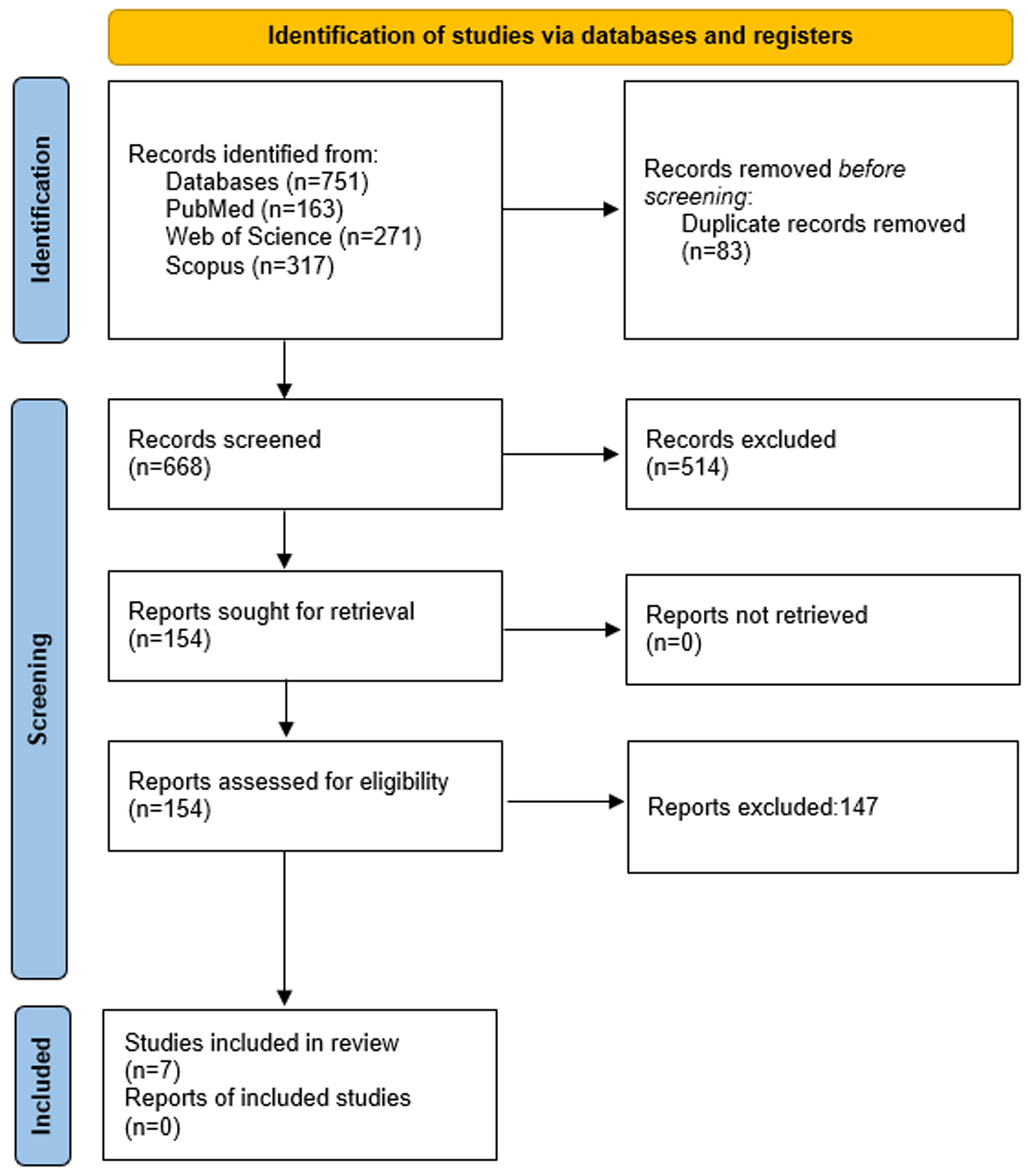
Figure 1. PRISMA flow diagram.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 5, May 2025, pages 285-296
Comparative Efficacy of Tirzepatide vs. Semaglutide in Reducing Body Weight in Humans: A Systematic Review and Meta-Analysis of Clinical Trials and Real-World Data
Figures

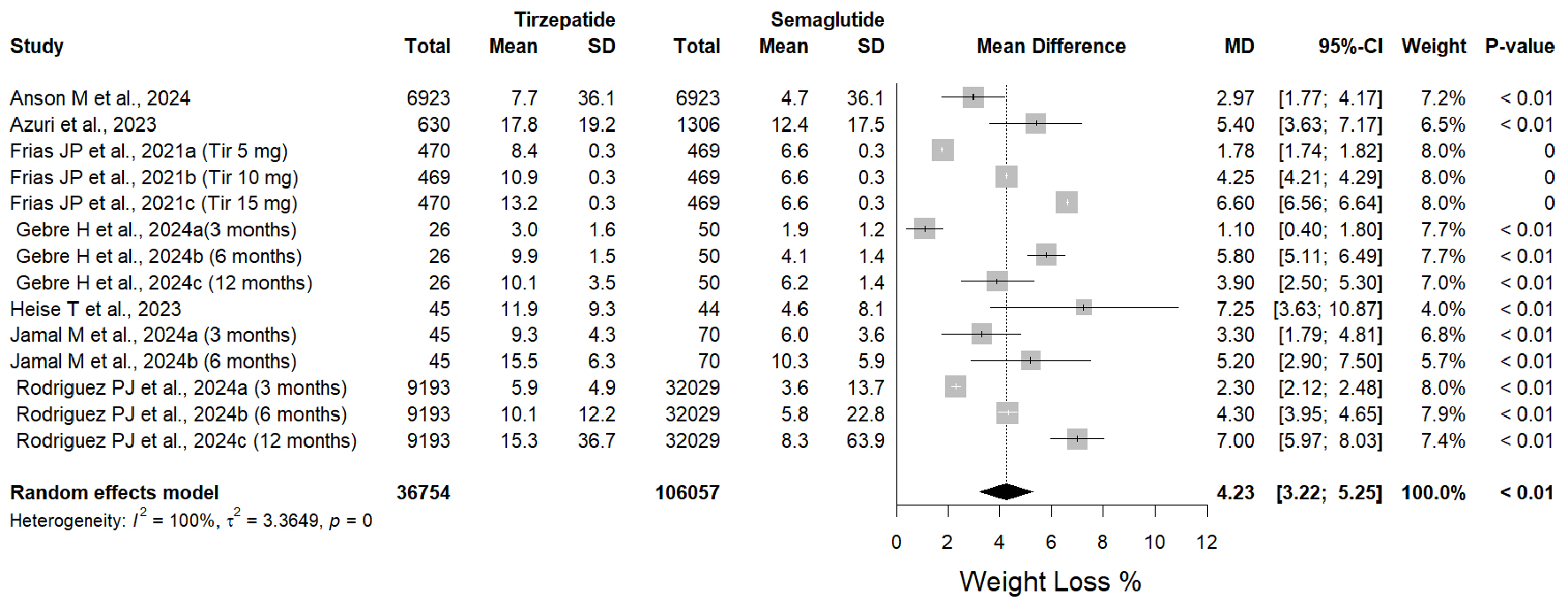
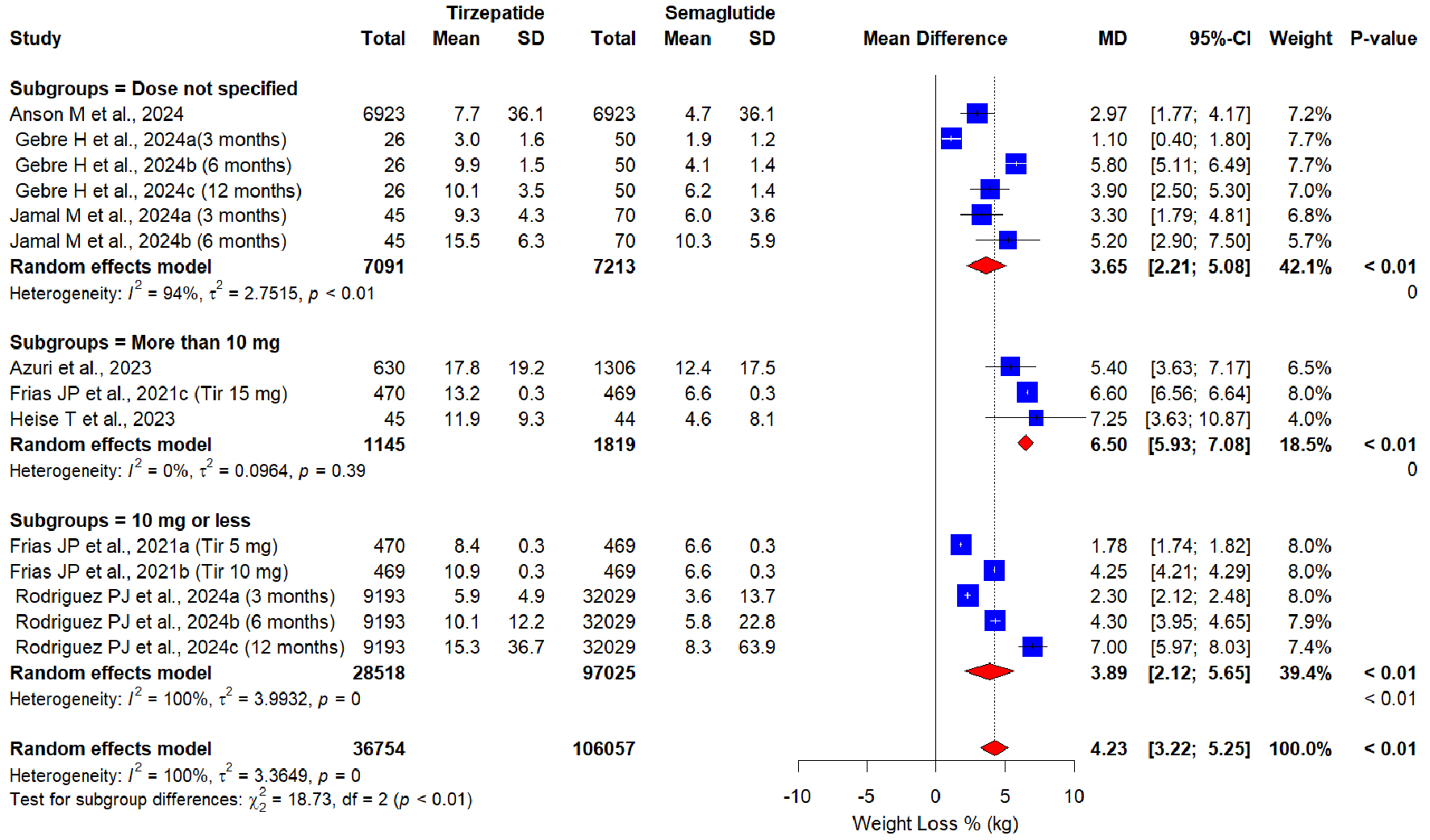
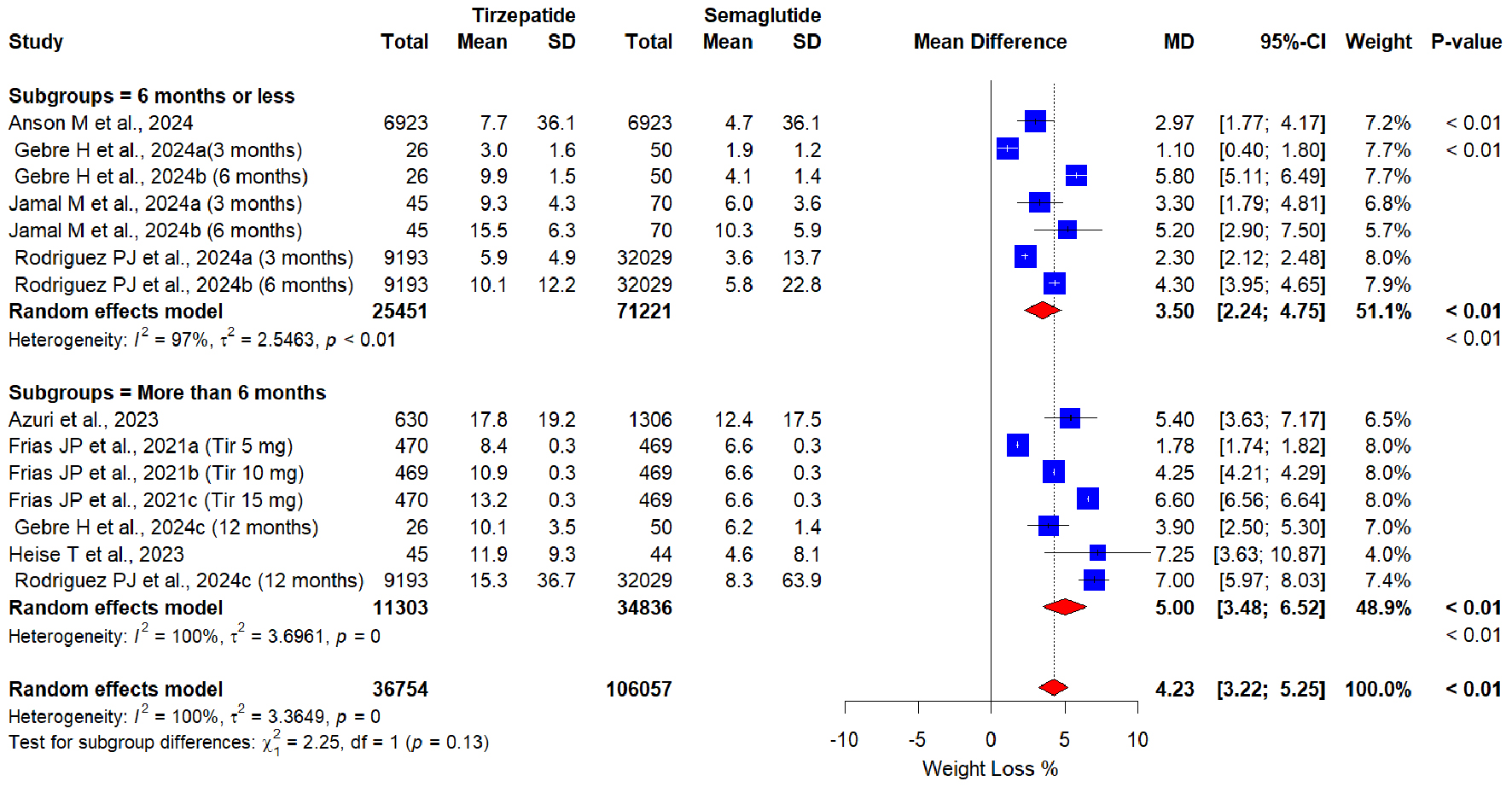

Tables
| Database | Search number | Query | Results |
|---|---|---|---|
| PubMed (all fields) | Search 1 | “tirzepatide” (MeSH terms) OR “tirzepatide” (all fields) OR “tirzepatide” (MeSH terms) OR “tirzepatide” (all fields) OR “mounjaro” (all fields) OR “tirzepatide” (MeSH terms) OR “tirzepatide” (all fields) OR “zepbound” (all fields) OR “tirzepatide” (MeSH terms) OR “tirzepatide” (all fields) OR “ly3298176” (all fields) | 821 |
| Search 2 | “semaglutide” (supplementary concept) OR “semaglutide” (all fields) OR “GLP-1 agonist” (all fields) OR “semaglutide” (supplementary concept) OR “semaglutide” (all fields) OR “ozempic” (all fields) OR “semaglutide” (supplementary concept) OR “semaglutide” (all fields) OR “rybelsus” (all fields) OR “semaglutide” (supplementary concept) OR “semaglutide” (all fields) OR “wegovy” (all fields) | 3,132 | |
| Search 3 | “body weight” (all fields) OR “Weight loss” (all fields) OR “loss in weight” (all fields) OR “body mass” (all fields) OR “Anti-obesity agents” (all fields) | 810,303 | |
| Search 4 | #1 AND #2 AND #3 | 163 | |
| Web of Science | Search 1 | ALL = (tirzepatide OR mounjaro OR zepbound OR ly3298176) | 1,123 |
| Search 2 | ALL = (semaglutide OR “GLP-1 agonist” OR ozempic OR rybelsus OR wegovy) | 4,332 | |
| Search 3 | ALL = (“body weight” OR “Weight loss” OR “loss in weight” OR “body mass” OR “Anti-obesity agents”) | 806,866 | |
| Search 4 | #1 AND #2 AND #3 | 271 | |
| Scopus | Search 1 | TITLE-ABS-KEY (“tirzepatide” OR “mounjaro” OR “zepbound” OR “ly3298176”) | 1,622 |
| Search 2 | TITLE-ABS-KEY (“GLP-1 agonist” OR “semaglutide” OR “ozempic” OR “rybelsus” OR “wegovy”) | 6,055 | |
| Search 3 | TITLE-ABS-KEY (“body weight” OR “Weight loss” OR “loss in weight” OR “body mass” OR “Anti-obesity agents”) | 25,765 | |
| Search 4 | #1 AND #2 AND #3 | 317 |
| Study ID; study design; blindness | Country; time period; funding; protocol registration (number/NR) | Study population (baseline) | Drug: route, frequency, dose Comparator: route, frequency, dose | Participants number (randomized/completed) | Eligibility criteria | ||
|---|---|---|---|---|---|---|---|
| BMI (kg/m2), mean ± SD | Age (years), mean ± SD | Female, n (%) | |||||
| BMI: body mass index; eGFR: estimated glomerular filtration rate; HbA1c: hemoglobin A1c; GI: gastrointestinal; GLP-1 RA: glucagon-like peptide-1 receptor agonist; NR: not reported; RCT: randomized controlled trial; SC: subcutaneous; Sem: semaglutide; SG: sleeve gastrectomy; Tir: tirzepatide; T1DM: type 1 diabetes mellitus; T2DM: type 2 diabetes mellitus. | |||||||
| Frias et al, 2021 [15]; RCT; open label | United States, Argentina, Australia, Brazil, Canada, Israel, Mexico, and the United Kingdom; July 30, 2019 - February 15, 2021; funded by Eli Lilley; Reg. NCT03987919 | Tir: 5 mg: 33.8 ± 6.85; 10 mg: 34.3 ± 6.60; 15 mg: 34.5 ± 7.11 Sem: 34.2 ± 7.15 | Tir: 5 mg: 56.3 ± 10.0; 10 mg: 57.2 ± 10.5; 15 mg: 55.9 ± 10.4 Sem: 56.9 ± 10.8 | Tir: 5 mg: 265 (56.4); 10 mg: 231 (49.3); 15 mg: 256 (54.5) Sem: 244 (52) | Tir: SC once weekly 5 mg, 10 mg, 15 mg for 40 weeks Sem: SC once weekly 1 mg for 40 weeks | Tir: 5 mg: 470/452; 10 mg: 469/412; 15 mg: 470/416 Sem: 469/443 | Inclusion: 18 years or older and T2DM that was inadequately controlled with metformin and had stable weight (± 5%) during the previous 3 months Exclusion: 1) T1DM; 2) an eGFR below 45 mL/min/1.73 m2; 3) a history of pancreatitis, non-proliferative/proliferative diabetic retinopathy or diabetic maculopathy |
| Heise et al, 2023 [16]; RCT; double-blind | Germany; time period NR; funded by Eli Lilley; Reg. NCT03951753 | Tir: 31.3 ± 5.0 Sem: 30.8 ± 3.8 | Tir: 61.1 ± 7.1 Sem: 63.7 ± 5.9 | Tir: 14 (33.3) Sem: 10 (22.7) | Tir: SC once weekly 15 mg for 28 weeks Sem: SC once weekly 1 mg for 28 weeks | Tir: 45/41 Sem: 44/43 | Inclusion: 20 - 74 years older with T2DM (HbA1c 7.0-9.5% if on metformin only or 6.5-9.0% if on metformin in combination with other oral antihyperglycemic medications) and a BMI 25 - 45 kg/m2 Exclusion: 1) T1DM; 2) had one or more episodes of severe hypoglycemia or ketoacidosis within 6 months before screening; 3) a history of proliferative retinopathy or maculopathy; 4) impaired renal eGFR < 45 mL/min/1.73 m2; 5) a history or current cardiovascular, respiratory, hepatic, renal, GI, endocrine, hematological or neurological disorders |
| Anson et al, 2024 [17]; retrospective cohort | North America; June 5, 2024; non-funded | Tir: NR Sem: NR | Tir: 47.5 ± 11.8 Sem: 47.5 ± 11.9 | Tir: 5,054 (73) Sem: 5,054 (73) | Tir: NR Sem: NR | Tir: 6,923 Sem: 6,923 | Inclusion: aged 18 or over without a pre-existing diagnosis of any diabetes mellitus who were prescribed either Tir or Sem Exclusion: individuals who were ever prescribed any GLP-1 RA or pramlintide |
| Azuri et al, 2023 [18]; retrospective cohort | Tir: Argentina, Brazil, China, India, Japan, Mexico, Russian Federation, Taiwan, and the United States; time period NR; funded by Eli Lilly Sem: Asia, Europe, North America, and South America; June - November 2018; funded by Novo Nordisk | Tir: 38.1 ± 6.69 Sem: 37.8 ± 6.7 | Tir: 44.9 ± 12.3 Sem: 46 ± 13 | Tir: 425 (67.5) Sem: 955 (73.1) | Tir: SC once weekly 15 mg for 72 weeks Sem: SC once weekly 2.4 mg for 68 weeks | Tir: 630/566 Sem: 1,306/1,240 | Inclusion: adults (18 years of age or older) with one or more self-reported unsuccessful dietary efforts to lose weight and either a BMI of 30 or greater or a BMI of 27 or greater with one or more treated or untreated weight-related coexisting conditions. Exclusion (Sem): 1) have T1DM or T2DM; 2) an HbA1c level of 48 mmol/mol (6.5%) or greater; 3) a history of chronic pancreatitis, acute pancreatitis within 180 days before enrollment, previous surgical obesity treatment, and use of anti-obesity medication within 90 days before enrollment; 4) have a self-reported change in body weight > 5 kg within 3 months before screening. |
| Gebre et al, 2024 [19]; retrospective cohort | Location NR; time period NR; funded by Novo Nordisk | Tir: 36.6 ± 5.3 Sem: 33.4 ± 6 | Tir: 42 ± 8 Sem: 42 ± 11 | Tir: 14 (54) Sem: 35 (70) | Tir: SC once weekly given for 9 months Sem: SC once weekly given for 9 months Remaining info NR | Tir: 26 Sem: 50 | Inclusion: patients with T1DM who were treated with Tir or Sem Exclusion criteria: not given |
| Jamal et al, 2024 [20]; retrospective cohort | Kuwait; January 2022; non-funded | Tir: 36.9 ± 7.1 Sem: 33.9 ± 6 | Tir: 40.2 ± 10.5 Sem: 38.1 ± 10.3 | Tir: 37 (82.2) Sem: 56 (80) | Tir: increasing dose regimen starting at 2.5 mg given for 6 months Sem: increasing dose regimen starting at 0.25 mg given for 6 months | Tir: 45 Sem: 70 | Inclusion: adult (> 18 years old) patients who sought management of weight recurrence after SG with Sem or Tir with BMI greater than 30 or 27 kg/m2 with at least one obesity-related complication Exclusion: patients on any other weight loss regimen, had personal history of pancreatitis, personal or family history of medullary thyroid cancer, or they were pregnant or breastfeeding, or if the patient discontinued the treatment in less than 12 weeks. |
| Rodriguez et al, 2024 [21]; retrospective cohort | United States; May 2022 - September 2023; non-funded | Tir: 39 ± 8.08 Sem: 38.6 ± 7.92 | Tir: 51.9 ± 12.7 Sem: 56.4 ± 13 | Tir: 6,484 (71) Sem: 21,060 (66) | Tir: SC once weekly 5 mg for 12 months Sem: SC once weekly 0.5 mg for 12 months | Tir: 9,193 Sem: 32,029 | Inclusion: 1) adults first dispensed Tir or Sem labeled for T2DM (as brand names Mounjaro (Eli Lilly) or Ozempic (Novo Nordisk), respectively), and who had BMI ≥ 27. 2) patients with regular interactions with the health care system Exclusion: history of GLP-1 RA use |
| Anson et al, 2024 [17] | Azuri et al, 2023 [18] | Gebre et al, 2024 [19] | Jamal et al, 2024 [20] | Rodriguez et al, 2024 [21] | |
|---|---|---|---|---|---|
| Scoring algorithms: ≥ 7 points were considered as “good”, 2 to 6 points were considered as “fair”, and ≤ 1 point was considered as “poor” quality. DM: diabetes mellitus. | |||||
| 1. Selection domain | |||||
| 1) Representativeness of the tirzepatide cohort | * | * | * | * | * |
| a) truly representative* | |||||
| b) somewhat representative* | |||||
| c) selected group of users | |||||
| d) no description | |||||
| 2) Selection of the semaglutide cohort | * | * | * | * | * |
| a) drawn from the same community* | |||||
| b) drawn from a different source | |||||
| c) no description of the derivation | |||||
| 3) Ascertainment of exposure to intervention | * | * | * | * | * |
| a) secure record (e.g., hospital records)* | |||||
| b) structured interview* | |||||
| c) written self-report | |||||
| d) no description | |||||
| 4) Demonstration that outcome of interest was not present at start of study (subjects were obese/overweight at beginning) | * | * | * | * | * |
| a) yes* | |||||
| b) no | |||||
| 1) Comparability of cohorts on the basis of the design or analysis | |||||
| a) study controls for _ (select the most important factor, e.g., age, gender, marital status)* | * | - | * | - | * |
| b) study controls for any additional factor* (weight) similar disease like DM or obesity) | * | - | * | - | * |
| Outcome | |||||
| 1) Assessment of outcome | |||||
| a) independent blind assessment* | * | * | * | * | * |
| b) record linkage* | |||||
| c) self-report | |||||
| d) no description | |||||
| 2) Was follow-up long enough for outcomes to occur | |||||
| a) yes (select an adequate follow up period for outcome of interest, a follow-up of 12 weeks at least)* | * | * | * | * | * |
| b) no | |||||
| 3) Adequacy of follow-up of cohorts | * | * | No statement | * | - |
| a) complete follow-up - all subjects accounted for* | |||||
| b) subjects lost to follow-up unlikely to introduce bias - numbers lost is ≤ 20%, or description of those lost suggested no different from those followed)* | |||||
| c) follow-up rate < 80% and no description of those lost | |||||
| d) no statement | |||||
| Total scores (quality) | 9 (good) | 7 (good) | 8 (good) | 7 (good) | 8 (good) |