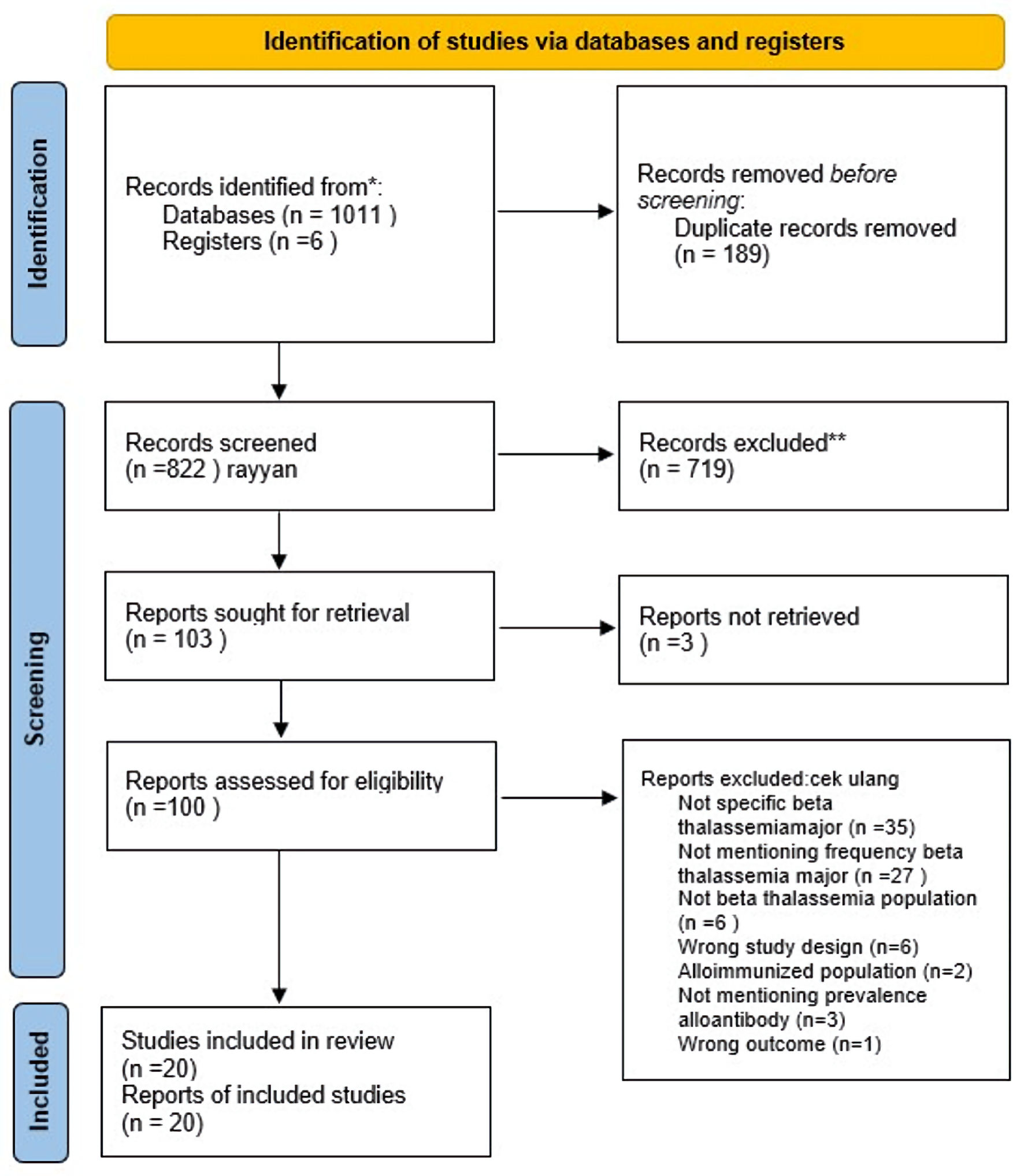
Figure 1. Study selection according to PRISMA flowchart. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 2, February 2025, pages 106-118
Prevalence of Alloimmunization Events in Thalassemia Patients With Repeated Transfusions in the Rhesus Blood Group System: A Systematic Review and Meta Analysis
Figures

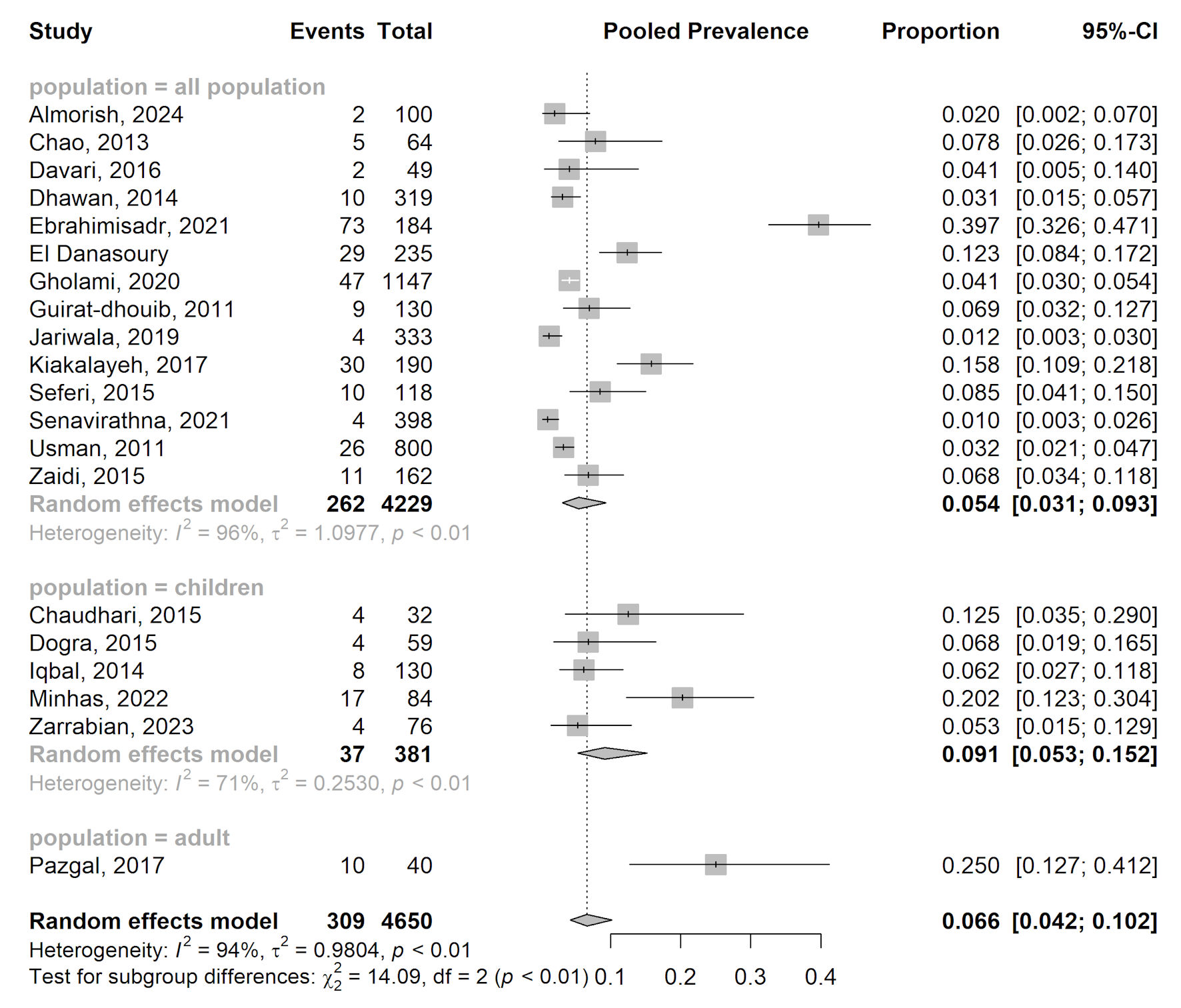
Tables
| Study | Study design | Sample size | Gender male, n (%) | Age (years), mean ± SD | Blood transfusion interval (days) | Number of transfusions | Duration of transfusion | First transfusion age | Population | Method | Total number of patients with alloantibody | Total number of patients with Rhesus alloantibody | Blood component |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N/A: not available; yr: years; RBC: red blood cell; IgG: immunoglobulin G. | |||||||||||||
| Almorish et al, 2024 [15] | Cross-sectional | 100 | 50 (50) | Range 1 - > 20 | N/A | > 12 units/year (88), ≥ 12 units/year (12) | < 160 month (71), ≥ 160 months (30) | < 1 yr (42), 1 - 10 yr (53), > 10 yr (5) | Sana’a City - Yemen | 11 cell identification panel | 6 | 2 | Leukoreduced |
| Chao et al, 2013 [16] | Retrospective cohort | 64 | 31 (48) | 19.2 ± 6.7 | N/A | N/A | 17.8 ± 4.3 years | 5.7 ± 2.2 month | China | LISS | 6 | 5 | Leukodepleted |
| Chaudhari et al, 2011 [17] | Cross-sectional | 32 | 22 (68.75) | 12.0 ± 3.8 | 21 - 35 | 2 units (1), 15-19 units (2), > 20 units (29) | 8.5 ± 4.0 year | 2.7 ± 1.3 years | India | 11 cell identification panel | 6 | 4 | Non leukodepleted whole blood or packed RBC |
| Davari et al, 2016 [18] | Cross-sectional | 49 | 25 (51) | 18.59 ± 8.16 | 14 - 35 | > 50 units | N/A | N/A | Iran | 10 cell identification panel | 8 | 2 | Leukoreduced and non leukoreduced |
| Dhawan et al, 2014 [19] | Cross-sectional | 319 | 235 (73.66) | 15.18 | 14 - 28 | 235.74 units | N/A | 14.93 months | India | 11 cell identification panel | 18 | 10 | Non leukodepleted |
| Dogra et al, 2015 [20] | Descriptive study | 59 | N/A | 9.27 ± 4.58 | 14 - 28 | Mean ± SD: 132 ± 38.44 units | N/A | 4.7 (3.8) years | India | 11 cell identification panel | 6 | 4 | Non leukodepleted |
| Ebrahimisadr et al, 2021 [21] | Descriptive study | 184 | 66 (35.86) | Range 0 - > 30 | 21 - 35 | N/A | N/A | N/A | Iran | 11 cell identification panel | 116 | 73 | Non leukodepleted |
| El Danasoury, et al, 2012 [22] | Prospective cohort | 235 | 126 (53.61) | 12 ± 6.1 | 14 - 28 | N/A | N/A | 2 ± 1.2 years | Egypt | 11 cell identification panel | 46 | 29 | Non leukodepleted |
| Gholami et al, 2021 [23] | Cross-sectional | 1147 | N/A | 14 ± 6 | N/A | > 20 units | N/A | N/A | Iran | IBTO homemade 11-cell panel in LISS solution | 97 | 47 | Non leukodepleted |
| Guirat-Dhouib et al, 2011 [24] | Prospective cohort | 130 | 73 (56.15) | 9.9 | N/A | N/A | N/A | N/A | Tunisian | Gel filtration test | 10 | 9 | Non leukodepleted |
| Iqbal et al, 2014 [25] | Cross-sectional | 130 | 76 (58.5) | 6.28 ± 3.18 | 5 - 20 | N/A | N/A | N/A | Pakistan | 3 cell panel followed by 11 cell panel | 11 | 8 | Non leukodepleted |
| Jariwala et al, 2019 [26] | Retrospective cohort | 333 | 188(56.45) | 11.3 (4 - 26) | N/A | 17.6 units/year | N/A | N/A | India | 11 cell identification panel | 4 | 4 | Non leukodepleted |
| Davoudi-Kiakalayeh et al, 2017 [27] | Retrospective cohort | 190 | N/A | 26 ± 5.9 | N/A | N/A | N/A | N/A | Iran | IBTO home-made, 3 RBC cells) and antibody identification panels | 47 | 30 | Leukodepleted |
| Minhas et al, 2022 [28] | Retrospective cross-sectional | 84 | 51 (59.5) | Median 7 (4 - 11) | N/A | Median 64 (26 - 127) units | N/A | N/A | Pakistan | Dia clon3 cell antigen panel | 22 | 17 | Non leukodepleted |
| Pazgal et al, 2020 [29] | Retrospective cohort | 40 | 21 (52.5) | 31.8 ± 6.9 | 14 - 21 | Mean 828.5 ± 239.3 units | N/A | 0.71 ± 0.93 years | Israel | Automated systems and LISS-IgG cards | 17 | 10 | Before 2004 non leukoreduced 2004: leukoreduced |
| Seferi et al, 2015 [30] | Retrospective study and prospective follow up | 118 | 59 (50) | 17 | 10 - 90 | N/A | N/A | N/A | Albania | DiaPanel, Diamed-Biorad | 14 | 10 | Pre- and post-storage leukoreduced red cell |
| Senavirathna et al, 2021 [31] | Retrospective descriptive study | 398 | 188 (47.24) | 18.41 ± 11.67 | N/A | N/A | N/A | N/A | Colombo | Tube technique | 6 | 4 | Non leukodepleted |
| Usman et al, 2011 [32] | Cross-sectional | 800 | 350 (43.75) | 11.5 | N/A | N/A | N/A | N/A | Pakistan | DiaMed | 30 | 26 | Non leukodepleted |
| Zaidi et al, 2015 [33] | Cross-sectional | 162 | 97 (60) | Median 6.7 (range: 0·5 - 25) | 14.2 - 31.8 | N/A | N/A | 0.98 ± 0.93 years | Pakistan | 3 cell antigen panel | 14 | 11 | Non leukodepleted |
| Zarrabian et al, 2023 [34] | Retrospective cohort | 76 | 44 (58) | 12.83 (5.48) | N/A | Mean (range) 145.5 (5 - 598) | 95.6 (63.3) months | N/A | Canada | 11 cell identification panel | 4 | 4 | Non leukodepleted |
| Rhesus bloood group system specificity | Almorish et al, 2024 [15] | Chao et al, 2013 [16] | Chaudhari et al, 2011 [17] | Davari et al, 2016 [18] | Dhawan et al, 2014 [19] | Dogra et al, 2015 [20] | Ebrahimisadr et al, 2021 [21] | El Danasoury et al, 2012 [22] | Gholami et al, 2021 [23] | Guirat-Dhouib et al, 2011 [24] | Iqbal et al, 2014 [25] | Jariwala et al, 2019 [26] | Davoudi-Kiakalayeh et al, 2017 [27] | Minhas et al, 2022 [28] | Pazgal et al, 2020 [29] | Seferi et al, 2015 [30] | Senavirathna et al, 2021 [31] | Usman et al, 2011 [32] | Zaidi et al, 2015 [33] | Zarrabian et al, 2023 [34] | Number of alloimmunized patients (total 292) | Antibody specificity among all studies (total 4,566) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||||||||||||||||||
| E | 1 | 4 | 2 | 1 | 2 | 3 | 10 | 11 | 21 | 3 | 5 | 0 | 11 | N/A | 8 | 1 | 3 | 7 | 4 | 4 | 101 | 34.58 | 101 | 2.21 |
| D | 0 | 0 | 0 | 0 | 2 | 1 | 11 | 6 | 0 | 1 | 2 | 0 | 6 | 2 | 0 | 0 | 8 | 1 | 0 | 40 | 13.69 | 40 | 0.87 | |
| C | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 7 | 8 | 3 | 0 | 2 | 2 | 0 | 0 | 0 | 5 | 0 | 0 | 30 | 10.27 | 30 | 0.66 | |
| c | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 11 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 23 | 7.87 | 23 | 0.50 | |
| D + C | 0 | 0 | 0 | 0 | 1 | 0 | 7 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 3 | 0 | 0 | 1 | 0 | 16 | 5.47 | 16 | 0.35 | |
| Cw | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 5 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 12 | 4.10 | 12 | 0.26 | |
| Kell + E | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 7 | 2.39 | 7 | 0.15 | |
| e | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 6 | 2.05 | 6 | 0.12 | |
| C + E | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 2.05 | 6 | 0.12 | |
| c + E | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 3 | 1.37 | 3 | 0.08 | |
| Cw + E | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1.02 | 3 | 0.06 | |
| E + Jkb | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1.02 | 3 | 0.06 | |
| C + e | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1.02 | 3 | 0.06 | |
| C + K | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0.68 | 2 | 0.04 | |
| E + K | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.68 | 2 | 0.04 | |
| Kell + C + E | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.68 | 2 | 0.04 | |
| Kell + C + D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.68 | 2 | 0.04 | |
| C + Kell | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.68 | 2 | 0.04 | |
| Kell + Cw + Kpa | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.68 | 2 | 0.04 | |
| E + C- + kell | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.68 | 2 | 0.04 | |
| E + S | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| Kpa + D | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| D + K | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| D + E + C | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0.34 | 1 | 0.02 | |
| E + K + C + M + Kpa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0.34 | 1 | 0.02 | |
| C + S | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| D + S | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| D + Kell | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| D + E | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| E + Jka | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| Rhesus bloood group system specificity | Almorish et al, 2024 [15] | Chao et al, 2013 [16] | Chaudhari et al, 2011 [17] | Davari et al, 2016 [18] | Dhawan et al, 2014 [19] | Dogra et al, 2015 [20] | Ebrahimisadr et al, 2021 [21] | El Danasoury et al, 2012 [22] | Gholami et al, 2021 [23] | Guirat-Dhouib et al, 2011 [24] | Iqbal et al, 2014 [25] | Jariwala et al, 2019 [26] | Davoudi-Kiakalayeh et al, 2017 [27] | Minhas et al, 2022 [28] | Pazgal et al, 2020 [29] | Seferi et al, 2015 [30] | Senavirathna et al, 2021 [31] | Usman et al, 2011 [32] | Zaidi et al, 2015 [33] | Zarrabian et al, 2023 [34] | Number of alloimmunized patients (total 293) | Antibody specificity among all studies (total 4,650) | ||
| N | % | N | % | |||||||||||||||||||||
| Cw + Kell | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| Cw + Kpa | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| E + C + Jka | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| E + Kell + Jkb | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| E + C- + Jkb | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| D + C + S | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| C- + kell + Cw | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| D + C + kell | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| E + S + Jkb | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| D + C + E + K + S + Jkb | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| E + C- + kell + M | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| E + kell + Fyb + S | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| D + kell + S + Jkb | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| C + D + E + kell | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |
| E + C + Fyb + Jka + S | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.34 | 1 | 0.02 | |