| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Case Report
Volume 17, Number 4, April 2025, pages 240-246
Intensive Granulocyte and Monocyte Adsorptive Apheresis Plus Upadacitinib for Induction Treatment of Refractory Crohn’s Disease
Satoshi Tanidaa, b, c, Naoto Imurab, Shun Sasohb, Yoshimasa Kubotab, Tesshin Banb, Tomoaki Andob, Makoto Nakamurab, Takashi Johb
aEducation and Research Center for Community Medicine, Nagoya City University Graduate School of Medical Sciences, Nagoya, Aichi 467-8601, Japan
bDivision of Gastroenterology, Gamagori City Hospital, Gamagori, Aichi 443-8501, Japan
cCorresponding Author: Satoshi Tanida, Education and Research Center for Community Medicine, Nagoya City University Graduate School of Medical Sciences, Nagoya, Aichi 467-8601, Japan
Manuscript submitted January 22, 2025, accepted March 28, 2025, published online April 5, 2025
Short title: Intensive GMA Plus UPA for Refractory CD
doi: https://doi.org/10.14740/jocmr6188
| Abstract | ▴Top |
Case 1 involved a 34-year-old woman who had been diagnosed with Crohn’s disease (CD) at 30 years old. After deciding to discontinue CD treatment, she was diagnosed with moderate flare-up of CD based on disease activity and endoscopic findings. Inadequate response was seen 7 days after starting oral prednisolone (PSL) at 30 mg/day, so combination therapy was started with intensive granulocyte and monocyte adsorptive apheresis (GMA) plus upadacitinib (UPA) at 45 mg/day. Twelve weeks after starting this combination therapy, clinical remission and endoscopic and histological improvements of the inflamed mucosa were achieved with no adverse events. Case 2 involved a 26-year-old man who had been diagnosed with CD at 13 years old. He was diagnosed with severe flare-up of CD based on disease activity and endoscopic findings due to loss of response to double doses of infliximab (IFX). Combination therapy was started with intensive GMA plus UPA at 45 mg/day. Twelve weeks after starting this therapy, clinical remission and endoscopic and histological improvements of the inflamed mucosa were achieved with no adverse events. The combination of intensive GMA plus UPA appears to have provided an effective therapeutic option for refractory CD in a patient with a 4-year history of CD and refractoriness to systemic corticosteroids, and in another patient with a 13-year history of CD and loss of response to IFX.
Keywords: Crohn’s disease; Upadacitinib; Intensive granulocyte and monocyte adsorptive apheresis; Clinical remission; Histological improvement
| Introduction | ▴Top |
Crohn’s disease (CD) often affects the entire digestive system and organs via chronic, progressive, transmural inflammation of the alimentary tract. Treatments for moderate-to-severe CD include 5-aminosalicylic acid (5-ASA), corticosteroids, thiopurines, and biologics such as neutralizing antibodies against tumor necrosis factor (TNF)-α [1, 2], interleukin (IL)-12 and/or -23 [3, 4], integrin α4β7 [5], and Janus kinase (JAK) inhibitors [6]. The therapy algorithm for luminal CD refractory to corticosteroids has recommended the use of biologics including anti-TNF-α antibodies, anti-IL-12 and/or -23 antibodies, or anti-integrin α4β7 antibodies. In addition, the algorithm for CD showing loss of response to anti-TNF-α antibodies has recommended changes to other anti-TNF-α antibodies or other classes of biologic agents [7].
Intensive granulocyte and monocyte adsorptive apheresis (GMA) is also available in Europe and Japan for the treatment of patients with active CD that may or may not be refractory to standard pharmacotherapy and biologics [8, 9]. Moreover, combination therapy with intensive GMA plus another biologic agent with a different mechanism of action as a switch from anti-TNF-α antibodies in patients with refractory CD who present with loss of response to anti-TNF-α antibodies has proven effective and allows rapid tapering off corticosteroids [10].
Upadacitinib (UPA) is a JAK inhibitor selective for JAK1 and was approved for the treatment of moderate-to-severe CD in Japan in June 2023. No reports appear to have described the efficacy of combination therapy with intensive GMA in combination with UPA in patients with refractory CD.
Here, we evaluated the efficacy and safety of intensive GMA in combination with UPA in two cases comprising one patient showing CD refractory to treatment with systemic corticosteroids and another who had shown loss of response to infliximab (IFX). These cases are discussed with regard to clinical remission, and endoscopic and histological improvements within 12 weeks.
| Case Reports | ▴Top |
Case 1
A 34-year-old woman had been diagnosed with colitis-type CD in April 2019, at 30 years old. At this time, oral prednisolone (PSL) was temporarily started at 50 mg/day and tapered off within a short period along with mesalazine at 3,000 mg/day. Azathioprine (AZA) was subsequently added at 100 mg/day. These treatments induced clinical remission. AZA was then decreased to 25 mg/day. The patient independently made the decision to discontinue the 25-mg/day AZA in 2019 and subsequently experienced exacerbation of CD. Oral PSL was temporarily re-started at 40 mg/day and tapered off in a short time, with addition of mesalazine at 3,000 mg/day and AZA at 25 mg/day when a flare occurred. She repeatedly experienced several cycles of clinical remission and flare.
The patient was referred to our division by another gastroenterologist in September 2023, at 34 years old, due to rapid exacerbation of CD symptoms with fever reaching 37.8 °C, and repeated episodes of severe abdominal pain and diarrhea (10 times/day) accompanied by bloody stool (CD activity index (CDAI), 376). The patient had no other medical history of note and no family history of CD. Her height was 152 cm and her weight was 56 kg (body mass index, 24.2 kg/m2). Body weight loss (-2 kg within the previous 1 month) had been observed. Laboratory investigations showed: white blood cell (WBC) count, 9,100/µL; red blood cell (RBC) count, 479 × 104/µL; hemoglobin (Hb), 14.1 g/dL; total protein (TP), 6.7 g/dL; albumin (Alb), 3.2 g/dL; aspartate aminotransferase (AST), 18 U/L; alanine aminotransferase (ALT), 26 U/L; and C-reactive protein (CRP), 10.67 mg/dL. A negative result was obtained from an antigenemia test for cytomegalovirus (C-7HRP test). Neither the pathogenic microbe Clostridium difficile (C. difficile) nor its A and/or B toxins were detected in stool cultures (Table 1).
 Click to view | Table 1. Baseline Laboratory Findings in Case 1 |
Colonoscopy at baseline in September 2023 showed marked erythema and multiple ulcers fused with adjacent ulcers and/or longitudinally aligned along the mucosa of the cecum and ascending, transverse, and descending colon and mild erythema in the rectum as discontinuous skipped lesions (Fig. 1a). Simple Endoscopic Score for Crohn’s disease (SES-CD) was 29, reflecting severe activity. Histopathological examinations revealed moderate-to-severe infiltration of neutrophils, eosinophils, and lymphocyte aggregates in the mucosa and submucosa with erosive and ulcerative epithelium. Immunohistochemical examination of colon biopsy specimens for cytomegalovirus also yielded negative results. Based on all these findings, moderately active CD was diagnosed.
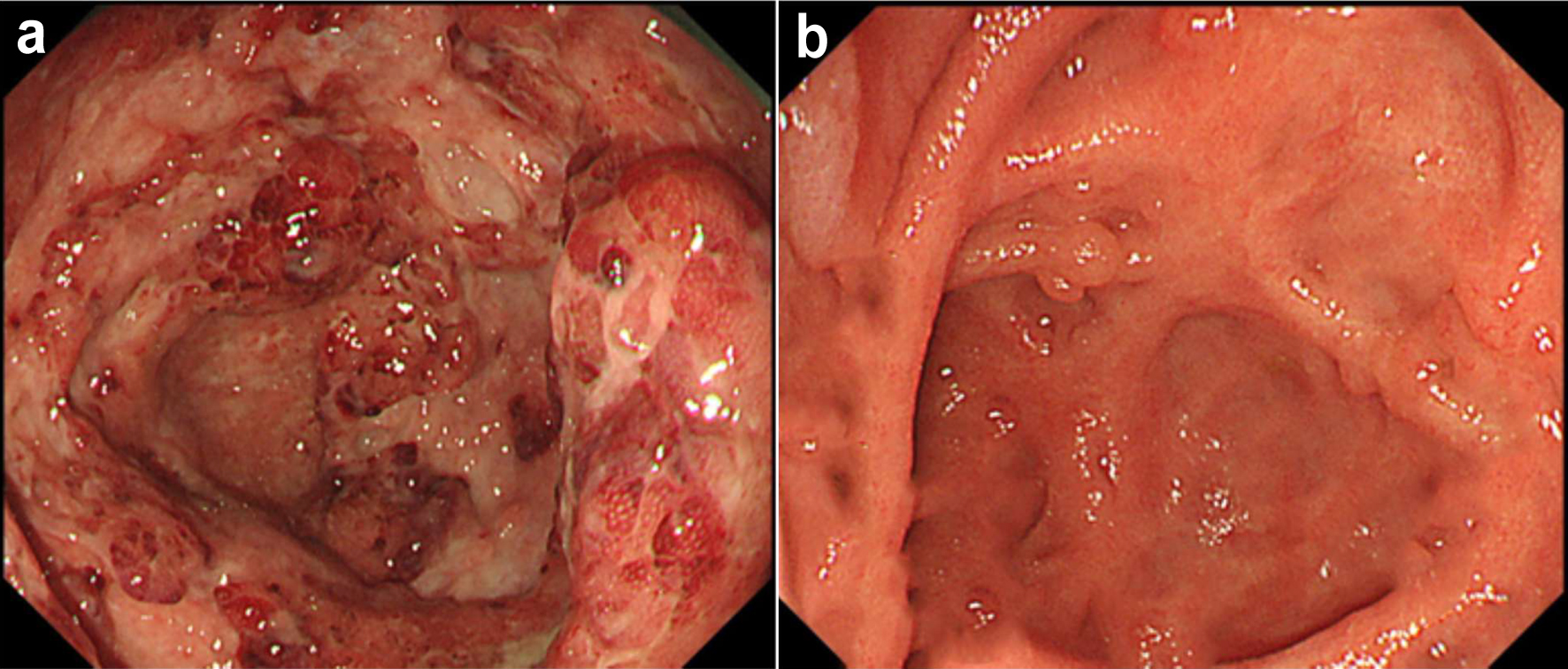 Click for large image | Figure 1. Colonoscopic findings at baseline and 12 weeks in case 1. (a) At baseline, colonoscopy shows marked erythema, edematous mucosa and multiple ulcers fused with the adjacent ulcer in the mucosa of the cecum. (b) At 12 weeks after starting combination therapy, colonoscopy shows decreased vascular patterns with mild erythema in a diffuse (50-75%) area in the cecum. |
As severe abdominal pain and bloody diarrhea persisted and the patient showed inadequate response at 7 days after starting oral PSL at 30 mg/day, intensive GMA (2 sessions a week; total, 10 times) in combination with UPA at 45 mg/day was started for corticosteroid-refractory CD. PSL was tapered off from 14 days after initiating this therapy. By 12 weeks after starting combination therapy with intensive GMA plus UPA, clinical remission had been achieved (CDAI, 33). Follow-up endoscopy after 12 weeks showed no ulcerated surfaces, no narrowed lumens, and decreased vascular pattern with mild erythema in a diffuse (50-75%) area of the cecum and ascending and descending colon, and a decreased vascular pattern in the transverse colon and rectum (SES-CD, 6), reflecting mild activity (Fig. 1b). Histopathological findings from colon biopsies taken from the area presenting with mild erythema revealed a mild increase in eosinophils and no increase in neutrophils in the lamina propria, and no non-caseating granuloma, reflecting histological improvement of the mucosa. Laboratory investigations at 12 weeks showed: WBC count, 4,500/μL; creatinine phosphokinase (CK), 110 U/L; total cholesterol (TCHO), 225 mg/dL; triglycerides (TG), 191 mg/dL; and CRP, 0.01 mg/dL. No adverse events were observed within the 12-week induction period.
Case 2
A 26-year-old man had been diagnosed with colitis-type CD in March 2011, at 13 years old, based on colonoscopic findings showing longitudinal ulcers and marked erythema with mucosal swelling in the transverse, descending, and sigmoid colon and aphthoid ulcers and marked erythema in the cecum and ascending colon. He had no other medical history of note and no family history of CD. As he was allergic to 5-ASA, this option was unavailable. Induction therapy with IFX at 5 mg/kg was started in April 2011, and was continued as maintenance therapy. Follow-up colonoscopy 24 weeks after initiating IFX showed multiple ulcers (0.5 -1 cm in diameter) and marked erythema in the transverse, descending, and sigmoid colon and diffuse marked erythema in the ascending colon. AZA was subsequently added and had been continued at 25 mg/day. Clinical remission was soon achieved. A moderate flare occurred in November 2020 and the dose of IFX was increased to 10 mg/kg with continuation of AZA at 25 mg/day, resulting in clinical remission. In February 2022, he was referred to our division by another gastroenterologist to continue maintenance IFX.
In May 2024, at 26 years old, he experienced rapid exacerbation of CD symptoms with slight fever (37.4 °C) and repeated episodes of severe abdominal pain and diarrhea (15 times/day) accompanied by bloody stool (CDAI, 470). He therefore visited our hospital before the next scheduled follow-up. His height was 176 cm and his weight was 60 kg (body mass index, 19.4 kg/m2). Body weight loss (-2 kg within the previous 1 month) had been observed. Laboratory investigations showed: WBC count, 16,900/µL; RBC count, 443 × 104 /µL; Hb, 13.5 g/dL; TP, 7.7 g/dL; Alb, 3.8 g/dL; AST, 17 U/L; ALT, 24 U/L; and CRP, 1.17 mg/dL. A negative result was obtained from the C-7HRP test for cytomegalovirus, and neither C. difficile nor its A and/or B toxins were detected from stool cultures (Table 2). Colonoscopy at baseline in May 2024 showed marked erythema and multiple ulcers fused with adjacent ulcers and/or longitudinally aligned in the mucosa of the sigmoid colon and rectum, longitudinally aligned ulcers, and a decreased vascular pattern in the transverse colon, and diffuse marked erythema and multiple small ulcers (1 cm in diameter) in the cecum and ascending colon (Fig. 2a). SES-CD was 23, reflecting severe activity. Maintenance IFX was discontinued because loss of response was considered present. Based on all these findings, severely active CD was diagnosed.
 Click to view | Table 2. Baseline Laboratory Findings in Case 2 |
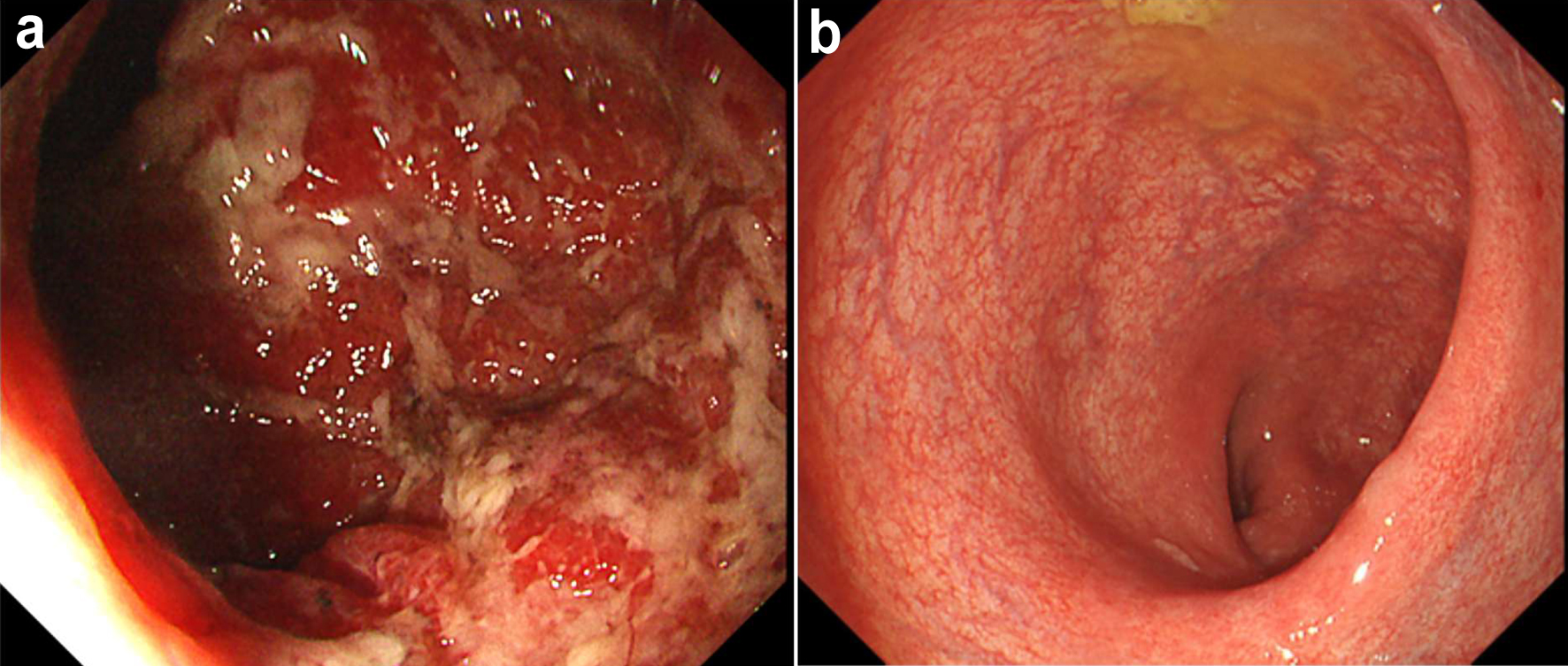 Click for large image | Figure 2. Colonoscopic findings at baseline and 12 weeks in case 2. (a) At baseline, colonoscopy shows marked erythema and multiple ulcers fused with the adjacent ulcer in the rectal mucosa. (b) At 12 weeks after starting combination therapy, colonoscopy shows a clear vascular pattern with no ulcerated surfaces around a decreased vascular pattern in part of the rectum. |
Oral PSL was started at 20 mg/day in May 2024. However, little improvement of symptoms (including diarrhea, bloody stools, and abdominal pain) was observed by 7 days after starting PSL. Induction combination therapy with intensive GMA plus UPA at 45 mg/day was therefore started for corticosteroid-refractory CD. PSL was subsequently tapered off after 3 weeks of combination therapy. As of the beginning of August, 12 weeks after starting combination therapy with intensive GMA plus UPA, clinical remission had been rapidly achieved (CDAI, 31). Follow-up endoscopy at 12 weeks showed a decreased vascular pattern in part of the rectum and a clear vascular pattern with no ulcerated surfaces in the remaining portions of colon (SES-CD, 1), reflecting endoscopic remission (SES-CD ≤ 4, a decrease of ≥ 2 points from baseline, and no subscore > 1 in any individual variable) (Fig. 2b) [6]. Histopathological findings from colon biopsies taken from the area presenting with a decreased vascular pattern revealed mild chronic inflammatory infiltrate from lymphocytes with mild fibrosis and no non-caseating granuloma in the lamina propria, reflecting histological improvement of the mucosa. Laboratory investigations at 12 weeks showed: WBC count, 7,100/µL; CK, 147 U/L; TCHO, 225 mg/dL; TG, 53 mg/dL; and C-reactive protein, 0.01 mg/dL. No adverse events were observed during the 12-week induction period.
| Discussion | ▴Top |
We have reported here two cases in which intensive GMA plus UPA rapidly achieved clinical remission and endoscopic and histological improvements of CD within 12 weeks in patients with refractory CD (Table 3).
 Click to view | Table 3. Clinical Course Through 12 Weeks |
Activation of signal transducers and activators of transcription (STATs) that are mediated by JAKs in T cells plays an important pathogenic role in CD [11, 12]. This suggested that down-regulation of STAT proteins might help inhibit cytokine-induced T-cell activation in CD, resulting in the discovery of novel drugs with a new mechanism of action [13]. UPA is a potent, orally active, and selective JAK1 inhibitor that suppresses the JAK-STAT pathway, downregulating expressions of multiple immune-relevant mediators and inflammatory cytokines that have been implicated in the pathogenesis of inflammatory bowel diseases [14, 15].
Comprehensive analyses of the phosphorylation of five STAT proteins (STAT1, 3, 4, 5, and 6) in response to 11 cytokines in immune cell populations from the mesenteric lymph node cells of 10 patients with ulcerative colitis and nine patients with CD and comparing the relative sensitivities to inhibition by titrated doses of two selective JAK1 inhibitors (filgotinib and UPA) have shown that UPA is a relatively more potent inhibitor than filgotinib for both JAK-3-dependent (IL-2, IL-21, and IL-7) and JAK-3-independent (interferon-β2a and IL-10) cytokines [16]. Next, two phase III clinical trials (U-EXCEL and U-EXCEED for induction phase) evaluated the efficacy of UPA for the treatment of moderately to severely active CD with a history of failure of one or more conventional or biologic therapies. In the U-EXCEL study, the rates of patients randomized to receive 45 mg of UPA or assigned to placebo who achieved clinical remission (CDAI < 150) and endoscopic response at 12 weeks were 49.5% vs. 29.1% and 45.5% vs. 13.1%, respectively (P < 0.001). In addition, in the U-EXCEED study, the rates of patients randomized to receive 45 mg of UPA or assigned to placebo who achieved clinical remission (CDAI < 150) and endoscopic response at 12 weeks were 38.9% vs. 21.1% and 34.6% vs. 3.5%, respectively (P < 0.001) [6]. UPA monotherapy thus appears clinically and endoscopically effective, but has been limited when higher induction remission rates are the goal. Additional treatment would be desirable for active CD patients who are biologically naive and have experienced loss of response to TNF-α antagonists. Based on the outcomes observed in the two cases presented herein with medication-refractory CD, intensive GMA combined with UPA in a short-term induction phase looks promising and has shown no safety concerns. However, the present case series included only two cases treated with combination therapy. Whether this combination therapy is more effective than either UPA or GMA monotherapy is thus unclear due to the limited number of cases and non-comparative design of the study. Our view is that additional comparative, controlled studies in larger cohorts of patients from multiple institutions are warranted to support the findings from the present two cases with refractory CD.
GMA is available in Europe and Japan for the treatment of patients with active CD that may or may not be refractory to standard pharmacotherapy, including TNF-α antagonists [9]. GMA depletes elevated and activated myeloid lineage leucocytes and has been associated with marked down-regulation of various inflammatory cytokines, including IL-1β, IL-6, IL-8, and TNF-α, which are released by myeloid leucocytes and lymphocytes [17]. Regarding remission rates, intensive GMA (35.2%) was not superior to weekly GMA (35.6%), but the time to remission was significantly shortened with intensive GMA (21.7 days) compared with weekly GMA (35.4 days; P = 0.0373). Regarding endoscopic responses, no outcomes or assessments of endoscopic findings during treatment with GMA alone were shown [9]. Interestingly, combination therapy with intensive GMA and thiopurine administration rapidly induced a high rate (77.2%) of clinical remission (CDAI < 150) at 6 weeks in 22 corticosteroid- and biologic-naive patients with active early-diagnosed CD within 2 years after diagnosis and with CDAI > 200, and no serious adverse effects were observed within 6 weeks. In addition, mucosal healing (SES-CD ≤ 2) by week 6 was observed in 22.7% of enrolled patients. This combination therapy thus might represent a rational option for patients with early-diagnosed CD [18].
On the other hand, the present two-case series showed that both patients with medication-refractory CD receiving intensive GMA plus UPA achieved endoscopic response (reduction of SES-CD score by ≥ 50% from baseline) [6], including one case (SES-CD, 1) with endoscopic remission by 12 weeks. Conversely, in a previous study evaluating the efficacy of induction combination therapy within 10 weeks with intensive GMA plus ustekinumab in three cases of medication-refractory CD, this combination treatment appeared effective regarding CDAI based on CD symptoms, and allowed rapidly tapering off corticosteroids without increasing the incidence of side effects. However, no endoscopic improvements of CD lesions based on SES-CD were observed [10]. This suggested that the short-term impact of UPA on endoscopic improvement during the induction period appears greater than that of ustekinumab. Moreover, intensive GMA, which normalizes imbalances in the immune profile among patients with CD, provides favorable and additional impacts to the efficacy of UPA. Indeed, combination therapy with intensive GMA plus UPA in the present case series rapidly ameliorated gut inflammation and subsequently induced clinical remission together with endoscopic and histological improvements, allowing tapering off steroids.
Regarding adverse events, the present two cases showed no hematopoietic dysfunctions such as leukocytopenia or erythrocytopenia within 12 weeks after UPA administration. UPA selectively inhibits JAK1 activity and thus avoids the inhibitory effects on JAK2-regulated hematopoietic function that are induced by less selective JAK inhibitors [19, 20]. In addition, no elevated values of creatine phosphokinase and lipid metabolism tests such as TCHO and TG were observed. During GMA therapy performed as five apheresis sessions in 5 consecutive weeks for 30 consecutive patients with actively distal ulcerative colitis, adverse events (6%) of slight headache, transient abdominal pain with tenesmus, fever, and mild liver dysfunction were reportedly described in eight of 30 patients [21]. In the present two cases, none of these adverse events associated with GMA treatments were observed. To the best of our knowledge, no previous reports have described intensive GMA plus UPA therapy achieving clinical and histological improvement of CD.
Learning points
Combination therapy comprising intensive GMA plus UPA was well tolerated and may be an effective therapeutic option to induce clinical remission and endoscopic and histological improvements in the mucosa for patients with refractory CD that has become refractory to systemic corticosteroids, or has shown loss of response to IFX.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
Satoshi Tanida has received speaker fees from JIMRO, Mitsubishi Tanabe Pharma, AbbVie, Janssen, Kissei Pharmaceutical Co. and writing fees from Pfizer. None of the other authors have any conflict of interest to declare.
Informed Consent
Permission for publication of the case details was obtained directly from each patient.
Author Contributions
ST designed and performed the study. ST and NI wrote the draft version of the manuscript. SS, YK, TB, and TA performed critical editing of the manuscript. ST prepared and wrote the final version of the manuscript. MN and TJ carefully supervised this study.
Data Availability
The data supporting the findings of this study are available from the corresponding authors upon reasonable request.
Abbreviations
Alb: albumin; ALT: alanine aminotransferase; 5-ASA: 5-aminosalicylic acid; AST: aspartate aminotransferase; AZA: azathioprine; CD: Crohn’s disease; CDAI: Crohn’s disease activity index; CK: creatinine phosphokinase; CRP: C-reactive protein; GMA: granulocyte and monocyte adsorptive apheresis; Hb: hemoglobin; IFX: infliximab; IL: interleukin; JAK: Janus kinase; PSL: prednisolone; RBC: red blood cell; SES-CD: Simple Endoscopic Score for Crohn’s disease; STAT: signal transducers and activators of transcription; TCHO: total cholesterol; TG: triglyceride; TNF: tumor necrosis factor; TP: total protein; WBC: white blood cell
| References | ▴Top |
- Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541-1549.
doi pubmed - Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130(2):323-333.
doi pubmed - Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, Blank MA, et al. Ustekinumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2016;375(20):1946-1960.
doi pubmed - D'Haens G, Panaccione R, Baert F, Bossuyt P, Colombel JF, Danese S, Dubinsky M, et al. Risankizumab as induction therapy for Crohn's disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399(10340):2015-2030.
doi pubmed - Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369(8):711-721.
doi pubmed - Loftus EV, Jr., Panes J, Lacerda AP, Peyrin-Biroulet L, D'Haens G, Panaccione R, Reinisch W, et al. Upadacitinib induction and maintenance therapy for Crohn's disease. N Engl J Med. 2023;388(21):1966-1980.
doi pubmed - Sulz MC, Burri E, Michetti P, Rogler G, Peyrin-Biroulet L, Seibold F, on behalf of the Swiss Ibdnet aowgotSSoG. Treatment algorithms for Crohn's disease. Digestion. 2020;101(Suppl 1):43-57.
doi pubmed - Fukuda Y, Matsui T, Suzuki Y, Kanke K, Matsumoto T, Takazoe M, Matsumoto T, et al. Adsorptive granulocyte and monocyte apheresis for refractory Crohn's disease: an open multicenter prospective study. J Gastroenterol. 2004;39(12):1158-1164.
doi pubmed - Yoshimura N, Yokoyama Y, Matsuoka K, Takahashi H, Iwakiri R, Yamamoto T, Nakagawa T, et al. An open-label prospective randomized multicenter study of intensive versus weekly granulocyte and monocyte apheresis in active crohn's disease. BMC Gastroenterol. 2015;15:163.
doi pubmed - Tanida S, Mizoshita T, Ozeki K, Katano T, Tanaka M, Nishie H, Shimura T, et al. Combination therapy with intensive granulocyte and monocyte adsorptive apheresis plus Ustekinumab in patients with refractory Crohn's disease. Ther Apher Dial. 2018;22(3):295-300.
doi pubmed - Mudter J, Neurath MF. The role of signal transducers and activators of transcription in T inflammatory bowel diseases. Inflamm Bowel Dis. 2003;9(5):332-337.
doi pubmed - Salas A, Hernandez-Rocha C, Duijvestein M, Faubion W, McGovern D, Vermeire S, Vetrano S, et al. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17(6):323-337.
doi pubmed - Danese S, Argollo M, Le Berre C, Peyrin-Biroulet L. JAK selectivity for inflammatory bowel disease treatment: does it clinically matter? Gut. 2019;68(10):1893-1899.
doi pubmed - Lovato P, Brender C, Agnholt J, Kelsen J, Kaltoft K, Svejgaard A, Eriksen KW, et al. Constitutive STAT3 activation in intestinal T cells from patients with Crohn's disease. J Biol Chem. 2003;278(19):16777-16781.
doi pubmed - Schreiber S, Rosenstiel P, Hampe J, Nikolaus S, Groessner B, Schottelius A, Kuhbacher T, et al. Activation of signal transducer and activator of transcription (STAT) 1 in human chronic inflammatory bowel disease. Gut. 2002;51(3):379-385.
doi pubmed - Hindmarch DC, Malashanka S, Shows DM, Clarke AS, Lord JD. Janus Kinase Inhibitors differentially inhibit specific cytokine signals in the mesenteric lymph node cells of inflammatory bowel disease patients. J Crohns Colitis. 2024;18(4):628-637.
doi pubmed - Saniabadi AR, Hanai H, Takeuchi K, Umemura K, Nakashima M, Adachi T, Shima C, et al. Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher Dial. 2003;7(1):48-59.
doi pubmed - Fukuchi T, Nakase H, Ubukata S, Matsuura M, Yoshino T, Toyonaga T, Shimazu K, et al. Therapeutic effect of intensive granulocyte and monocyte adsorption apheresis combined with thiopurines for steroid- and biologics-naive Japanese patients with early-diagnosed Crohn's disease. BMC Gastroenterol. 2014;13:124.
doi pubmed - Ghaffari S, Kitidis C, Fleming MD, Neubauer H, Pfeffer K, Lodish HF. Erythropoiesis in the absence of janus-kinase 2: BCR-ABL induces red cell formation in JAK2(-/-) hematopoietic progenitors. Blood. 2001;98(10):2948-2957.
doi pubmed - Mohamed MF, Beck D, Camp HS, Othman AA. Preferential Inhibition of JAK1 Relative to JAK3 by Upadacitinib: exposure-response analyses of ex vivo data from 2 phase 1 clinical trials and comparison to tofacitinib. J Clin Pharmacol. 2020;60(2):188-197.
doi pubmed - Yamamoto T, Umegae S, Kitagawa T, Yasuda Y, Yamada Y, Takahashi D, Mukumoto M, et al. Granulocyte and monocyte adsorptive apheresis in the treatment of active distal ulcerative colitis: a prospective, pilot study. Aliment Pharmacol Ther. 2004;20(7):783-792.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.