute urine retention. J Urol. 2003;170(6 Pt 1):2339-2341.
doi pubmed - Luo GC, Foo KT, Kuo T, Tan G. Diagnosis of prostate adenoma and the relationship between the site of prostate adenoma and bladder outlet obstruction. Singapore Med J. 2013;54(9):482-486.
doi pubmed - Rosenberg MT, Riley JB, Miner MM. Clinical assessment and diagnosis of lower urinary tract symptoms/benign prostatic hyperplasia. Male Lower Urinary Tract Symptoms and Benign Prostatic Hyperplasia. 2014;47-58.
doi - Roehrborn CG, Bruskewitz R, Nickel GC, Glickman S, Cox C, Anderson R, Kandzari S, et al. Urinary retention in patients with BPH treated with finasteride or placebo over 4 years. Characterization of patients and ultimate outcomes. The PLESS Study Group. Eur Urol. 2000;37(5):528-536.
doi pubmed - Welch G, Weinger K, Barry MJ. Quality-of-life impact of lower urinary tract symptom severity: results from the Health Professionals Follow-up Study. Urology. 2002;59(2):245-250.
doi pubmed - Thiruchelvam N. Benign prostatic hyperplasia. Oxford Textbook of Urological Surgery. Oxford Textbooks in Surgery. 2017.
doi - Lulic Z, Son H, Yoo SB, Cunnington M, Kapse P, Miller D, Cortes V, et al. Free combination of dutasteride plus tamsulosin for the treatment of benign prostatic hyperplasia in South Korea: analysis of drug utilization and adverse events using the National Health Insurance Review and Assessment Service database. BMC Urol. 2021;21(1):178.
doi pubmed pmc - Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Wein A. The standardization of terminology of lower urinary tract function: Report from the Standardization Sub-Committee of the International Continence Society. Urogynecology and Reconstructive Pelvic Surgery. 2007;562-573.
doi - Filson CP, Wei JT, Hollingsworth JM. Trends in medical management of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Urology. 2013;82(6):1386-1392.
doi pubmed pmc - Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, N'Dow J, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2013;64(1):118-140.
doi pubmed - Roehrborn CG, Siami P, Barkin J, Damiao R, Major-Walker K, Nandy I, Morrill BB, et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol. 2010;57(1):123-131.
doi pubmed - Cohen SA, Parsons JK. Combination pharmacological therapies for the management of benign prostatic hyperplasia. Drugs Aging. 2012;29(4):275-284.
doi pubmed - Debruyne F, Barkin J, van Erps P, Reis M, Tammela TL, Roehrborn C, Aria A, et al. Efficacy and safety of long-term treatment with the dual 5 alpha-reductase inhibitor dutasteride in men with symptomatic benign prostatic hyperplasia. Eur Urol. 2004;46(4):488-494; discussion 495.
doi pubmed - Hong SK, Min GE, Ha SB, Doo SH, Kang MY, Park HJ, Yoon CY, et al. Effect of the dual 5alpha-reductase inhibitor, dutasteride, on serum testosterone and body mass index in men with benign prostatic hyperplasia. BJU Int. 2010;105(7):970-974.
doi pubmed - The Editors of the Adis. Retraction-Current evidence supports the use of combination therapy in the management of benign prostatic hyperplasia. Drugs & Therapy Perspectives. 2012;28(12):5-8.
doi - McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, Foster HE, Jr., et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185(5):1793-1803.
doi pubmed - Hashimoto M, Shimizu N, Sugimoto K, Hongoh S, Minami T, Nozawa M, Yoshimura K, et al. Efficacy of Adding Dutasteride to alpha-Blocker Therapy Treated Benign Prostatic Hyperplasia Patients with Small Volume Prostate (<30 mL). Low Urin Tract Symptoms. 2017;9(3):157-160.
doi pubmed - Zhang H, Frendl DM, Wang Z, Olumi AF. High Real-World Medication Adherence and Durable Clinical Benefit in Medicare Patients Treated with 5-Alpha Reductase Inhibitors for Benign Prostatic Hyperplasia. J Urol. 2020;204(2):325-331.
doi pubmed pmc - Kurokawa S, Kamei J, Sakata K, Sugihara T, Fujisaki A, Ando S, Takayama T, et al. The cutoff value of transitional zone index predicting the efficacy of dutasteride on subjective symptoms in patients with benign prostate hyperplasia. Low Urin Tract Symptoms. 2022;14(4):261-266.
doi pubmed - Shigehara K, Kato Y, Kawaguchi S, Izumi K, Kadono Y, Mizokami A. A comparison of the efficacy of dutasteride on reducing lower urinary tract symptoms among patients with small versus large benign prostatic hyperplasia. Curr Urol. 2024;18(3):199-202.
doi pubmed pmc - Montorsi F, Henkel T, Geboers A, Mirone V, Arrosagaray P, Morrill B, Black L. Effect of dutasteride, tamsulosin and the combination on patient-reported quality of life and treatment satisfaction in men with moderate-to-severe benign prostatic hyperplasia: 4-year data from the CombAT study. Int J Clin Pract. 2010;64(8):1042-1051.
doi pubmed - Roehrborn CG, Barkin J, Siami P, Tubaro A, Wilson TH, Morrill BB, Gagnier RP. Clinical outcomes after combined therapy with dutasteride plus tamsulosin or either monotherapy in men with benign prostatic hyperplasia (BPH) by baseline characteristics: 4-year results from the randomized, double-blind Combination of Avodart and Tamsulosin (CombAT) trial. BJU Int. 2011;107(6):946-954.
doi pubmed - Yamanishi T, Asakura H, Seki N, Tokunaga S. A 52-week multicenter randomized controlled study of the efficacy and safety of add-on dutasteride and imidafenacin to tamsulosin in patients with benign prostatic hyperplasia with remaining overactive bladder symptoms (DIrecT study). Low Urin Tract Symptoms. 2019;11(3):115-121.
doi pubmed pmc - Hariton E, Locascio JJ. Randomised controlled trials - the gold standard for effectiveness research: Study design: randomised controlled trials. BJOG. 2018;125(13):1716.
doi pubmed pmc - Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
doi pubmed pmc - Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;134:103-112.
doi pubmed - Vrabel M. Preferred reporting items for systematic reviews and meta-analyses. Oncol Nurs Forum. 2015;42(5):552-554.
doi pubmed - Munn Z, Stern C, Aromataris E, Lockwood C, Jordan Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol. 2018;18(1):5.
doi pubmed pmc - Roy A, Singh A, Sidhu DS, Jindal RP, Malhotra M, Kaur H. New Visual Prostate Symptom Score versus International Prostate Symptom Score in Men with Lower Urinary Tract Symptoms: A Prospective Comparision in Indian Rural Population. Niger J Surg. 2016;22(2):111-117.
doi pubmed pmc - Taneja Y, Ram P, Kumar S, Raj K, Singh CK, Dhaked SK, Jaipuria J. Comparison of Visual Prostate Symptom Score and International Prostate Symptom Score in the evaluation of men with benign prostatic hyperplasia: A prospective study from an Indian population. Prostate Int. 2017;5(4):158-161.
doi pubmed pmc - Rivera-Oliva DD. Visual prostate symptom score (VPSS). Is it an effective alternative for the assessment of lower urinary tract symptoms? Experience in a reference center in southeastern Mexico. Revista Mexicana de Urologia. 2022;81(6):1-9.
doi - Yuan C, Ryan PB, Ta CN, Kim JH, Li Z, Weng C. From clinical trials to clinical practice: How long are drugs tested and then used by patients? J Am Med Inform Assoc. 2021;28(11):2456-2460.
doi pubmed pmc - Lee JY, Kang DH, Park SY, Lee SW, Kim YT, Choi HY, Moon HS. Effect of discontinuation of tamsulosin in korean men with benign prostatic hyperplasia taking tamsulosin and dutasteride: an open-label, prospective, randomized pilot study. Low Urin Tract Symptoms. 2012;4(1):35-40.
doi pubmed - Ngu H, Neo SH, Koh EYL, Ho H, Tan NC. Making shared decisions with older men selecting treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia (LUTS/BPH): a pilot randomized trial. J Patient Rep Outcomes. 2022;6(1):112.
doi pubmed pmc - Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet. 2002;359(9308):781-785.
doi pubmed - Reese JH, Lombard CM, Krone K, Stamey TA. Phyllodes type of atypical prostatic hyperplasia: a report of 3 new cases. J Urol. 1987;138(3):623-626.
doi pubmed - The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Chapter 8: Risk of Bias in Randomized Trials. 2021. Retrieved from https://training.cochrane.org/handbook/current.
- Higgins JPT, Altman DG, Gotzsche PC. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ. 2011;343:d5928.
doi - Chung BH, Lee SH, Roehrborn CG, Siami PF, Major-Walker K, Wilson TH, Montorsi F, et al. Comparison of the response to treatment between Asian and Caucasian men with benign prostatic hyperplasia: long-term results from the combination of dutasteride and tamsulosin study. Int J Urol. 2012;19(11):1031-1035.
doi pubmed - Roehrborn CG, Barkin J, Tubaro A, Emberton M, Wilson TH, Brotherton BJ, Castro R. Influence of baseline variables on changes in International Prostate Symptom Score after combined therapy with dutasteride plus tamsulosin or either monotherapy in patients with benign prostatic hyperplasia and lower urinary tract symptoms: 4-year results of the CombAT study. BJU Int. 2014;113(4):623-635.
doi pubmed - Roehrborn CG, Oyarzabal Perez I, Roos EP, Calomfirescu N, Brotherton B, Wang F, Palacios JM, et al. Efficacy and safety of a fixed-dose combination of dutasteride and tamsulosin treatment (Duodart((R)) ) compared with watchful waiting with initiation of tamsulosin therapy if symptoms do not improve, both provided with lifestyle advice, in the management of treatment-naive men with moderately symptomatic benign prostatic hyperplasia: 2-year CONDUCT study results. BJU Int. 2015;116(3):450-459.
doi pubmed - Chung JH, Oh CY, Kim JH, Ha US, Kim TH, Lee SH, Han JH, et al. Efficacy and safety of tamsulosin 0.4 mg single pills for treatment of Asian patients with symptomatic benign prostatic hyperplasia with lower urinary tract symptoms: a randomized, double-blind, phase 3 trial. Curr Med Res Opin. 2018;34(10):1793-1801.
doi pubmed - Haque N, Masumori N, Sakamoto S, Ye Z, Yoon SJ, Kuo HC, Brotherton B, et al. Superiority of dutasteride 0.5 mg and tamsulosin 0.2 mg for the treatment of moderate-to-severe benign prostatic hyperplasia in Asian men. Int J Urol. 2018;25(11):944-951.
doi pubmed - Choubey VK, Sankhwar SN, Carlus SJ, Singh AN, Dalela D, Thangaraj K, Rajender S. SRD5A2 gene polymorphisms and the risk of benign prostatic hyperplasia but not prostate cancer. Asian Pac J Cancer Prev. 2015;16(3):1033-1036.
doi pubmed - Kardasevic A, Milicevic S. The Correlation Between Prostate Volume in Patients with Benign Prostatic Hyperplasia in Relation to Erectile Dysfunction. Med Arch. 2016;70(6):449-452.
doi pubmed pmc - Jin B, Turner L, Zhou Z, Zhou EL, Handelsman DJ. Ethnicity and migration as determinants of human prostate size. J Clin Endocrinol Metab. 1999;84(10):3613-3619.
doi pubmed - Lam JS, Cheung YK, Benson MC, Goluboff ET. Comparison of the predictive accuracy of serum prostate specific antigen levels and prostate specific antigen density in the detection of prostate cancer in Hispanic-American and white men. J Urol. 2003;170(2 Pt 1):451-456.
doi pubmed - Lyseng-Williamson KA, Jarvis B, Wagstaff AJ. Tamsulosin: an update of its role in the management of lower urinary tract symptoms. Drugs. 2002;62(1):135-167.
doi pubmed - Kim JJ, Han DH, Sung HH, Choo SH, Lee SW. Efficacy and tolerability of tamsulosin 0.4 mg in Asian patients with lower urinary tract symptoms secondary to benign prostatic hyperplasia refractory to tamsulosin 0.2 mg: a randomized placebo controlled trial. Int J Urol. 2014;21(7):677-682.
doi pubmed - Lepor H. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Tamsulosin Investigator Group. Urology. 1998;51(6):892-900.
doi pubmed - Overland GB, Vatten L, Rhodes T, DeMuro C, Jacobsen G, Vada K, Angelsen A, et al. Lower urinary tract symptoms, prostate volume and uroflow in norwegian community men. Eur Urol. 2001;39(1):36-41.
doi pubmed - Han JH, Lee JY, Kwon JK, Lee JS, Cho KS. Clinical Significance of Periurethral Calcification According to the Location in Men With Lower Urinary Tract Symptoms and a Small Prostate Volume. Int Neurourol J. 2017;21(3):220-228.
doi pubmed pmc - Rosen RC, Roehrborn CG, Manyak MJ, Palacios-Moreno JM, Wilson TH, Lulic Z, Giuliano F. Evaluation of the impact of dutasteride/tamsulosin combination therapy on libido in sexually active men with lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH): A post hoc analysis of a prospective randomised placebo-controlled study. Int J Clin Pract. 2019;73(9):1-9.
doi pubmed pmc - Simon RM, Howard LE, Moreira DM, Roehrborn C, Vidal AC, Castro-Santamaria R, Freedland SJ. Does Prostate Size Predict the Development of Incident Lower Urinary Tract Symptoms in Men with Mild to No Current Symptoms? Results from the REDUCE Trial. Eur Urol. 2016;69(5):885-891.
doi pubmed - Kibel AS. Effect of dutasteride on the risk of prostate cancer. Yearbook of Urology. 2010;70-71.
doi - Frost JM, Smith LA, Sharma P, de Riese WT. Possible clinical implications of peripheral zone changes depending on prostate size. Int Urol Nephrol. 2019;51(10):1721-1726.
doi pubmed - Sellers J, Wagstaff RG, Helo N, de Riese WTW. Quantitative measurements of prostatic zones by MRI and their dependence on prostate size: possible clinical implications in prostate cancer. Ther Adv Urol. 2021;13:17562872211000852.
doi pubmed pmc - Guzman JA, Sharma P, Smith LA, Buie JD, de Riese WT. Histological changes of the peripheral zone in small and large prostates and possible clinical implications. Res Rep Urol. 2019;11:77-81.
doi pubmed pmc - Lorenzo G, Hughes TJR, Dominguez-Frojan P, Reali A, Gomez H. Computer simulations suggest that prostate enlargement due to benign prostatic hyperplasia mechanically impedes prostate cancer growth. Proc Natl Acad Sci U S A. 2019;116(4):1152-1161.
doi pubmed pmc - Holder K, Galvan B, Sakya J, Frost J, de Riese W. Anatomical Changes of the Peripheral Zone Depending on Benign Prostatic Hyperplasia Size and Their Potential Clinical Implications:A Review for Clinicians. Urol Pract. 2021;8(2):259-263.
doi pubmed - Barkin J, Guimaraes M, Jacobi G, Pushkar D, Taylor S, van Vierssen Trip OB. Alpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5alpha-reductase inhibitor dutasteride. Eur Urol. 2003;44(4):461-466.
doi pubmed - Piercy GB, Deber R, Trachtenberg J, Ramsey EW, Norman RW, Goldenberg SL, Nickel JC, et al. Impact of a shared decision-making program on patients with benign prostatic hyperplasia. Urology. 1999;53(5):913-920.
doi pubmed - Braddock CH, 3rd. The emerging importance and relevance of shared decision making to clinical practice. Med Decis Making. 2010;30(5 Suppl):5S-7S.
doi pubmed - Stacey D, Kryworuchko J, Bennett C, Murray MA, Mullan S, Legare F. Decision coaching to prepare patients for making health decisions: a systematic review of decision coaching in trials of patient decision AIDS. Med Decis Making. 2012;32(3):E22-33.
doi pubmed - Hanckel FS. The problem of induction in clinical decision making. Med Decis Making. 1984;4(1):59-68.
doi pubmed - Gummesson C, Sunden A, Fex A. Clinical reasoning as a conceptual framework for Interprofessional Learning: A Literature Review and a case study. Physical Therapy Reviews. 2018;23(1):29-34.
doi - D'Agate S, Chavan C, Manyak M, Palacios-Moreno JM, Oelke M, Michel MC, Roehrborn CG, et al. Impact of early vs. delayed initiation of dutasteride/tamsulosin combination therapy on the risk of acute urinary retention or BPH-related surgery in LUTS/BPH patients with moderate-to-severe symptoms at risk of disease progression. World J Urol. 2021;39(7):2635-2643.
doi pubmed pmc - Taguchi K, Cho SY, Ng AC, Usawachintachit M, Tan YK, Deng YL, Shen CH, et al. The Urological Association of Asia clinical guideline for urinary stone disease. Int J Urol. 2019;26(7):688-709.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.