| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Review
Volume 17, Number 8, August 2025, pages 423-436
Prevention of Chromium-Induced Radiation-Chemical Oncogenesis, Including in Offspring, in an Experimental Model: A Systematic Review
Marat Iztleuova, g , Yerbolat Iztleuovb
, Talgar Abilovc
, Gulmira Iztleuovad
, Elyanora Kydyrbayevae
, Nauryzbay Imanbayevf
aDepartment of Natural Sciences, NJSC “Marat Ospanov West Kazakhstan Medical University”, Aktobe, Kazakhstan
bDepartment of Radiology, NJSC “Marat Ospanov West Kazakhstan Medical University”, Aktobe, Kazakhstan
cFaculty of General Medicine, NJSC “Marat Ospanov West Kazakhstan Medical University”, Aktobe, Kazakhstan
dDepartment of Phthisiology and Dermatovenereology, NJSC “Marat Ospanov West Kazakhstan Medical University”, Aktobe, Kazakhstan
eDepartment of Obstetrics and Gynecology, NJSC “Marat Ospanov West Kazakhstan Medical University”, Aktobe, Kazakhstan
fDepartment of Oncology, NJSC “Marat Ospanov West Kazakhstan Medical University”, Aktobe, Kazakhstan
gCorresponding Author: Marat Iztleuov, Department of Natural Sciences, NJSC “Marat Ospanov West Kazakhstan Medical University”, Aktobe, Kazakhstan
Manuscript submitted April 25, 2025, accepted August 9, 2025, published online August 31, 2025
Short title: Chromium-Induced Oncogenesis Prevention
doi: https://doi.org/10.14740/jocmr6265
| Abstract | ▴Top |
Radiation and chemical-induced cancer are of increasing concern as the various activities of humans continuously elevate the levels of radiation and toxic chemicals in the environment. The prevention of this incidence using alternative medicines-phytopreparations, therefore, becomes pertinent as conventional approaches tend to produce various unwanted side effects. To achieve this, there is a need to understand the various mechanisms of action through which phytopreparations exhibit their protective effects. This systematic review, therefore, aims to explore the mechanism of action of various phytopreparations in the prevention of induced radiation and chemical (chromium) cancer. A systematic review approach following the stipulated guidelines by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was used to identify research papers published between 2015 and 2025. Four databases, namely Europe PubMed Central (PMC), PubMed, Springer Open, and Wiley Online Library were used to search for related open access papers. A total of 621 research papers were reviewed for suitability to the review objective; however, only five papers met the inclusion criteria, and an additional two papers were sourced from ResearchGate. Thus, a total of seven papers were finally included in the analysis. This review highlights the mechanisms of action of various phytopreparations in the prevention of radiation and chromium-induced cancer. The major mechanisms of phytopreparations’ action in the prevention of induced radiation and chromium oncogenesis majorly involve regulating pro and anti-inflammatory cytokines, improving cell-to-cell communication, and preventing damage to DNA structure.
Keywords: Hexavalent chromium; Gamma-irradiation; Induced oncogenesis; Prevention; Phytopreparations
| Introduction | ▴Top |
Radiation and chemicals are agents that have been recognized to cause apparent and dangerous alterations in the germ line of higher plants and animals [1]. This effect is on the increase due to various human activities that are continuously altering the natural ecological systems at an extraordinary rate [2]. Nowadays, environmental pollution is directly related to various changes in human health and directly impacts social status. Epidemiological research and analysis have revealed a daily increase in harm to human health as a consequence of damage to the ecological sphere. Swift urban development and industrialized societies particularly compromise air quality, an assessment can be made by considering the accumulated levels of air pollutants [3].
Ionizing and non-ionizing forms of radiation exists. Non-ionizing radiations have low photon energy which makes them harmless, examples include radio waves, microwaves; however, ionizing radiation (including X-rays, gamma rays, alpha-particles, beta-particles, etc.) carries high photon energy, which is required to break tightly bound electrons from atoms in living cells, thereby creating ions that damage the genetic materials of the cell [4]. Ultraviolet radiation (UVR), although non-ionizing, can be detrimental to cells, having been classified as both a mutagen and a nonspecific damaging agent with tumor initiating and tumor promoting properties [5]. The negative impacts of UVR on the skin have been extensively explored, particularly its association with skin cancers including most common human malignancy, such as basal cell and squamous cell carcinomas, cutaneous melanomas, among others [6, 7].
Ionizing radiation, although a rare but potentially dangerous pollutant, occurs in the environment at low levels as a natural event (such as cosmic and terrestrial radiation) and usually does no harm to living things. However, some specific human tasks, such as the testing of weapons and unprecedented events at nuclear power plants, may release ionizing radiation beyond safety limits. This type of radiation may destroy organic molecules such as DNA and result in malfunctions in cell activities, consequently leading to cellular and organismal death. Radioactive contamination can destroy DNA and other biological molecules [8, 9]. Anthropogenic sources of radioactive contamination are evident in the event at Chornobyl in 1986 in the nuclear power plants, which resulted in long-term radioactive pollution [2]. A consequence of radiation from X-rays and gamma rays is the indirect destruction of biomolecules after synthesizing reactive oxygen species (ROSs), particularly superoxide and hydroxide radicals, from intracellular radiolysis. This can have numerous consequences, such as the oxidation of biomolecules and initiation of diverse intracellular signaling pathways [10], and radiation exposure can elevate the incidence of cancer [11].
The plausible unwanted health impacts resulting from exposure to chromium (Cr) necessitated the Environmental Protection Agency (EPA) to establish a threshold contaminant level of 0.1 mg/L total Cr in potable water. Cr (VI) is mostly present in occupational environments due to industrial activities involving leather processing, smelting, welding, and plating of metals [12]. It is also in automobile exhaust and tobacco products, including traditional and electronic cigarettes and hookahs [12, 13]. An estimated 66% of current or past hazardous waste sites on the National Priorities List also contain Cr. Systemic poisoning associated with Cr has been reported in the respiratory, pulmonary, gastrointestinal, dermal and renal systems [12]. The International Agency for Research on Cancer has identified metallic Cr and Cr (III) compounds as group 3 human carcinogens; specific Cr alloys are categorized as group 2B, while hexavalent Cr compounds are classed as group 1 human carcinogens. Cr (IV) is a potent and highly cytotoxic mutagen. DNA mutilation was taught to be the main mechanism of Cr genotoxicity and carcinogenicity; it has however been established that dichromate ions also cause methylation in DNA molecules and gene silencing, suggesting that epigenetic mechanisms may also be connected with these events. Most of the cellular destruction induced by Cr arises from the production of ROS [14].
Cancer is a major cause of death in childhood in the developed nations of the world. An elevated incidence of blood cancer, as well as defects at birth, has been documented in the offspring of fathers who experienced exposure to radiation from nuclear plants and from various diagnostic procedures involving irradiation [15]. Parent exposure - that is, exposure of sex cells to radiation and chemicals - elevates the occurrence of tumors and abnormalities in the child, and gamete cell transformations that cause cancer are transferrable to the next generations [1]. This phenomenon is often referred to as transgenerational carcinogenesis, which is simply the transfer of cancer risk to the untreated offspring of parents exposed to oncogenes before mating [16].
Mutation is a multiple-stage event that involves entry, distribution, biological transformation, and discharge of the genotoxicant from the body, as well as their invasion of cells and cell nuclei, direct or indirect relation of the genotoxicant and/or its metabolites with DNA molecules, and consequently damage to the DNA. The various mechanisms of radiation and chemical (Cr)-induced oncogenesis is discussed in Table 1 [4, 17-39].
 Click to view | Table 1. Mechanism of Radiation and Chromium-Induced Oncogenesis |
One basic medical philosophy states that “prevention is better than cure”. This ancient principle remains relevant with modern approaches to chemoprophylaxis of numerous diseases utilizing antimutagens (amidst other medications) to annihilate the destructive effects of environmental and endogenous genotoxicants on human health [40]. Cancer prevention takes place across the complete disease scope, starting with the primary up to the tertiary prevention and includes multiple schemes, such as molecular prevention, which involves the use of natural or man-made agents that disrupt the primary drivers, principal derangements, or the scope in which these agents function and in which the derangements take place before the penetration of the basement membrane. In essence, molecular prevention includes chemopreventive agents (such as conventional drugs, micronutrients, etc.), vaccines and therapeutic interventions in individuals at elevated risk of cancer due to microbial or some underlying diseases, as all these eventually operate at the molecular level, and possess the tendency to minimize precancer or cancer incidence and death [41]. Chemoprevention is a plausible scheme used to control cancer by halting and impeding its progression. It is one of the most demanding fields of cancer research and is focused on various interferences, including biological, nutritional, and pharmacological aspects [42]. It appraises using biological or synthetic substances to suppress oncogenesis before invading non-tumor cells. It involves primary, secondary and tertiary approaches. The application of dietary phytochemicals is an active approach to primary and secondary chemoprevention [43, 44].
Medicinal plants filled with antioxidants protect cells from harm, thus preventing and controlling cancer and other ailments [45]. The anti-cancer properties of plants are linked to some major bioactive constituents: alkaloids, tannins, flavonoids, phenols, steroids, terpenoids, and saponins [46]. These phytochemicals have both antioxidant and pro-oxidant properties, thereby annealing oxidative DNA damage following mutation in DNA caused by exposure to carcinogenic agents [47-49]. Also, dietary phytochemicals have been observed to trigger many signaling pathways simultaneously, thus reducing apoptosis, blocking cellular proliferation and invasion and stimulating immune system functionality [50-53]. It is, however, still important to further understand the mechanism of action of these vital agents in order to better harness their numerous benefits. One study [54] concluded that there is a need to understand the underlying mechanism of action of the radioprotective agent-Tulsi (a medicinal plant native to Southeast Asia); another study [55] also concluded that an understanding of the mechanisms of action of the traditional Chinese medicine (TCM) is important, and this knowledge is essential to develop new anti-radiation medicines.
Research aim and question
This review aims to evaluate the mechanisms of action of various phytopreparations in the prevention of radiation- and chemically (Cr)-induced oncogenesis, with a particular focus on the mechanisms underlying the protective effects of phytopreparations against radiation- and Cr-induced cancer.
| Methods | ▴Top |
Description of method
A systematic literature review was conducted using four major databases to identify research papers relevant to the review theme.
Data sources
The databases used for the search included Europe PubMed Central (PMC), PubMed, Springer Open, and Wiley Online Library.
Data selection
The following keywords were used to retrieve articles relevant to the review topic: “phytochemical preparations for radiation- and chemical-induced cancer prevention”, “phytochemical preparations for radiation cancer”, and “prevention of induced chromium-radiation oncogenesis”. The search strategy followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Data objectivity
Only open-access research articles published between 2015 and 2025 were considered. Inclusion criteria encompassed studies focusing on radiation-induced oncogenesis, chemoprevention, and experimental models involving animals or in vitro (using cell lines) systems. Studies were excluded if they did not involve animal models, in vitro experiments, or did not address radiation- or Cr-induced oncogenesis. The database search was conducted on March 1, 2025.
The initial search in the Wiley Online Library yielded 2,579 results, including journal articles, books, and conference papers. Springer Open returned 61 results, Europe PMC yielded 227 results, and PubMed yielded 22 results. In the second stage of the review, filters were applied to include studies published between 2015 and 2025 from open-access journals. This refinement yielded 545 results from Wiley Online Library, 50 from Springer Open, 12 from Europe PMC, and 14 from PubMed. The third stage of filtering involved the removal of duplicates and an assessment of titles and abstracts to determine relevance to the research theme. This process resulted in five relevant papers from Wiley Online Library, zero from Springer Open, eight from Europe PMC, and four from PubMed. A final selection was made based on the predefined inclusion and exclusion criteria, resulting in a total of five eligible papers: three from Wiley Online Library, one from PubMed, and one from Europe PMC (Fig. 1).
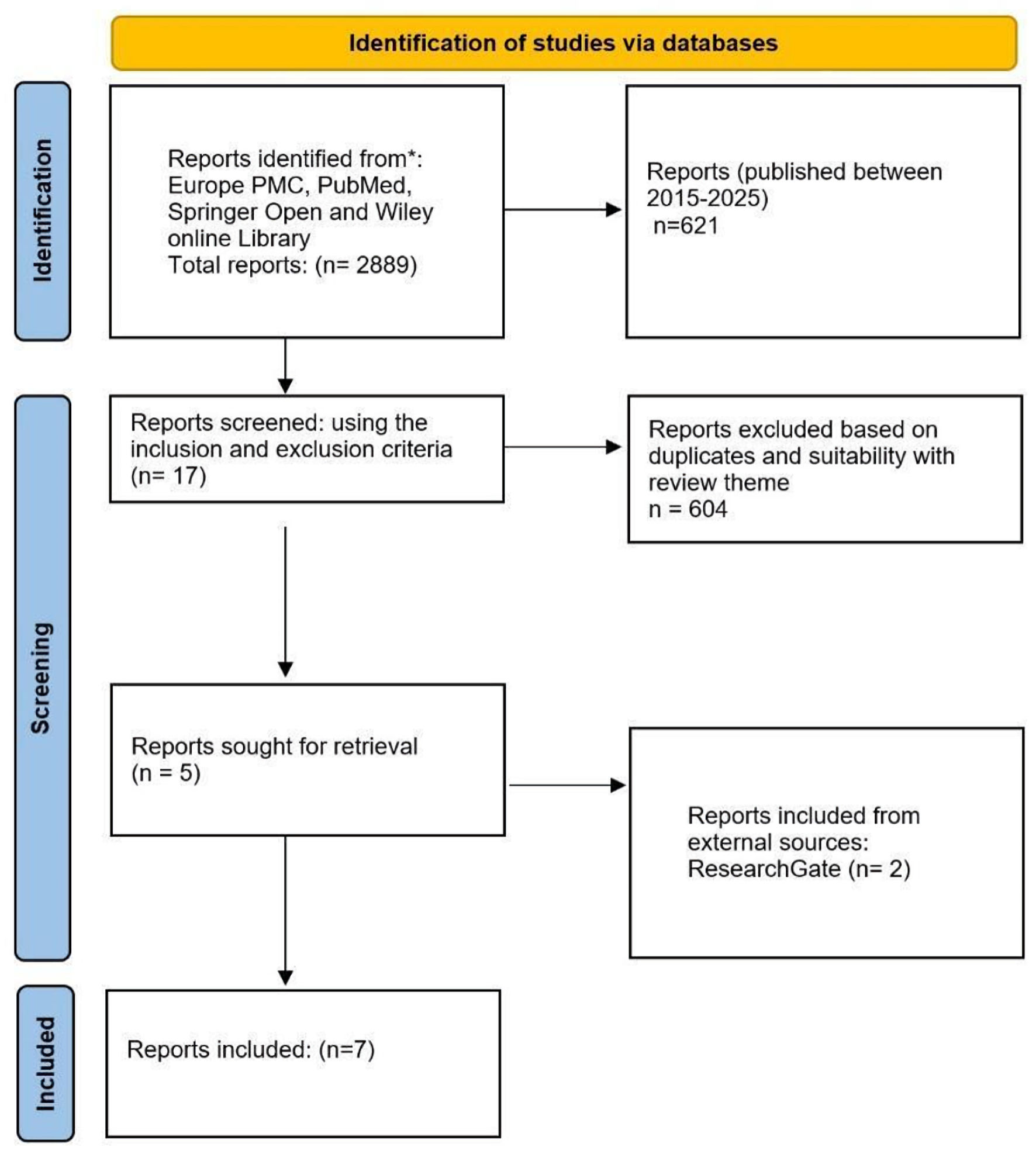 Click for large image | Figure 1. Flow chart for the systematic literature review on the prevention of radiation- and chemical-induced oncogenesis. Source: authors’ development in accordance with PRISMA 2020 recommendations. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; ROS: reactive oxygen species. |
| Results | ▴Top |
The findings from the reviewed research articles (Table 2 [56-62]) highlight several mechanisms of action through which phytopreparations derived from medicinal plants exert protective effects against radiation- and Cr-induced oncogenesis. These phytopreparations were shown to upregulate anti-inflammatory cytokines while inhibiting pro-inflammatory cytokines, thereby reducing inflammation and preventing cellular injury. Furthermore, the phytopreparations enhanced cell viability and metabolic activity by modulating oxidative stress markers and ROSs, which improved intercellular communication and minimized oxidative damage, ultimately preventing apoptosis.
 Click to view | Table 2. Overview of the Included Research Articles |
In addition, these compounds promoted DNA repair processes, preventing permanent genetic damage and thereby contributing to genomic stability. The primary mechanisms by which phytopreparations prevent cancer include: 1) modulation of pro- and anti-inflammatory cytokine expression; 2) enhancement of cell viability through improved intercellular communication; 3) preservation of DNA integrity, which supports gene stability. These mechanisms are illustrated and discussed in detail based on the research studies identified during the systematic review (Fig. 2).
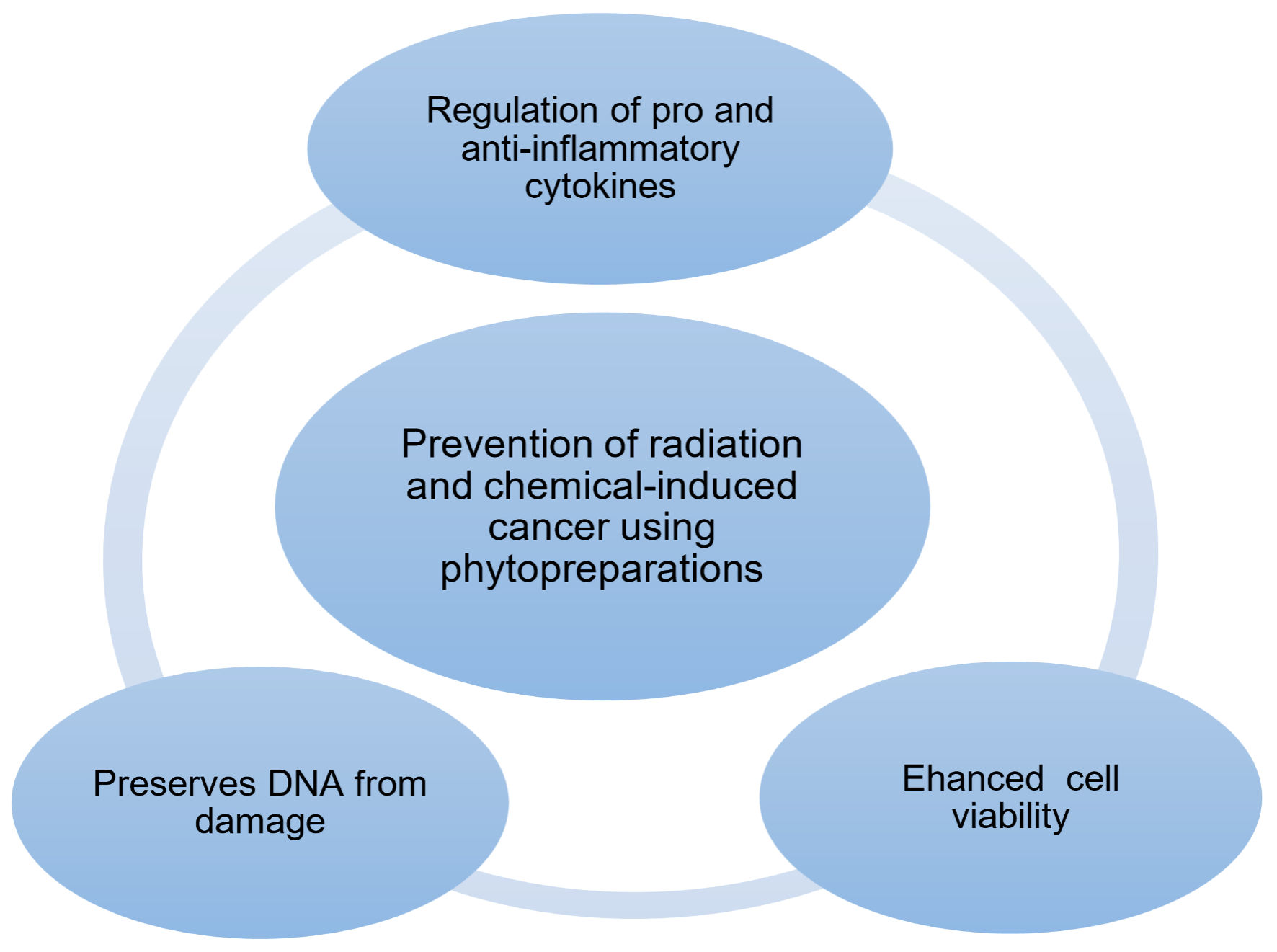 Click for large image | Figure 2. Mechanism for the prevention of radiation-induced oncogenesis using phytopreparations. Source: authors’ development. |
| Discussion | ▴Top |
Regulation of pro- and anti-inflammatory cytokines
Pro-inflammatory cytokines, mostly interleukin-1 (IL-1) family, IL-6, and tumor necrosis factor alpha (TNF-α), are crucial factors in generating chronic inflammation. This act is made possible via the stimulation of pro-tumorigenic or anti-tumorigenic functions, which depends on the cancer type, tumor microenvironment (TME), and other associated factors. Following the interplay between IL-6 and its receptor, a number of signaling pathways are activated. This interplay stimulates signal transducer and activates some transcription factors followed closely by the transcription of target genes, resulting in inflammation, metastasis and so on [63]. Based on the milieu of events that occur during inflammation, the synthesis of IL-6 by immune cells is commonly caused by infections and tissue injuries. In addition, TNF-α is an inflammatory cytokine that takes part in the control of numerous signaling processes by binding to TNF-α R-1 and TNF-α R-2. The cytokine stimulates the growth of tumor through several mechanisms including enhancing epithelial-to-mesenchymal transition (EMT), boosting the cell differentiation rate and intensifying the rate of angiogenesis amidst other functions [64-67]. TNF-α exhibits pro-tumorigenic activity by promoting angiogenesis, cell proliferation, migration, and the survival of tumor cells. It is a well-recognized inflammatory mediator involved in both malignant and non-malignant conditions [63]. In a study by Dumlu et al [56], elevated levels of the cytokines IL-6 and TNF-α were observed in the first-generation offspring of rats exposed to gamma irradiation, as well as those exposed to both gamma irradiation and hexavalent Cr, with more pronounced cytokine levels in the latter group. However, normalized cytokine values were recorded in the offspring of rats that received burdock root oil treatment prior to exposure to either gamma irradiation alone or in combination with Cr (VI). Notably, the levels of IL-6 and TNF-α were significantly lower in the offspring of gamma-irradiated rats, suggesting that burdock root oil effectively suppressed cytokine production in the treated groups.
A similar finding was reported by Lyer et al [57], who observed elevated TNF-α levels in rats exposed to gamma irradiation. However, pretreatment with umbelliferone prior to irradiation significantly reduced TNF-α levels, bringing them close to those observed in the control (non-irradiated) groups.
In contrast to the pro-inflammatory activity of IL-6 and TNF-α, IL-10 is a potent anti-inflammatory cytokine that plays a crucial role in attenuating the host immune response to pathogens, thereby minimizing tissue damage and maintaining homeostasis [68, 69]. High levels of IL-10 signaling can interfere with the synthesis of pro-inflammatory cytokines by directly targeting immune effector cells [57]. IL-10 is typically produced later in the inflammatory cascade, following the early release of pro-inflammatory cytokines. Its localized synthesis at the site of inflammation helps establish a balance between effective pathogen clearance and the prevention of excessive immune-mediated damage, ultimately supporting the restoration of normal tissue integrity [69].
The anti-inflammatory activity of IL-10 on gamma-irradiated as well as gamma-irradiated and Cr (IV)-exposed rats was demonstrated by Dumlu et al [56]. IL-10 levels in offsprings of rats, which were administered burdock oil before exposure to gamma irradiation and Cr (IV), were similar to those of the control levels (levels of offsprings of rats not exposed to gamma irradiation as well as gamma irradiation and Cr (IV)). There was significant reduction in the IL-6 and TNF-α values. Thus, phytopreparation can help regulate pro- and anti-inflammatory cytokines, eliminating the cascade of events that results in inflammation, which is necessary for tumorigenesis, thereby eliminating the incidence of induced cancer and transgenerational oncogenesis.
Efficient and increased cell to cell communication
Cell-to-cell communication is a crucial activity that remarkably determines an organism’s homeostasis, growth, and disease processes. This occurs through direct contact or via the activation of specific cell signaling pathways at a distance using ligand-receptor interactions, which represents a principal means of cell communication and is intricately connected with numerous disease processes and tissue degenerative activities [70]. Cell communication has a foundational role in permitting cancer cells to coopt and regulate stromal and immune cells. The effective communication between the tumor cells and the surrounding cells aids angiogenesis, immune escape, EMT, generation of a pre-metastatic niche, metastases and multi-drug resistance [71].
Adenosine triphosphate (ATP) hydrolysis supplies the energy required to fuel numerous and important activities in cells: intracellular signaling, DNA replication, RNA synthesis, active transport, and signal transduction, among others [72]. ATP acts as a signaling molecule for apt responses to various cytotoxic agents and performs a crucial role in mediating the radiation stress-induced responses that function to control or repair the deleterious impacts of gamma irradiation on the body [73]. ATP serves as a homeostatic messenger and mediator in cell-to-cell communication [74] and also participates in the activities that result in all types of cell death. During the late phase of apoptosis, ATP levels reduce substantially, majorly due to the loss of mitochondrial function and utilization by ATP-dependent proteases [75-77]. In a study by Chen et al [58], the levels of ATP in HaCaT cells (keratinocytes) and skin melanoma cell line 1 (SKMEL-1, melanocytes) exposed to ultraviolet B radiation (UVB) were significantly lower than in the control and treatment groups. It was further reported that ATP levels in HaCaT cells treated with linearthin, aspalathin and nothofagin from Aspalathus linearis (A. linearis) prior to UVB exposure were significantly higher than in the unexposed cells. Also, the extracts from A. linearis were tested for their anti-apoptotic potential via the activation of caspases 3/7. It was observed that the phytopreparation reduced caspase activation in the UVB-exposed cells. The reduction in apoptotic effects was contingent on the levels of cell damage by UVB exposure, as cells unexposed to irradiation appeared to have a dose-dependent elevation in caspase activity and, consequently, apoptotic cell death. This implies that the phytopreparation enhanced cell viability, thus preventing apoptosis. It was further confirmed that the phytopreparation was not cytotoxic to the cells at low concentrations. In another study by Kim et al [59], the preventive effect of ferulic acid (FA), a phenolic compound present in large amounts in most plants, was investigated. It was reported that human lens epithelial cell (HLEC) line (FHL124) exposed to X-ray and without FA treatment were swollen and showed distorted morphology. However, treatment with FA prior to exposure to irradiation improved cell morphology.
Although the morphology of the treated cells was not identical to that of the control group (cells not exposed to X-rays), the preservation of structural integrity suggests a protective effect of FA, which prevented the complete loss of form in the X-ray-exposed cells. To further assess whether FA protected cells against X-ray-induced apoptosis, flow cytometry was employed to quantify the rate of cell death. A substantial increase in apoptosis was observed in HLECs following exposure to 4 Gy of X-ray radiation compared with the control group. However, HLECs pretreated with folic acid exhibited dose-dependent resistance to X-ray-induced apoptosis.
In a related study, Perillo et al [60] investigated the effects of Clerodendrum trichotomum extract on human epidermal keratinocytes (HEKa) prior to UVB exposure. The extract demonstrated a protective effect by inhibiting UVB-induced cellular alterations. Treated epidermal cells also exhibited increased hydration and enhanced abundance and density of collagen fibers. In addition, mouse skin cells pretreated with the same extract prior to UVB exposure showed significantly reduced levels of biomarkers associated with photoaging. The authors concluded that the extract modulated signaling pathways responsible for the expression of these biomarkers.
Furthermore, moderate increases in ROS contribute to numerous pathological conditions, including tumor initiation and progression. In tumor cells, ROS synthesis is elevated due to increased metabolic activity, genetic mutations, and localized hypoxia. Through interactions with lipids, ROS can promote oxidative stress via a feedback loop initiated by fatty acid peroxidation, which alters the lipid bilayer of cell membranes and leads to the generation of free radicals. This process is harmful to cells. Additionally, ROS interactions with proteins can affect multiple signaling pathways involved in the regulation of cell proliferation and apoptosis [78].
Excessive ROS can be neutralized by elevated levels of antioxidants. Several studies have reported the ability of various phytopreparations to produce a wide range of antioxidants, which have been shown to prevent oxidative stress in cells, thereby reducing cellular damage and inhibiting apoptosis. In a study conducted by Patil et al [61], human dermal fibroblast (HDF) cells exposed to UVB radiation exhibited a significant increase in intracellular ROS generation. However, pretreatment of HDFs with ursolic acid prior to UVB exposure resulted in a reduction in ROS levels, suggesting that the polyphenolic compounds present in ursolic acid were effective in eliminating intracellular ROS generated by UVB-induced oxidative stress.
A similar observation was reported by Chen et al [58], who demonstrated the protective effect of FA in reducing ROS and malondialdehyde (MDA), a lipid peroxidation product, in HKECs exposed to UVB radiation. In a pilot clinical study by Sanders et al [62], a curcumin-based mouthrinse was used to treat radiotherapy-induced oral mucositis in patients. The results showed that curcumin alleviated ulceration caused by radiotherapy, indicating the potential of this phytopreparation to promote oral epithelial cell repair.
Other studies have also demonstrated the efficacy of various phytopreparations in suppressing oxidative stress, enhancing antioxidant levels, and preventing apoptosis in cells exposed to different types of radiation [56, 57].
Preservation of DNA structure and gene stability
The three-dimensional structure of chromosomes plays a crucial role in the regulation of gene expression and also influences the repair of radiation-induced DNA damage. Genomic aberrations that disrupt chromosomal spatial domains can lead to various pathological conditions, including cancer [79]. Ionizing radiation directly affects DNA structure by inducing strand breaks, particularly double-strand breaks [80]. Mutations in genes and their associated signaling pathways are key factors that determine cell apoptosis, proliferation, survival, and differentiation [70].
The effects of most notable antimutagens depend on their ability to prevent major DNA lesions. These effects generally begin after mutations have occurred, either through the interception and binding of reactive genotoxic molecules by dismutagens or by regulating the synthesis of genotoxic metabolites through the limitation of genotoxicant bioavailability [40]. The antimutagenic effect of burdock root oil was demonstrated by Dumlu et al [56], offspring of adult rats exposed to gamma irradiation and hexavalent Cr exhibited pronounced mutagenic effects, as evidenced by chromosomal aberrations in the bone marrow. However, when the parent rats received burdock root oil prior to exposure to the mutagenic agents (gamma irradiation and Cr), their offspring showed significant improvements in chromosomal structure. Offspring from parents exposed to gamma irradiation alone, who received treatment prior to exposure, exhibited no chromosomal anomalies, with chromosomal aberration values remaining within the control range.
Similarly, Patil et al [61] reported that no DNA damage was observed in HDF cells treated with 20 µM ursolic acid prior to UVB exposure. All tested concentrations of ursolic acid significantly reduced DNA damage, as indicated by the levels of intact DNA. The World Health Organization has supported and reinforced the integration, recognition, and use of traditional, complementary, and integrative medicines (TCIMs) in national health systems at all levels, including primary health care, specialized care, and hospital care [81].
The discovery and production of safe and potent molecular preventive agents is a promising prospect for cancer prevention [40]. Many researchers are now working on developing new cancer treatment methods, but radiotherapy is currently one of the most powerful and preferred methods of cancer treatment. Unfortunately, although the number of patients treated with this method is increasing by the day, the side effects of the method have not been reduced [57]. Now, the world has focused on natural medicines, and the demand for plant-based medicines is rising. Utilizing novel drug delivery systems with phytoconstituents can result in improved bioavailability, enhanced solubility and permeability, thus minimizing the dose and side effects [82]. An overview of the major phytoconstituents with the mechanism by which they exert their anti-cancer activity is discussed in Table 3 [51, 82, 83, 87-95].
 Click to view | Table 3. Major Phytoconstituents With Anti-Cancer Properties and Their Mechanism of Activity |
| Limitation of the Study | ▴Top |
This review utilized research papers with open access and only four databases, and as such the number of research papers included in the study was relatively small. Papers where phytopreparations were used in combination with nanoparticles were not included in this study, limiting the breadth of the study.
| Conclusions | ▴Top |
Cancer prevention has become pertinent considering the adverse effects of radiotherapy and chemotherapy, the rate of industrialization and levels of various environmental pollutants, as well as various economic activities that predispose humans to carcinogenic agents, especially radiation and chemicals (Cr). Phytopreparations from medicinal plants could be a vital key to cancer prevention, particularly those that are radiation and chemical (Cr) induced. It is worthy of note that no observed adverse effect of the phytopreparations on cells were reported by most of the papers included in this systematic review; however, one paper reported toxicity of the phytopreparation to the skin cells (melanocytes and keratinocytes) at the highest concentration experimented but the degree of toxicity and related damage to cell was not reported. Interestingly, the same paper also reported pronounced cytoprotective activity of the phytopreparation at the lowest concentration experimented. More research still needs to be conducted to understand the molecular mechanisms underlying the activity of phytopreparations with particular attention on the role of this agent in genome stability and DNA integrity.
Acknowledgments
None to declare.
Financial Disclosure
The work was carried out within the framework of a scientific project with grant funding from the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan, IRN AP23489880 “Prevention of induced radiation-chemical (chromium) oncogenesis, including in experimental offspring” (Contract No. 308 GF 24-26 dated September 9, 2024, Reg. No. 0124RK00949).
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
All authors made an equal and significant contribution to the preparation of the article. Marat Iztleuov, Yerbolat Iztleuov, Talgar Abilov, Gulmira Iztleuova, Elyanora Kydyrbayeva, and Nauryzbay Imanbayev participated in the conceptual development of the research, collection and analysis of data, drafting of the manuscript, and critical scientific revision. All authors have read and approved the final version of the manuscript for publication and agree to be accountable for its content.
Data Availability
The authors confirm that the data supporting the findings of this study are included within the article.
Abbreviations
ATP: adenosine triphosphate; CAT: catalase activity; Cr (VI): hexavalent chromium; E: erythema score; EMT: epithelial-to-mesenchymal transition; FA: ferulic acid; HDF: human dermal fibroblast; H&E: hematoxylin and eosin; HEKa: human epidermal keratinocytes; HKEC: human keratinocyte cells; HLEC: human lens epithelial cells; H2O2: hydrogen peroxide; IL: interleukin; MAPK: mitogen-activated protein kinase; MDA: malonic dialdehyde; MMP: matrix metalloproteinase; NF-κB: nuclear factor kappa; NRS: numerical rating scale; OH: hydroxyl radical; PMC: PubMed Central; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; ROS: reactive oxygen species; SH group: sulfhydryl; SKMEL-1: skin melanoma cell line 1; TAC: total antioxidant capacity; TCIM: traditional, complementary and integrative medicine; TCM: traditional Chinese medicine; TME: tumor microenvironment; TNF-α: tumor necrosis factor alpha; TOS: total oxidative status; TXB2: thromboxane B2; U: ulceration score; UVB: ultraviolet B radiation; WHO: World Health Organization
| References | ▴Top |
- Nomura T, Baleva L, Ryo H, Adachi S, Sipyagina A, Karakhan N. Transgenerational effects of radiation on cancer and other disorders in mice and humans. J Radiat Cancer Res. 2017;8(3):123.
doi - Videvall E, Burraco P, Orizaola G. Impact of ionizing radiation on the environmental microbiomes of Chornobyl wetlands. Environ Pollut. 2023;330:121774.
doi pubmed - Gavito-Covarrubias D, Ramirez-Diaz I, Guzman-Linares J, Limon ID, Manuel-Sanchez DM, Molina-Herrera A, Coral-Garcia MA, et al. Epigenetic mechanisms of particulate matter exposure: air pollution and hazards on human health. Front Genet. 2023;14:1306600.
doi pubmed - Saini S, Gurung P. A comprehensive review of sensors of radiation-induced damage, radiation-induced proximal events, and cell death. Immunol Rev. 2025;329(1):e13409.
doi pubmed - D'Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14(6):12222-12248.
doi pubmed - Lo JA, Fisher DE. The melanoma revolution: from UV carcinogenesis to a new era in therapeutics. Science. 2014;346(6212):945-949.
doi pubmed - Slominski RM, Chen JY, Raman C, Slominski AT. Photo-neuro-immuno-endocrinology: How the ultraviolet radiation regulates the body, brain, and immune system. Proc Natl Acad Sci U S A. 2024;121(14):e2308374121.
doi pubmed - Han WK, Yu KN. Ionizing radiation, DNA double strand break and mutation. Adv Genet Res. 2010;4:197-210. Available from: https://www.researchgate.net/publication/266462167_Ionizing_Radiation_DNA_Double_Strand_Break_and_Mutation.
- Reisz JA, Bansal N, Qian J, Zhao W, Furdui CM. Effects of ionizing radiation on biological molecules—mechanisms of damage and emerging methods of detection. Antioxid Redox Signal. 2014;21(2):260-292.
doi pubmed - Panganiban RA, Snow AL, Day RM. Mechanisms of radiation toxicity in transformed and non-transformed cells. Int J Mol Sci. 2013;14(8):15931-15958.
doi pubmed - Irsal M, Sutoro SG, Widiatmoko ME, Cahya I. Assessment awareness and knowledge of apron to protect radiographer during radiographic examination. Futurity Med. 2023;2(4):10-16. Available from: https://futurity-medicine.com/index.php/fm/article/view/47.
- Chen QY, Murphy A, Sun H, Costa M. Molecular and epigenetic mechanisms of Cr(VI)-induced carcinogenesis. Toxicol Appl Pharmacol. 2019;377:114636.
doi pubmed - Williams M, Bozhilov K, Ghai S, Talbot P. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS One. 2017;12(4):e0175430.
doi pubmed - Manic L, Wallace D, Onganer PU, Taalab YM, Farooqi AA, Antonijevic B, Buha Djordjevic A. Epigenetic mechanisms in metal carcinogenesis. Toxicol Rep. 2022;9:778-787.
doi pubmed - Nomura T. Transgenerational carcinogenesis: induction and transmission of genetic alterations and mechanisms of carcinogenesis. Mutat Res. 2003;544(2-3):425-432.
doi pubmed - Cheng RY, Hockman T, Crawford E, Anderson LM, Shiao YH. Epigenetic and gene expression changes related to transgenerational carcinogenesis. Mol Carcinog. 2004;40(1):1-11.
doi pubmed - Behjati S, Gundem G, Wedge DC, Roberts ND, Tarpey PS, Cooke SL, Van Loo P, et al. Mutational signatures of ionizing radiation in second malignancies. Nat Commun. 2016;7:12605.
doi pubmed - Sharma D, Singh VP, Singh RK, Joshi CS, Sharma V. Isolation and characterization of bioactive compounds from natural resources: Metabolomics and molecular approaches. In: Evolutionary Diversity as a Source for Anticancer Molecules. Academic Press; 2021. p. 77-101.
doi - Fedeles BI, Essigmann JM. Impact of DNA lesion repair, replication and formation on the mutational spectra of environmental carcinogens: Aflatoxin B(1) as a case study. DNA Repair (Amst). 2018;71:12-22.
doi pubmed - Evans HH, DeMarini DM. Ionizing radiation-induced mutagenesis: radiation studies in Neurospora predictive for results in mammalian cells. Mutat Res. 1999;437(2):135-150.
doi pubmed - Sage E, Shikazono N. Radiation-induced clustered DNA lesions: Repair and mutagenesis. Free Radic Biol Med. 2017;107:125-135.
doi pubmed - Johann To Berens P, Molinier J. Formation and recognition of UV-Induced DNA damage within genome complexity. Int J Mol Sci. 2020;21(18):6689.
doi pubmed - Timmins J. Recognition of DNA Lesions. Int J Mol Sci. 2023;24(11):9682.
doi pubmed - Ward JF. Radiation chemical mechanisms of cell death. In: Fielden EM, Fowler JF, Hendry JH, Scott D, editors. Proc 8th Int Congr Radiat Res. Vol. 2. Edinburgh: Taylor & Francis; 1987. p. 162-168. https://www.worldcat.org/title/19775426.
- Nickoloff JA, Sharma N, Allen CP, Taylor L, Allen SJ, Jaiswal AS, Hromas R. Roles of homologous recombination in response to ionizing radiation-induced DNA damage. Int J Radiat Biol. 2023;99(6):903-914.
doi pubmed - Ward JF. The yield of DNA double-strand breaks produced intracellularly by ionizing radiation: a review. Int J Radiat Biol. 1990;57(6):1141-1150.
doi pubmed - Ward JF. The complexity of DNA damage: relevance to biological consequences. Int J Radiat Biol. 1994;66(5):427-432.
doi pubmed - Roots R, Okada S. Estimation of life times and diffusion distances of radicals involved in x-ray-induced DNA strand breaks of killing of mammalian cells. Radiat Res. 1975;64(2):306-320.
pubmed - May JM, Bylicky M, Chopra S, Coleman CN, Aryankalayil MJ. Long and short non-coding RNA and radiation response: a review. Transl Res. 2021;233:162-179.
doi pubmed - Cannan WJ, Pederson DS. Mechanisms and consequences of double-strand DNA break formation in chromatin. J Cell Physiol. 2016;231(1):3-14.
doi pubmed - O'Brien TJ, Ceryak S, Patierno SR. Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms. Mutat Res. 2003;533(1-2):3-36.
doi pubmed - Hirose T, Kondo K, Takahashi Y, Ishikura H, Fujino H, Tsuyuguchi M, Hashimoto M, et al. Frequent microsatellite instability in lung cancer from chromate-exposed workers. Mol Carcinog. 2002;33(3):172-180.
doi pubmed - Belli M, Tabocchini MA. Ionizing radiation-induced epigenetic modifications and their relevance to radiation protection. Int J Mol Sci. 2020;21(17):5993.
doi pubmed - Ma L, Zhang Y, Xu J, Yu Y, Zhou P, Liu X, Guan H. Effects of ionizing radiation on DNA methylation patterns and their potential as biomarkers. Int J Mol Sci. 2025;26(7):3342.
doi pubmed - Margueron R, Trojer P, Reinberg D. The key to development: interpreting the histone code? Curr Opin Genet Dev. 2005;15(2):163-176.
doi pubmed - Tharmalingam S, Sreetharan S, Kulesza AV, Boreham DR, Tai TC. Low-dose ionizing radiation exposure, oxidative stress and epigenetic programing of health and disease. Radiat Res. 2017;188(4.2):525-538.
doi pubmed - Friedl AA, Mazurek B, Seiler DM. Radiation-induced alterations in histone modification patterns and their potential impact on short-term radiation effects. Front Oncol. 2012;2:117.
doi pubmed - Katz LM, Hielscher T, Liechty B, Silverman J, Zagzag D, Sen R, Wu P, et al. Loss of histone H3K27me3 identifies a subset of meningiomas with increased risk of recurrence. Acta Neuropathol. 2018;135(6):955-963.
doi pubmed - Wang Y, Han Y, Jin Y, He Q, Wang Z. The advances in epigenetics for cancer radiotherapy. Int J Mol Sci. 2022;23(10):5654.
doi pubmed - Eremina NV, Zhanataev AK, Durnev AD. Induced cell death as a possible pathway of antimutagenic action. Bull Exp Biol Med. 2021;171(1):1-14.
doi pubmed - Maresso KC, Tsai KY, Brown PH, Szabo E, Lippman S, Hawk ET. Molecular cancer prevention: Current status and future directions. CA Cancer J Clin. 2015;65(5):345-383.
doi pubmed - Sheikh IVS, Singh HT, Diwakar A, Atul S, Pritesh V, Anil KS, Anupam B. Cancer chemoprevention by flavonoids, dietary polyphenols and terpenoids. Biointerface Res Appl Chem. 2020;11(1):8502-8537.
doi - Jain A, Madu CO, Lu Y. Phytochemicals in chemoprevention: a cost-effective complementary approach. J Cancer. 2021;12(12):3686-3700.
doi pubmed - Abdoul-Latif FM, Ainane A, Houmed Aboubaker I, Mohamed J, Ainane T. An overview of cancer in djibouti: current status, therapeutic approaches, and promising endeavors in local essential oil treatment. Pharmaceuticals (Basel). 2023;16(11):1617.
doi pubmed - Sharma E, Tewari M, Sati P, Sharma I, Attri DC, Rana S, et al. Serving up health: How phytochemicals transform food into medicine in the battle against cancer. Food Front. 2024.
doi - Situmorang PC, Ilyas S, Nugraha SE, Syahputra RA, Nik Abd Rahman NMA. Prospects of compounds of herbal plants as anticancer agents: a comprehensive review from molecular pathways. Front Pharmacol. 2024;15:1387866.
doi pubmed - Martemucci G, Costagliola C, Mariano M, D’Andrea L, Napolitano P, D’Alessandro AG. Free radical properties, source and targets, antioxidant consumption and health. Oxygen. 2022;2(2):48-78.
doi - Situmorang PC, Ilyas S, Siahaan DAS, Restuati M, Sari ER, Chairunisa C, et al. Effect of rhodomyrtus tomentosa Hassk. on HIF1α and VEGF expressions on hypertension placental. J Pharm Pharmacogn Res. 2022;10(6):1076-1086.
doi - Yang L, Zhang W, Chopra S, Kaur D, Wang H, Li M, Chen P, et al. The epigenetic modification of epigallocatechin gallate (EGCG) on cancer. Curr Drug Targets. 2020;21(11):1099-1104.
doi pubmed - Hun Lee J, Shu L, Fuentes F, Su ZY, Tony Kong AN. Cancer chemoprevention by traditional chinese herbal medicine and dietary phytochemicals: targeting nrf2-mediated oxidative stress/anti-inflammatory responses, epigenetics, and cancer stem cells. J Tradit Complement Med. 2013;3(1):69-79.
doi pubmed - George BP, Chandran R, Abrahamse H. Role of phytochemicals in cancer chemoprevention: insights. Antioxidants (Basel). 2021;10(9):1455.
doi pubmed - Chang YJ, Hsu SL, Liu YT, Lin YH, Lin MH, Huang SJ, Ho JA, et al. Gallic acid induces necroptosis via TNF-alpha signaling pathway in activated hepatic stellate cells. PLoS One. 2015;10(3):e0120713.
doi pubmed - Ilyas S, Simanullang RH, Hutahaean S, Rosidah R, Situmorang PC. Correlation of Myc expression with Wee1 expression by zanthoxylum acanthopodium in cervical carcinoma histology. Pak J Biol Sci. 2022;25(11):1014-1020.
doi pubmed - Baliga MS, Jimmy R, Thilakchand KR, Sunitha V, Bhat NR, Saldanha E, Rao S, et al. Ocimum sanctum L (Holy Basil or Tulsi) and its phytochemicals in the prevention and treatment of cancer. Nutr Cancer. 2013;65(Suppl 1):26-35.
doi pubmed - Hong N, Tang Z, Chi Q, Du L, Jin Y. Formulations of traditional Chinese medicine for the prevention and treatment of radiation-induced injury. Acupunct Herb Med. 2024;4(4):463-474.
doi - Dumlu N, Geyikoglu F, Colak S. Radioprotective effect of umbelliferon against radiation-induced myocardial damages. J Sci Technol. 2024;17(3):811-825.
doi - Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32(1):23-63.
doi pubmed - Chen Y, Zhu L, Meng H, Sun X, Xue C. Ferulic acid protects human lens epithelial cells against ionizing radiation-induced oxidative damage by activating Nrf2/HO-1 signal pathway. Oxid Med Cell Longev. 2022;2022:6932188.
doi pubmed - Kim KM, Im AR, Shim KS, Lee AY, Kim T, Choi SA, Nam KW, et al. Clerodendrum trichotomum extract attenuates UV-B-induced skin impairment in hairless mice by inhibiting MAPK signaling. Photodermatol Photoimmunol Photomed. 2024;40(6):e13011.
doi pubmed - Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G, Castoria G, et al. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52(2):192-203.
doi pubmed - Patil K, Guledgud MV, Kulkarni PK, Keshari D, Tayal S. Use of curcumin mouthrinse in radio-chemotherapy induced oral mucositis patients: a pilot study. J Clin Diagn Res. 2015;9(8):ZC59-62.
doi pubmed - Sanders JT, Freeman TF, Xu Y, Golloshi R, Stallard MA, Hill AM, San Martin R, et al. Radiation-induced DNA damage and repair effects on 3D genome organization. Nat Commun. 2020;11(1):6178.
doi pubmed - Li B, Vincent A, Cates J, Brantley-Sieders DM, Polk DB, Young PP. Low levels of tumor necrosis factor alpha increase tumor growth by inducing an endothelial phenotype of monocytes recruited to the tumor site. Cancer Res. 2009;69(1):338-348.
doi pubmed - Kumar M, Allison DF, Baranova NN, Wamsley JJ, Katz AJ, Bekiranov S, Jones DR, et al. NF-kappaB regulates mesenchymal transition for the induction of non-small cell lung cancer initiating cells. PLoS One. 2013;8(7):e68597.
doi pubmed - Cao Y. Tumor angiogenesis and therapy. Biomed Pharmacother. 2005;59(Suppl 2):S340-343.
doi pubmed - Lan T, Chen L, Wei X. Inflammatory cytokines in cancer: comprehensive understanding and clinical progress in gene therapy. Cells. 2021;10(1):100.
doi pubmed - Iztleuov Y, Iztleuov M, Dushmanov A, Kydyrbayeva E, Mutigulina G, Imanbayev N, Iztleuova G. Prevention of mutagenesis, oxidative stress and inflammation in first generation male rats whose parents are exposed to gamma radiation and hexavalent chromium. Salud Cienc Tecnol. 2025;5:1259.
doi - Carlini V, Noonan DM, Abdalalem E, Goletti D, Sansone C, Calabrone L, Albini A. The multifaceted nature of IL-10: regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front Immunol. 2023;14:1161067.
doi pubmed - Su J, Song Y, Zhu Z, Huang X, Fan J, Qiao J, Mao F. Cell-cell communication: new insights and clinical implications. Signal Transduct Target Ther. 2024;9(1):196.
doi pubmed - Chiodoni C, Di Martino MT, Zazzeroni F, Caraglia M, Donadelli M, Meschini S, Leonetti C, et al. Cell communication and signaling: how to turn bad language into positive one. J Exp Clin Cancer Res. 2019;38(1):128.
doi pubmed - Dunn J, Grider MH. Physiology, adenosine triphosphate. In: StatPearls. Treasure Island (FL) ineligible companies. 2025.
pubmed - Kojima S, Ohshima Y, Nakatsukasa H, Tsukimoto M. Role of ATP as a key signaling molecule mediating radiation-induced biological effects. Dose Response. 2017;15(1):1559325817690638.
doi pubmed - Chen YH, Lin S, Jin SY, Gao TM. Extracellular ATP is a homeostatic messenger that mediates Cell-Cell communication in physiological processes and psychiatric diseases. Biol Psychiatry. 2025;97(1):41-53.
doi pubmed - Lemasters JJ, Qian T, He L, Kim JS, Elmore SP, Cascio WE, Brenner DA. Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid Redox Signal. 2002;4(5):769-781.
doi pubmed - Skulachev VP. Bioenergetic aspects of apoptosis, necrosis and mitoptosis. Apoptosis. 2006;11(4):473-485.
doi pubmed - Zhou Y, Tozzi F, Chen J, Fan F, Xia L, Wang J, Gao G, et al. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 2012;72(1):304-314.
doi pubmed - Akinfenwa AO, Abdul NS, Marnewick JL, Hussein AA. Protective effects of linearthin and other chalcone derivatives from aspalathus linearis (Rooibos) against UVB induced oxidative stress and toxicity in human skin cells. Plants (Basel). 2021;10(9):1936.
doi pubmed - Samivel R, Nagarajan RP, Subramanian U, Khan AA, Masmali A, Almubrad T, Akhtar S. Inhibitory effect of ursolic acid on ultraviolet B radiation-induced oxidative stress and proinflammatory response-mediated senescence in human skin dermal fibroblasts. Oxid Med Cell Longev. 2020;2020:1246510.
doi pubmed - Borrego-Soto G, Ortiz-Lopez R, Rojas-Martinez A. Ionizing radiation-induced DNA injury and damage detection in patients with breast cancer. Genet Mol Biol. 2015;38(4):420-432.
doi pubmed - Mao JJ, Pillai GG, Andrade CJ, Ligibel JA, Basu P, Cohen L, Khan IA, et al. Integrative oncology: Addressing the global challenges of cancer prevention and treatment. CA Cancer J Clin. 2022;72(2):144-164.
doi pubmed - Jangdey MS, Gupta A, Saraf S. Fabrication, in-vitro characterization, and enhanced in-vivo evaluation of carbopol-based nanoemulsion gel of apigenin for UV-induced skin carcinoma. Drug Deliv. 2017;24(1):1026-1036.
doi pubmed - Rudzinska A, Juchaniuk P, Oberda J, Wisniewska J, Wojdan W, Szklener K, Mandziuk S. Phytochemicals in cancer treatment and cancer prevention-review on epidemiological data and clinical trials. Nutrients. 2023;15(8):1896.
doi pubmed - Martinez-Perez C, Ward C, Cook G, Mullen P, McPhail D, Harrison DJ, Langdon SP. Novel flavonoids as anti-cancer agents: mechanisms of action and promise for their potential application in breast cancer. Biochem Soc Trans. 2014;42(4):1017-1023.
doi pubmed - Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674.
doi pubmed - Schnekenburger M, Dicato M, Diederich M. Plant-derived epigenetic modulators for cancer treatment and prevention. Biotechnol Adv. 2014;32(6):1123-1132.
doi pubmed - Alam MN, Almoyad M, Huq F. Polyphenols in colorectal cancer: current state of knowledge including clinical trials and molecular mechanism of action. Biomed Res Int. 2018;2018:4154185.
doi pubmed - Mollakhalili Meybodi N, Mortazavian AM, Bahadori Monfared A, Sohrabvandi S, Aghaei Meybodi F. Phytochemicals in cancer prevention: a review of the evidence. Int J Cancer Manag. 2017;10(1):e7219.
doi - Deiters A, Martin SF. Synthesis of oxygen- and nitrogen-containing heterocycles by ring-closing metathesis. Chem Rev. 2004;104(5):2199-2238.
doi pubmed - Su C, Zhang P, Song X, Shi Q, Fu J, Xia X, Bai H, et al. Tetrachlorobenzoquinone activates Nrf2 signaling by Keap1 cross-linking and ubiquitin translocation but not Keap1-Cullin3 complex dissociation. Chem Res Toxicol. 2015;28(4):765-774.
doi pubmed - Chikamori K, Grozav AG, Kozuki T, Grabowski D, Ganapathi R, Ganapathi MK. DNA topoisomerase II enzymes as molecular targets for cancer chemotherapy. Curr Cancer Drug Targets. 2010;10(7):758-771.
doi pubmed - Mondal A, Gandhi A, Fimognari C, Atanasov AG, Bishayee A. Alkaloids for cancer prevention and therapy: Current progress and future perspectives. Eur J Pharmacol. 2019;858:172472.
doi pubmed - Rajasekar N, Sivanantham A, Ravikumar V, Rajasekaran S. An overview on the role of plant-derived tannins for the treatment of lung cancer. Phytochemistry. 2021;188:112799.
doi pubmed - Ramprasath VR, Awad AB. Role of phytosterols in cancer prevention and treatment. J AOAC Int. 2015;98(3):735-738.
doi pubmed - Thoppil RJ, Bishayee A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J Hepatol. 2011;3(9):228-249.
doi pubmed - Elekofehinti OO, Iwaloye O, Olawale F, Ariyo EO. Saponins in cancer treatment: current progress and future prospects. Pathophysiology. 2021;28(2):250-272.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.