| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 4, April 2025, pages 223-230
Examining the Role of Corticosteroids in the Management of Acute Interstitial Pneumonia: A Systematic Review
Carlos Valladaresa, b, e , Aalia Narvelb, c
, Bianna Koutsenkob, c
, Alexa Simonettib, c
, Fatima Mossolemb, c
, Alexis Chowb, c
, Samrat Gollapudib, c
, Usmaan Al-Shehabb, c
, Nicholas Averellb, c
, Katherine Chiapaikeo-Pocod
, Adam Kaplana
aDepartment of Internal Medicine, Rutgers Health - Community Medical Center, Toms River, NJ 08755, USA
bRowan-Virtua School of Osteopathic Medicine, Stratford, NJ 08084, USA
cFutures Forward Research Institute, Toms River, NJ 08753, USA
dDepartment of Critical Care Medicine, Rutgers Health - Community Medical Center, Toms River, NJ 08755, USA
eCorresponding Author: Carlos Valladares, Department of Internal Medicine, Rutgers Health - RWJBH Community Medical Center, Toms River, NJ 08755, USA
Manuscript submitted February 1, 2025, accepted March 24, 2025, published online April 5, 2025
Short title: Corticosteroids in the Management of AIP
doi: https://doi.org/10.14740/jocmr6186
| Abstract | ▴Top |
Background: Acute interstitial pneumonia (AIP), also known as Hamman-Rich syndrome, is a rapidly progressive interstitial lung disease. In addition to being challenging to diagnose, AIP is also difficult to treat. The mortality rate of AIP is greater than 70% due to the disease’s rapid progression. Furthermore, survivors are likely to develop chronic interstitial lung disease as a sequela. Treatment primarily focuses on supportive care, which consists of oxygenation through mechanical ventilation, administration of broad-spectrum antibiotics, and the use of corticosteroids. Although the use of steroids as empiric treatment is controversial and results on its mortality benefit are variable, some studies have shown high-dose pulse steroid therapy to be associated with better health outcomes. This review aimed to evaluate cases of AIP to better understand the role of corticosteroids in the management plan of these cases.
Methods: A systematic review was conducted using the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 guidelines. Outcomes of interest included patient age, sex, autoimmune condition, corticosteroid use, survival or expiration of patients, and time from hospitalization to expiration.
Results: Initial querying of the five databases yielded 376 articles. Following a thorough review, only 30 articles remained, comprising 42 patient cases. Of these cases, 62% of the patients survived, 36% expired, and 2% were unknown. The average stay from hospitalization to death was 20.2 days, and corticosteroid pulse doses were used as a first- or second-line treatment in 31% of patients.
Conclusion: The limitations of the evidence used in this study highlight the need for a greater output of higher-level evidence in the form of controlled trials and retrospective studies to help further elucidate the proper role and dosage of corticosteroids in the management plan of AIP with the ultimate goal of enhancing clinical decision-making and patient care. The findings of this systematic review, primarily based on observational data from case reports, highlight the critical need for treatment guidelines for this condition. The compilations of these cases also illustrated the diverse strategies employed by clinicians globally to save patients afflicted by this condition. While specific recommendations cannot be made based solely on these results, we anticipate that this comprehensive overview of varied clinical approaches from around the world will serve as a valuable resource for healthcare providers navigating the complexities of managing this condition.
Keywords: Acute interstitial pneumonia; Hamman-Rich syndrome; Corticosteroids; Pneumonia
| Introduction | ▴Top |
Acute interstitial pneumonia (AIP), also called Hamman-Rich syndrome, is a rapidly progressive interstitial lung disease with a mortality rate greater than 70%. The American Thoracic Society/European Respiratory Society classifies AIP as an idiopathic interstitial pneumonia [1]. In 1935, Louis Hamman and Arnold Rich first described this disease in a case report of four patients with rapidly developing fibrosis of the lungs that was characterized by the following: acute onset with severe hypoxia or respiratory failure, bilateral lung infiltrates on radiographic image, the absence of an identifiable trigger or predisposing condition, and diffuse alveolar damage (DAD) on histology [2]. Though the precise origin of a pulmonary insult remains elusive, the progression of the disease involves distinct stages: acute exudative, organizing proliferative, and fibrotic/healed [3, 4]. Complications associated with AIP include respiratory failure and acute respiratory distress syndrome (ARDS) [5].
Patients who develop AIP are typically around 55 years old without preexisting lung disease. Those affected present with a viral-like prodromal illness lasting 7 - 14 days before seeking medical attention [5]. Symptoms of AIP encompass dyspnea, cough, and fever, with notable physical exam findings including hypoxia, tachypnea, and widespread crackles on lung auscultation [5]. Given the non-specific presentation, a thorough clinical workup is necessary to rule out causes of pulmonary edema, screen for autoimmune and connective tissue diseases, and investigate microbiological causes that may lead to ARDS [5]. AIP is a diagnosis of exclusion, as clinical evaluation typically reveals the characteristic chest X-ray pattern of ARDS, marked by bilateral diffuse airspace opacities [5]. High-resolution computed tomography (HRCT) will reveal bilateral, symmetric lung infiltrates. Other findings may include ground-glass opacities and a restrictive pattern on pulmonary function testing [1]. Bronchoalveolar lavage (BAL) can aid in differentiating AIP from other pneumonias. BAL will reveal neutrophilia with atypical type II pneumocytes and hyaline membranes [5]. However, the final diagnosis of AIP is confirmed by histology findings of DAD on lung biopsy [6].
In addition to being challenging to diagnose, AIP is also difficult to treat [6, 7]. Treatment primarily focuses on supportive care, which consists of oxygenation through mechanical ventilation, administration of broad-spectrum antibiotics, and the use of corticosteroids. Although the use of steroids as empiric treatment is controversial and results on their mortality benefit are variable, data regarding their efficacy remain scarce, leaving clinicians with limited evidence to guide their use. Some studies have shown high-dose pulse steroid therapy to be associated with better health outcomes [8]. Nonetheless, opinions on the efficacy of steroids in AIP treatment are divided, contributing to the ongoing debate surrounding their use in clinical practice. The objective of this systematic review was to assess the role of steroid usage in the treatment of AIP through an analysis of case reports, case series, and case-control studies to gain deeper insights into their implication in managing this condition.
| Materials and Methods | ▴Top |
A systematic review was conducted using the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 guidelines.
Inclusion and exclusion criteria
Studies that consisted of primary clinical data on the use of corticosteroids in the management of AIP were analyzed. Case reports, case series, and case-control studies were included in the inclusion criterion. Outcomes of interest included patient sex, age, autoimmune condition, corticosteroid use, survival or expiration of patients, and time from hospitalization to expiration.
Only adult patients diagnosed with AIP and who were administered corticosteroid therapy, regardless of age, comorbid conditions, or organ dysfunction were included. The inclusion of patients with comorbidities, such as autoimmune conditions and diabetes, is detailed in the “Results” section. Due to limited data available, articles published more than 10 years ago were included for analysis in this review.
Articles were excluded from analysis if they did not contain AIP diagnosis, corticosteroid treatment, or a specific outcome of interest, as well as cases in which AIP was drug-induced. Articles were not excluded based on nation of origin. Articles were excluded if they were not in English or if they contained infectious or chronic causes of interstitial pneumonia. Studies were not differentiated based on the dose of corticosteroids used. Articles without full text available, studies still in progress without any reported data or animal studies were also excluded.
Information sources and search strategy
This systematic review utilized five medical databases to search for articles on the use of corticosteroid treatment in patients with AIP. These databases include PubMed, Embase, Scopus, Web of Science, and the Cochrane Library. Search terms utilized were: (“acute interstitial pneumonia” OR “Interstitial Pneumonia, Acute” OR “Acute Interstitial Pneumonitis”) OR (“Hamman Rich Syndrome” OR “Hamman-Rich Disease”) AND (“steroids” OR “glucocorticoids” OR “corticosteroids” OR “Corticoids” OR “Corticoid” OR “Glucocorticoid”). The initial article search was conducted by CV on January 21, 2024. Additionally, a gray literature search was performed to identify any unpublished studies, conference abstracts, and government reports relevant to this topic. However, this search did not yield any additional studies that met the inclusion criteria.
Duplicate studies were identified and resolved utilizing Rayyan.ai online software. Following the identification of duplicate articles, two reviewers (CV and NA) manually sorted through the retrieved articles to ensure that no further duplicates existed.
Study selection
Once duplicates were identified and sorted through, title and abstract analysis were conducted for inclusion. Following title and abstract analysis, a full-text appraisal was completed by two trained reviewers (CV and NA). In the event of debate over the inclusion of a study, a third reviewer was brought in to break ties. Studies determined to be eligible for data analysis were subjected to data extraction.
Data collection and analysis
Full-text appraisal was performed through an initial critical appraisal, followed by data extraction. Extracted data were examined for relevance, significance, and generalizability. The primary measure extracted by the reviewers was the outcome of patients diagnosed with AIP treated with corticosteroids. Articles were then subjected to critique of design (Fig. 1) [9].
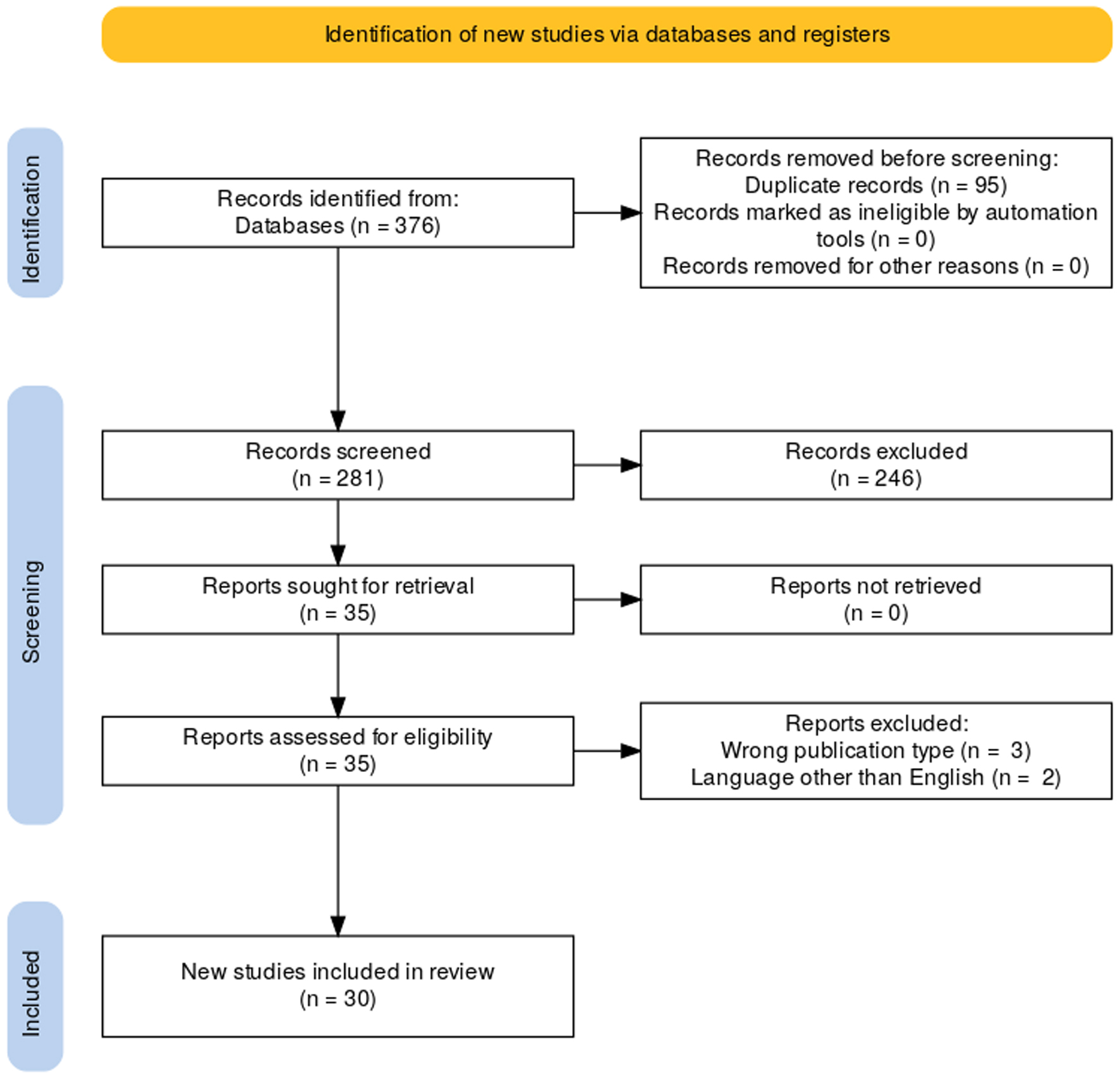 Click for large image | Figure 1. Article selection flow sheet per Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 guidelines [9]. |
Certainty of evidence and risk of bias assessment
Included articles were subjected to scoring for the certainty of evidence using Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria by two reviewers (AS and AC) [10]. Articles were then assessed for bias based on their respective study design.
The methodological quality of the studies was evaluated utilizing a modified version of the Newcastle-Ottawa Scale (NOS), tailored for case series, case reports, and case-control studies, as devised by Murad et al [11]. The trained reviewers employed an identical procedure to reach a consensus on the methodological quality, also termed as risk of bias, of the studies, mirroring the approach utilized for the GRADE quality of evidence assessment. The summarized results can be found in Table 1 [2, 6, 7, 12-38].
Institutional Review Board approval
This study is a systematic review of published case reports and case series and does not involve human subjects directly; therefore, Institutional Review Board (IRB) approval was not required.
Ethical compliance with human/animal study
Ethical compliance was not applicable, as this study does not involve human or animal subjects.
| Results | ▴Top |
Querying of the four databases yielded 376 articles. Detection of duplicates via Rayyan.ai’s automatic function and subsequent removal yielded 281 original articles. Following duplicates, articles were then subjected to abstract and title appraisal. Out of the 281 articles, only 35 remained. Full-text appraisal was then conducted on the 35 articles with 30 satisfying inclusion criteria. Patient data were then extracted from the included articles and analyzed (Table 2).
Full-text articles excluded from analysis
Of the articles subjected to full-text appraisal, five were excluded from the final analysis. Three of the articles were of the wrong publication type [39-41]. This meant that the articles were not case-control studies, case series, or case reports. Two articles were also removed as they were written in a language different from English [42, 43].
Results of quality of evidence rating and risk of bias assessment
Regarding GRADE scoring, all studies were rated as low and very low evidence quality, primarily due to their status as case reports, case series, or case-control studies.
Regarding methodological quality for risk of bias, most studies answered “yes” to all questions. Summarized results can be found in Supplementary Material 1 (jocmr.elmerjournals.com). However, not all studies satisfied all the assessments of bias and are listed as follows. Fleishman et al and Quintero et al both did not report follow-up [13, 25].
Overview of outcomes
Of the included articles, a total of 42 patient cases were identified, spanning 1958 to 2023 (n = 42). Twenty patients were reported to be female (47.6%), 19 were male (45.2%), and three (7.1%) had unknown gender. Patient ages ranged from 20 to 78 years old. The average age was 51 years old (median: 52). Four patients had a history of autoimmune disease (polymyalgia rheumatica, psoriasis, and Sjogren) (9.52%) and another four patients had diabetes mellitus (9.52%). Twenty-six patients survived (62%) and 15 expired (36%) which represented a mortality rate of 33,000 per 100,000 individuals. One patient (2%) was transferred to a different facility and the final outcome was not reported. Patients who expired had a mean of 20.2 days from hospitalization to death.
Pulse dose steroid
Of the 13 cases in which pulse dose steroid (methylprednisolone 1 g daily) was used, nine cases [6, 18-21, 26, 28, 29] reported using it as first-line treatment, out of which five patients survived, while four expired. On the other hand, four cases [2, 15, 22, 33] reported using pulse dose steroid as second-line treatment which resulted in the survival of three patients with one expiring (Table 3).
Additional immunosuppressive therapy
A total of eight cases were reported using cyclophosphamide, cyclosporine, and mycophenolate, either alone or in combination, as additional immunosuppressive therapy [15, 30, 32, 33, 35]. Temmesfeld-Wollbruck et al reported three cases in which cyclophosphamide was used, and all of them resulted in fatal outcomes [30]. Villafuerte et al reported using a combination of cyclophosphamide and cyclosporine, also resulting in the expiration of the patient [32]. Two cases were reported in which mycophenolate was used and both resulted in patient survival (Table 4) [33, 35].
| Discussion | ▴Top |
We present the most recent systematic review of case reports on this topic; however, as of May 2024, there is still no consensus on the role of corticosteroids in the treatment of AIP.
Reviewal of results and implications
To understand whether steroids can reduce the mortality rate of AIP, 42 patient cases were reviewed dating from 1958 to 2023, with a mix of genders and ages. Notably, 26 patients survived (62%) while 15 expired (36%), resulting in a mortality rate of 33,000 per 100,000 individuals. It is important to note that the gender distribution was balanced with 47.6% female patients and 45.2% male patients. Comorbidities were present in a few of the patients, namely 9.52% with autoimmune diseases and 9.52% with diabetes mellitus. Methylprednisolone used as first- or second-line treatment did not show much of a difference in terms of outcome as both groups had higher rates of survival. Additional immunosuppressive therapy did show a markable difference favoring mycophenolate over cyclophosphamide.
Common adverse effects of corticosteroid therapy, such as hyperglycemia, increased infection risk, hypertension, psychosis, and osteoporosis, were not consistently reported in the included cases. However, clinicians should be vigilant regarding these potential complications, particularly in patients with preexisting diabetes or immunosuppression.
Treatment modalities differed between patients including the use of different corticosteroids (methylprednisolone, prednisone, prednisolone, hydrocortisone, dexamethasone, corticotropin, and cortisone), time courses of treatment, additional treatments used (cyclophosphamide, mycophenolate, cyclophosphamide + cyclosporine, and no additional treatment), and points in disease course where treatment was initiated. For instance, in the case series included by Bonaccorsi et al in 2003, patients were given methylprednisolone at different times in their disease course, with the patient who survived getting treatment earliest in the disease course [35]. Many of the studies did not include the dosage or type of corticosteroid used, creating further ambiguity regarding the effectiveness of a certain dose or type of corticosteroid for the treatment of AIP. The patient in the study by Horn et al in 2018 had a do-not-resuscitate (DNR) order, which may have affected the chances of survival [37]. This may also be true for other studies that had a higher risk of bias and may not have reported whether the patient was DNR.
In addition, the patients included in this study had varying medical histories, including patients with and without prior lung disease, heart disease, metabolic conditions, diabetes mellitus, and autoimmune conditions; however, the data were insufficient to establish a correlation between these conditions and steroid therapy outcomes. Some of the patients were smokers while others were nonsmokers, introducing another confounding variable. There was also a significant age range among the patients, spanning from 20 to 78. The effectiveness of corticosteroids as treatment may be affected by these factors. For example, in the study by Quefatieh et al in 2003, the patients were aged 20 - 78 and the patient aged 78 was the patient who expired [34].
Based on the synthesized data from this review, we propose a decision-making flowchart to assist clinicians in managing AIP with corticosteroids (Fig. 2). This flowchart presents a simplified treatment algorithm in the absence of standardized guidelines and is designed to assist in clinical decision-making.
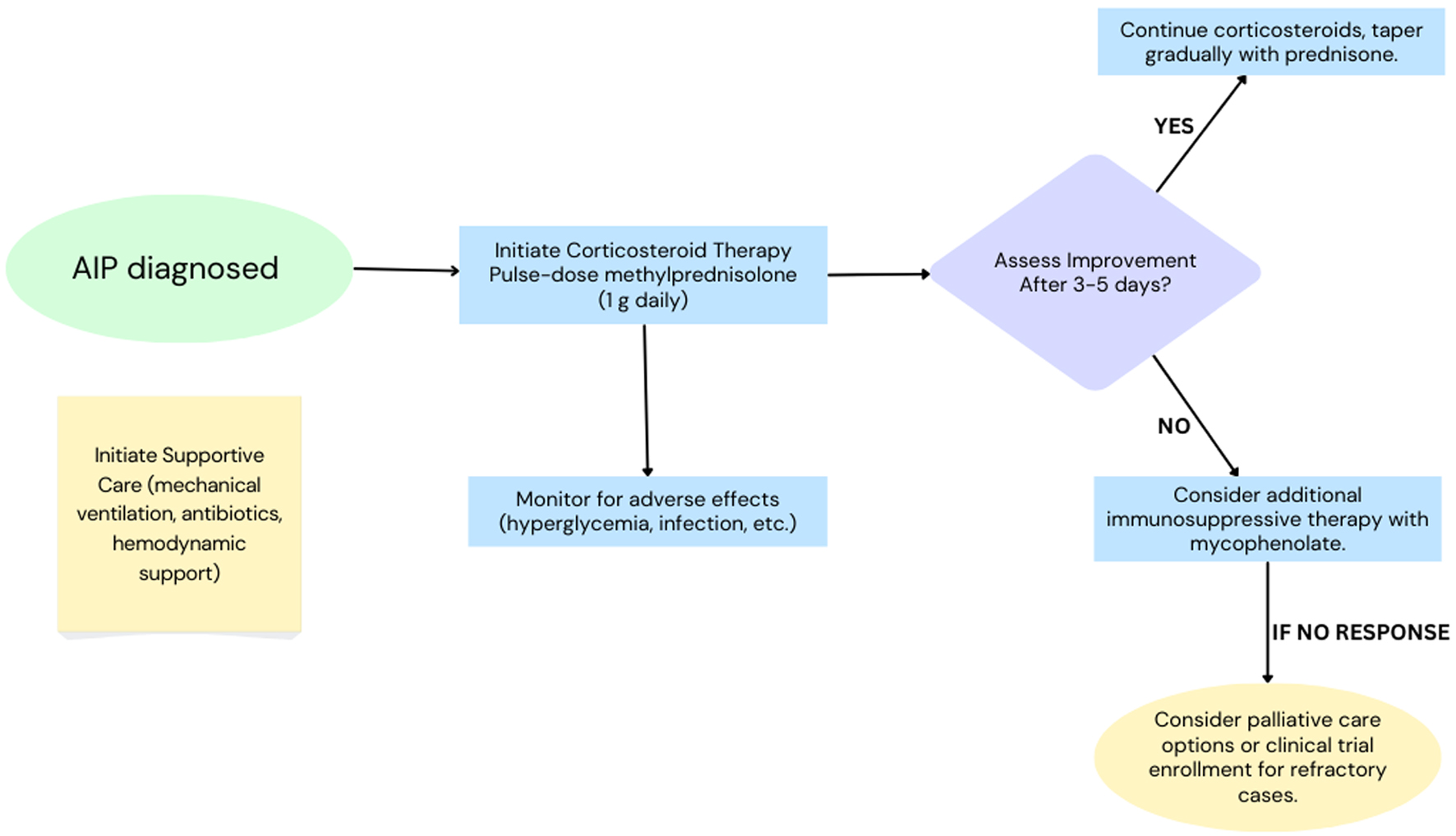 Click for large image | Figure 2. Proposed clinical decision-making pathway for management of acute interstitial pneumonia (AIP). |
Limitations
This study was not without limitations. The limited sample size of the study, coupled with the variability in steroid types, doses, and durations of administration among the 42 patients, complicates the interpretation of the results. When analyzing the included studies using GRADE and NOS, all studies had very low quality of evidence. A majority of the studies included were case reports or case series, which lacked control groups for comparative analysis. The sole case-control study conducted by Quefatieh et al in 2003 did not compare the use of corticosteroids to other treatment modalities. Instead, it compared AIP to ARDS, and therefore, lacked a control relevant to the research question [34]. In addition to the low quality of evidence, the study publications spanned from 1957 to 2023, a period during which medical practices have changed significantly, potentially affecting treatment outcomes.
Conclusion
The analysis of the data revealed significant challenges in drawing definitive conclusions regarding the impact of how different doses of steroids affect AIP mortality rates. While this review provides valuable insights, the variability in the data and the small sample size highlight the need for further research to understand the role of steroids in the management of AIP. Additional studies with larger patient populations are warranted to establish a clearer understanding of the effectiveness of steroids in treating AIP.
Future considerations
Future research should further delineate the role of corticosteroids in AIP management, particularly with respect to dose, duration, and timing of initiation. Studies should also investigate the impact of comorbid conditions, such as autoimmune diseases, diabetes mellitus, chronic kidney disease, and cardiovascular disease, on corticosteroid efficacy and patient prognosis. Stratifying patients based on these factors could help to identify subgroups that may benefit from specific treatment protocols or closer monitoring. In addition, research is needed to evaluate the efficacy and safety of additional immunosuppressive agents, such as mycophenolate, and to determine the optimal timing and dosing of these therapies.
The proposed decision-making flowchart (Fig. 2) offers an interim framework to aid clinicians in standardizing treatment decisions for AIP until formal guidelines are developed. Future studies should validate this approach and explore its applicability in diverse clinical settings.
| Supplementary Material | ▴Top |
Suppl 1. Risk of bias for included studies.
Acknowledgments
The authors would like to thank David F. Lo for editing this manuscript. We also appreciate the contributions of all individuals who assisted in the literature review and data extraction process.
Financial Disclosure
No financial support, funding, or sponsorship was received for the conduct of this study or the preparation of this manuscript.
Conflict of Interest
The authors declare no conflict of interest related to this study.
Informed Consent
Not applicable, as this study is a systematic review and does not involve individual patient data.
Author Contributions
Carlos Valladares: conceptualization, methodology, data extraction, writing - original draft, supervision, and final manuscript approval. Aalia Narvel: data extraction, literature review, and writing - review and editing. Bianna Koutsenko: data analysis and writing - review and editing. Alexa Simonetti: literature search, methodology, and data verification. Fatima Mossolem: writing - review and editing, and figure/table preparation. Alexis Chow: data extraction and statistical analysis. Samrat Gollapudi: writing - review and editing, and data visualization. Usmaan Al-Shehab: literature review and methodology. Nicholas Averell: writing - review and editing, and supervision. Katherine Chiapaikeo-Poco: data analysis and figure/table preparation. Adam Kaplan: conceptualization, supervision, and final manuscript approval.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
AIP: acute interstitial pneumonia; ARDS: acute respiratory distress syndrome; BAL: bronchoalveolar lavage; DAD: diffuse alveolar damage; DNR: do-not-resuscitate; GRADE: Grading of Recommendations, Assessment, Development, and Evaluation; HRCT: high-resolution computed tomography; NOS: Newcastle-Ottawa Scale; PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses
| References | ▴Top |
- Travis WD, Costabel U, Hansell DM, King TE, Jr., Lynch DA, Nicholson AG, Ryerson CJ, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733-748.
doi pubmed - William N, Puopolo A, Weiler M, Casserly B. Hamman-rich syndrome: a forgotten entity. Monaldi Archives for Chest Disease. 2017;87(1).
doi - Olson J, Colby TV, Elliott CG. Hamman-Rich syndrome revisited. Mayo Clin Proc. 1990;65(12):1538-1548.
doi pubmed - Katzenstein AL, Myers JL, Mazur MT. Acute interstitial pneumonia. A clinicopathologic, ultrastructural, and cell kinetic study. Am J Surg Pathol. 1986;10(4):256-267.
pubmed - Mrad A, Huda N. Acute Interstitial Pneumonia. In: StatPearls. Treasure Island (FL) companies. 2025.
pubmed - Mastan A, Murugesu N, Hasnain A, O'Shaughnessy T, Macavei V. Hamman-Rich syndrome. Respir Med Case Rep. 2018;23:13-17.
doi pubmed - Gomercic Palcic M, Turalija I, Vrbanic L, Folnozic I, Basioli Kasap E, Ulamec M. Successfully treated acute interstitial pneumonia. Acta Clin Croat. 2022;61(4):722-726.
doi pubmed - Chertoff J, Alnuaimat H. A 45-year-old woman with acute interstitial pneumonia (Hamman-Rich Syndrome). Turk J Anaesthesiol Reanim. 2017;45(4):244.
doi pubmed - Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
doi pubmed - Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383-394.
doi pubmed - Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60-63.
doi pubmed - Khan A, Humayun M, Haider I, Ayub M, Shah Z, Ajmal F. Primary Sjogren's Syndrome presenting as Acute Interstitial Pneumonitis/Hamman-Rich Syndrome. Case Rep Med. 2016;2016:4136765.
doi pubmed - Fleishman SJ, Bosman AR, Fuller DN. Diffuse interstitial fibrosis of the lungs; successful treatment of a case, with adrenal steroids. Am J Med. 1958;24(5):823-830.
doi pubmed - Freedman A, Deaton WR, Jr. Diffuse interstitial fibrosis of the lungs (Hamman-Rich syndrome); successful palliation of a case with steroid therapy. Dis Chest. 1958;34(5):557-561.
doi pubmed - Markoska F, Lestan D, Turel M, Harlander M. Acute interstitial pneumonia triggered by strenuous exercise. Respir Med Case Rep. 2020;30:101077.
doi pubmed - Anton E, De Miguel J, Hermida JA, Aramburu JA, Jara B, Juretschke MA. Acute interstitial pneumonia: Favorable outcome with corticosteroid therapy. Arch Bronconeumol. 2001;37(7):286-288.
doi - Bolton J, Garl J. A case of acute interstitial pneumonitis with a very atypical clinical presentation, radiologic findings, and response to corticosteroid therapy. Chest. 2014;146(4).
doi - Haque UD, Close J, Modugula S, Harris RJ. A Case of Acute Interstitial Pneumonia during the COVID-19 Pandemic. American Journal of Respiratory and Critical Care Medicine. 2021;203(9).
doi - Jarrett S, Matta A, Benzaquen S. A case of acute interstitial pneumonia (AIP). American Journal of Respiratory and Critical Care Medicine. 2022;205(1).
doi - Lin N, Hippensteel JA, Cool C, Janssen WJ. Early diagnosis and treatment of acute interstitial pneumonia. American Journal of Respiratory and Critical Care Medicine. 2018;197.
- Nguyen M, Prendergast A, Mohan N, Cheema RS, Benzaquen S, Aurelio A Diaz Caballero Luis. Diffuse alveolar damage in a 37-year-old man with acute shortness of breath. Chest. 2023;164(4):A3340-A3341.
doi - Ni X, She J, Gu C, Zhu L. Successful treatment of acute interstitial pneumonia in an immunocompromised lymphoma patient with “induced inflammation”. American Journal of Respiratory and Critical Care Medicine. 2018;2018:197.
- Imokawa S, Sano T, Todate A, Uchiyama H, Yasuda K, Nagayama M, Enomoto N, et al. [Two cases of acute respiratory failure due to idiopathic interstitial pneumonia successfully treated with sivelestat sodium hydrate and steroid therapy]. Nihon Kokyuki Gakkai Zasshi. 2006;44(1):27-33.
pubmed - Podugu A, Dasari C, Boutros N. A case of acute interstitial pneumonia (AIP) presenting as crazy paving pattern on HRCT (high resolution CT scan). American Journal of Respiratory and Critical Care Medicine. 2014;189.
- Quintero J, Akkineni S, Patel H. A rare case of acute interstitial pneumonia (hamman-rich syndrome). Journal of Hospital Medicine. 2018;13(4).
- Salim MU, Mustahsan SM, Fatima A. Abruptio placentae with type II respiratory failure secondary to acute interstitial pneumonia responsive to steroids. J Coll Physicians Surg Pak. 2017;27(9):S106-S107.
pubmed - Schmidt B, Khanna V, Banthiya S, Soueidan AS. A rare case of acute interstitial pneumonia in pregnancy. Chest. 2023;164(4):A3235-A3236.
doi - Sprague K, Klenz J. Hamman-rich syndrome: An atypical presentation with recurrence and indolence. American Journal of Respiratory and Critical Care Medicine. 2010;181(1).
- Tamama K, Yamato T, Yoshida M, Sakahira K, Suzuki T, Ishii T, Saito N, et al. A case of probable acute interstitial pneumonia with a dramatic response to pulse corticosteroid administration. J Med. 2000;31(1-2):77-89.
pubmed - Temmesfeld-Wollbruck B, Morr H, Suttorp N, Altmannsberger M, Seeger W. [Three cases of a fulminant course of idiopathic pulmonary fibrosis (Hamman-Rich syndrome)]. Pneumologie. 1993;47(10):573-578.
pubmed - Van Slyck EJ. Case report: diffuse interstitial pulmonary fibrosis (Hamman-Rich syndrome); diagnosis by lung biopsy; treated with cortico-steroids. Dis Chest. 1957;31(5):593-598.
doi pubmed - Villafuerte D, Koch K, Amalakuhan B, Simpson T. Fatal outcome in a patient with acute interstitial pneumonia despite immunosuppressive therapy. American Journal of Respiratory and Critical Care Medicine. 2017;195.
doi - Wu S, Lang EA. COVID-19 mimicker: Treatment of acute interstitial pneumonia during the pandemic. American Journal of Respiratory and Critical Care Medicine. 2021;203(9).
doi - Quefatieh A, Stone CH, DiGiovine B, Toews GB, Hyzy RC. Low hospital mortality in patients with acute interstitial pneumonia. Chest. 2003;124(2):554-559.
doi pubmed - Bonaccorsi A, Cancellieri A, Chilosi M, Trisolini R, Boaron M, Crimi N, Poletti V. Acute interstitial pneumonia: report of a series. Eur Respir J. 2003;21(1):187-191.
doi pubmed - Bruminhent J, Yassir S, Pippim J. Acute interstitial pneumonia (hamman-rich syndrome) as a cause of idiopathic acute respiratory distress syndrome. Case Rep Med. 2011;2011:628743.
doi pubmed - Horn C, Shah SF, Staiano P, Seeram V. A case of rapidly progressing respiratory failure. Journal of Investigative Medicine. 2018;66(2):539.
doi - Mangoni AA, Desai SR, Shaikh H, Barker RD, Mufti GJ, Jackson SH. An unusual case of pneumonia. Int J Clin Pract. 2003;57(2):153-154.
pubmed - Feuillet S, Tazi A. [Acute interstitial pneumonia: diagnostic approach and management]. Rev Mal Respir. 2011;28(6):809-822.
doi pubmed - Vourlekis JS, Brown KK. Acute interstitial pneumonia: A clinical review. J Respir Dis. 2001;22(1):14-21.
- Avnon LS, Pikovsky O, Sion-Vardy N, Almog Y. Acute interstitial pneumonia-Hamman-Rich syndrome: clinical characteristics and diagnostic and therapeutic considerations. Anesth Analg. 2009;108(1):232-237.
doi pubmed - Lee KY, Jee YK, Kim YS, Myong NH, Park JS. Efficacy of early steroid therapy in acute interstitial pneumonia. Tuberc Respir Dis (Seoul). 2002;52(5):519-528.
doi - Wang J, Chen Y, Sun Q. [Acute interstitial pneumonia: report of a case]. Zhonghua Nei Ke Za Zhi. 1997;36(11):744-747.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.