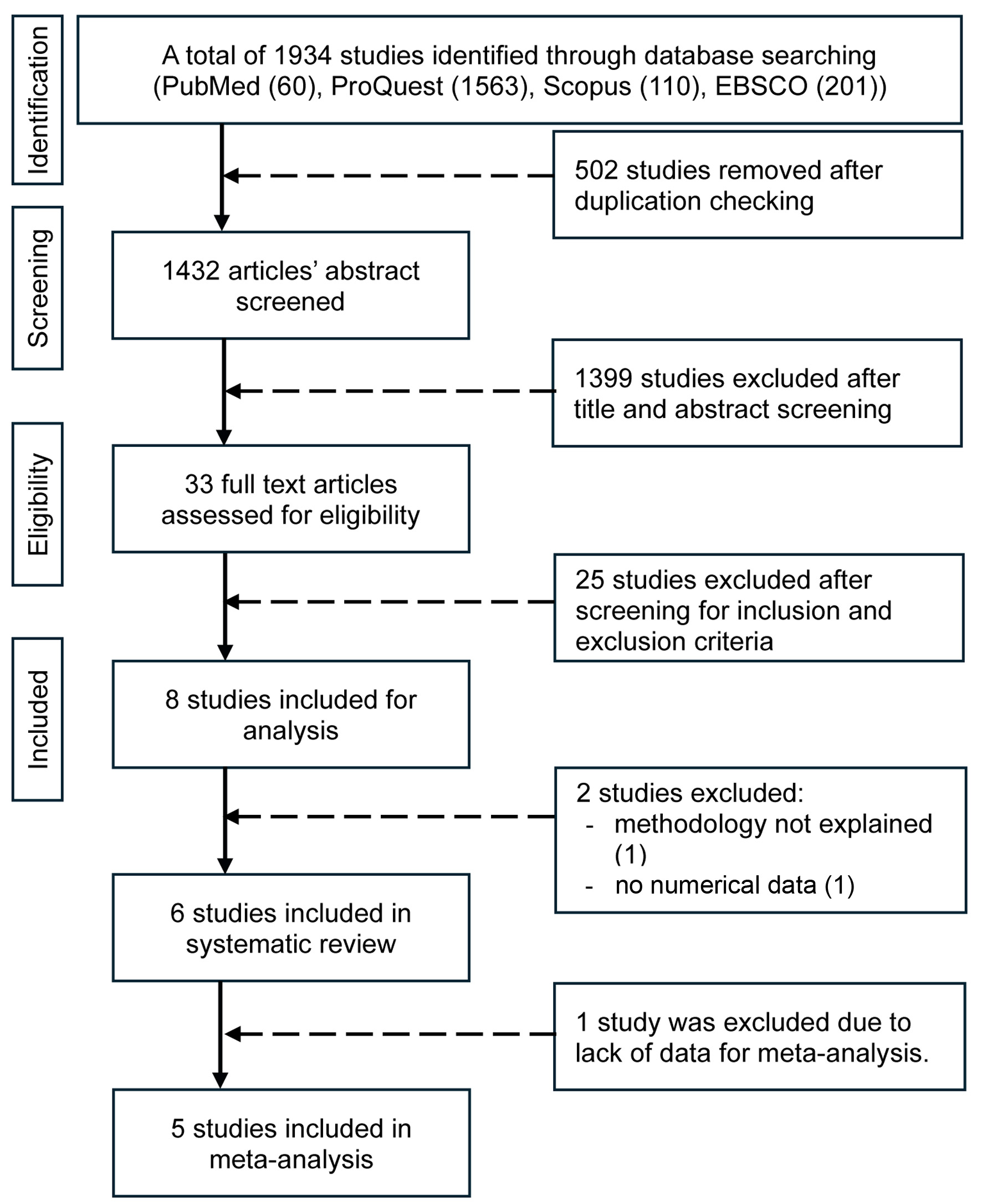
Figure 1. Flow diagram of the search process.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 8, August 2025, pages 445-459
The Elimination Effect of Medical-Grade Honey on Pseudomonas aeruginosa Biofilms: A Systematic Review and Meta-Analysis
Figures

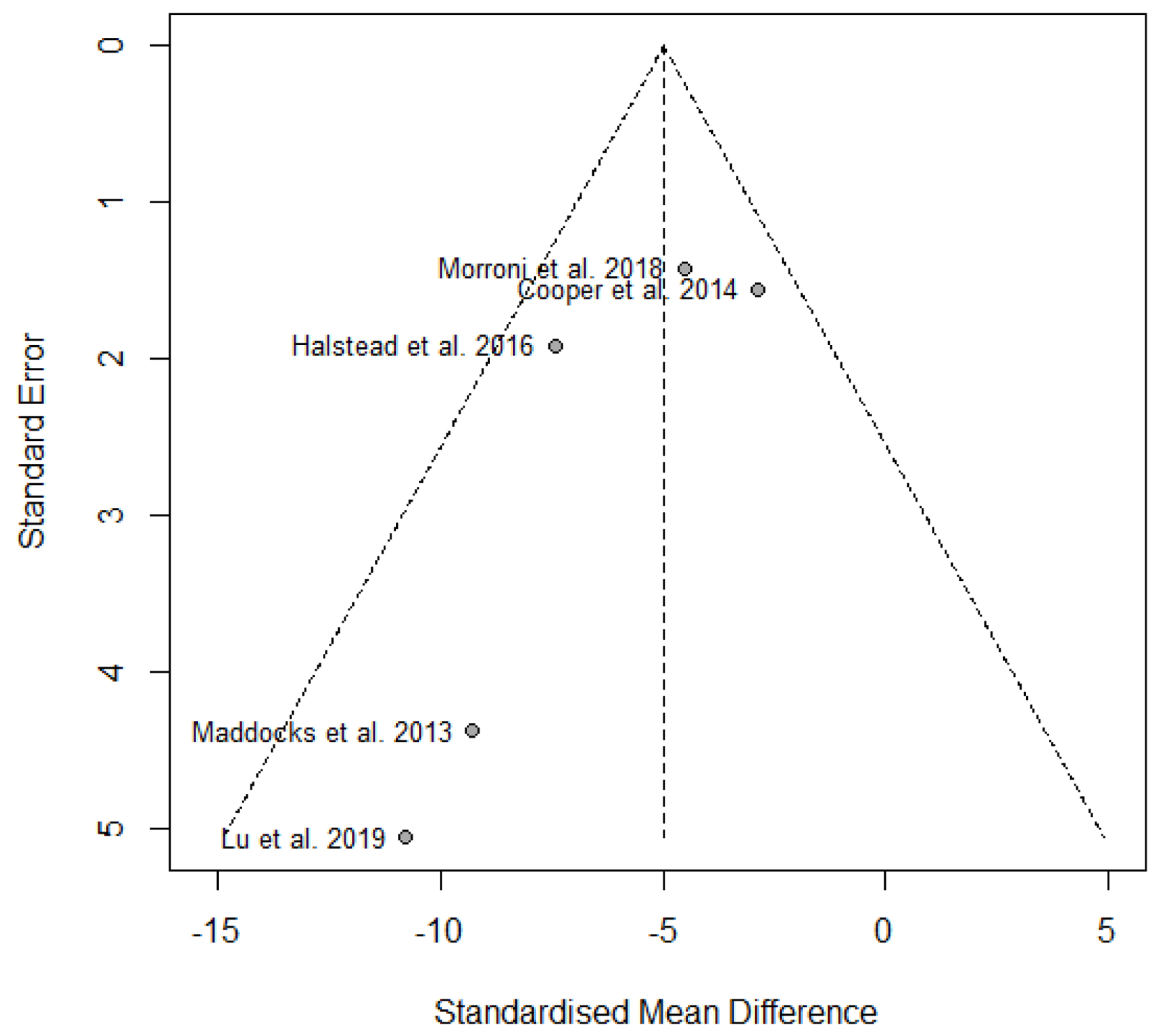
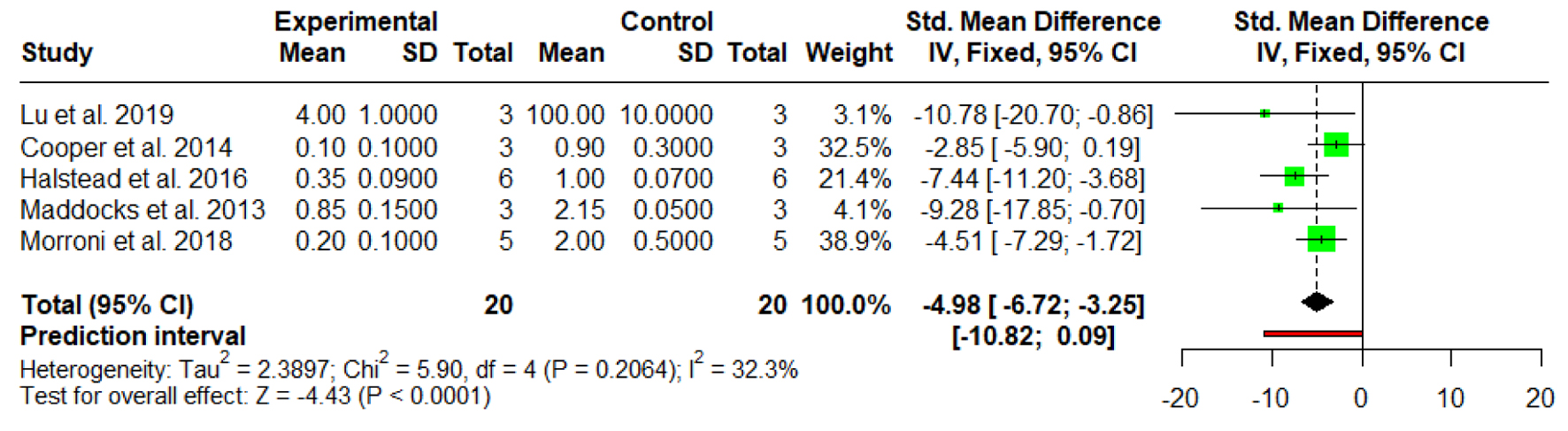
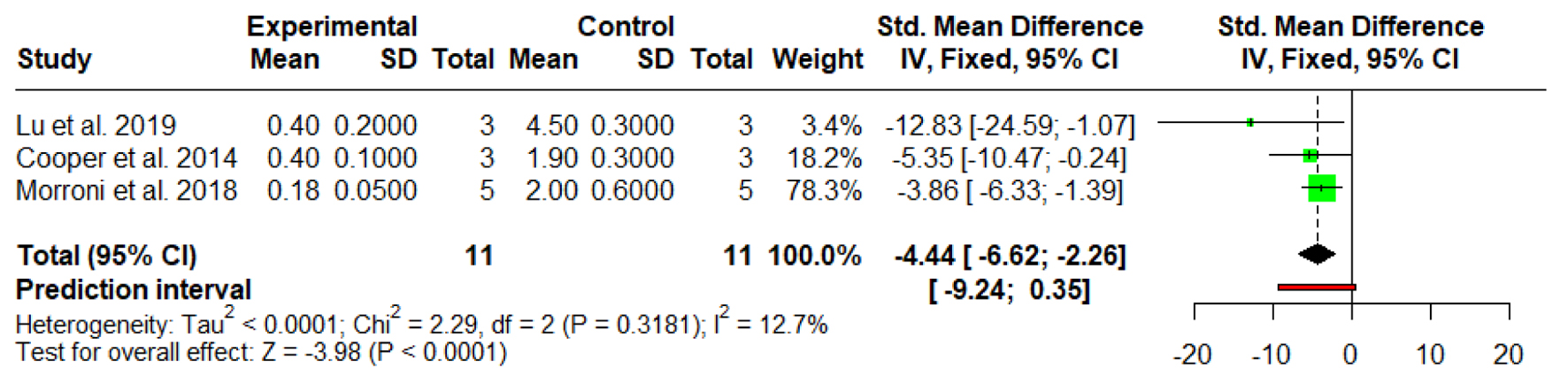
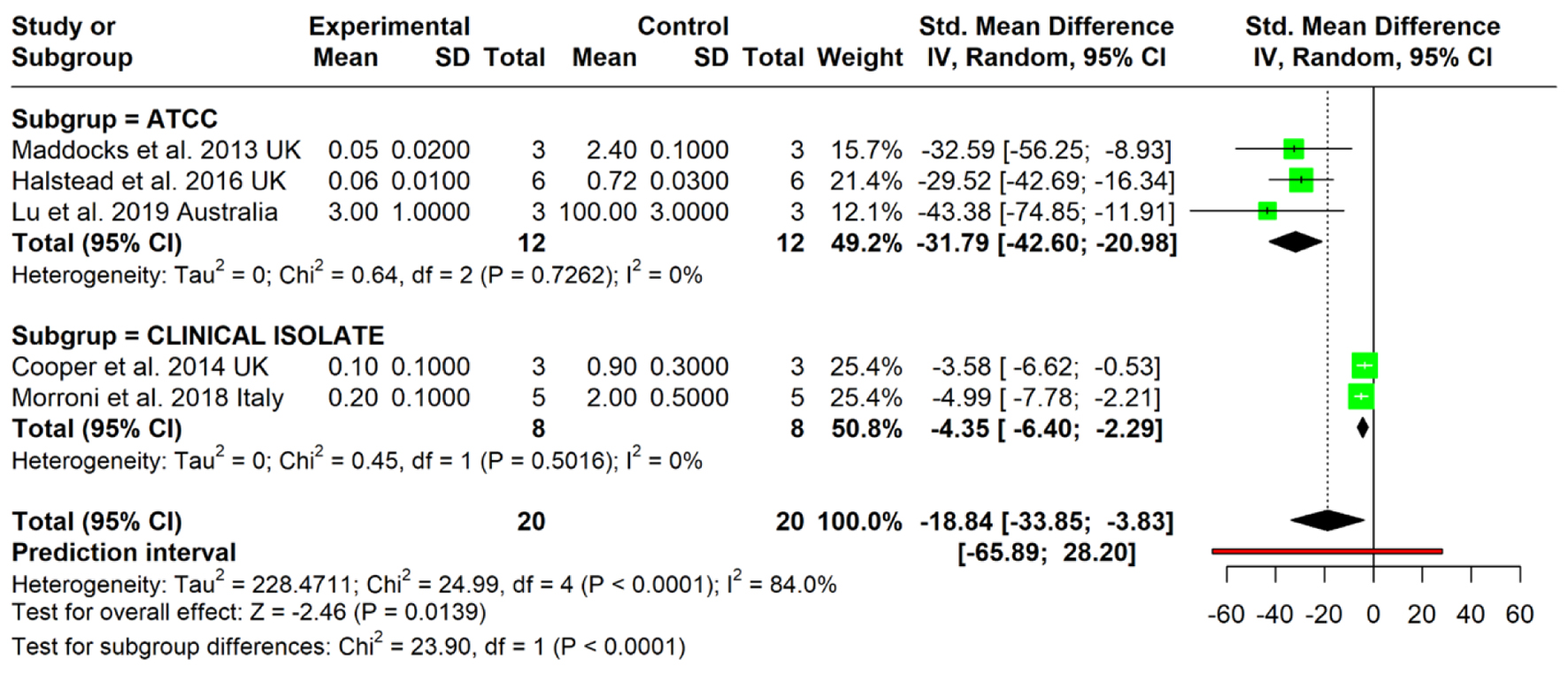
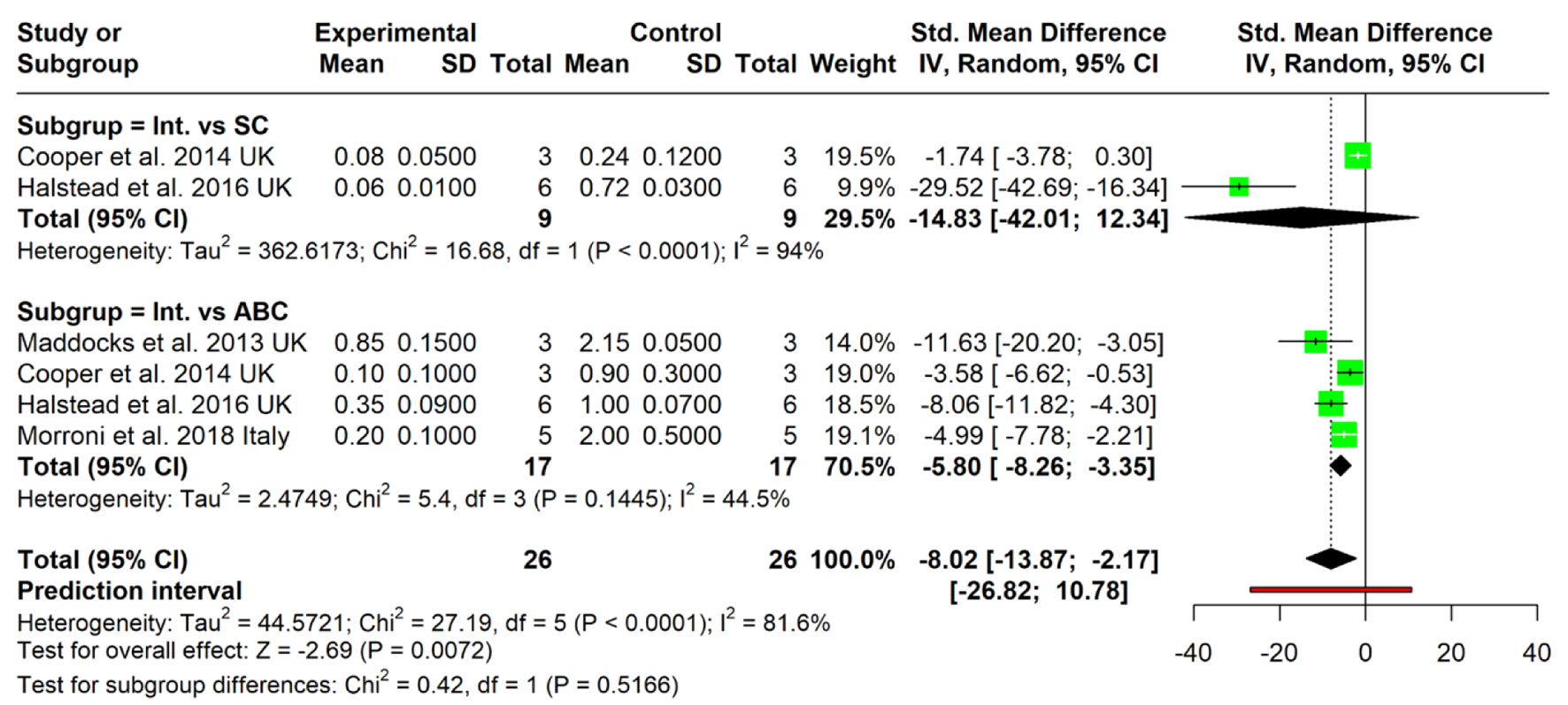
Tables
| No. | Study | P. aeruginosa strain | Medical-grade honey | Honey concentrations tested | CV assay | Outcomes measured | Key findings |
|---|---|---|---|---|---|---|---|
| ATCC: American Type Culture Collection; CV: crystal violet; MBEC: minimum biofilm eradication concentration; MBIC: minimal biofilm inhibitory concentration; MIC: minimum inhibitory concentration; NCTC: National Collection of Type Cultures; PBS: phosphat-buffered saline; UCBPP: University of California Berkeley Plant Pathology. | |||||||
| 1 | Camplin and Maddocks, 2014 [10] | ATCC 9027, clinical isolate 867 | Medihoney | 0-80% w/v | Stained with 0.25% w/v CV for 10 min. Two washes with PBS. Destaining with 7% acetic acid. | MIC, MBEC, resistance development | MIC: 15.3% to 25.6%. MBEC: 43.3% and 48.3% |
| 2 | Cooper et al, 2014 [27] | Clinical isolate | Medihoney | 5-50% w/v | Stained with 0.25% w/v CV for 15 min. Three washes with PBS. Destaining with 7% acetic acid. | MIC50, MIC90, MBEC, biofilm structure | MIC50: 16.8%, MBEC: 35.3%; ≥ 40% honey significantly alter biofilm structure and viability |
| 3 | Halstead et al, 2016 [28] | ATCC 15692, NCTC 6749, clinical isolate | Surgihoney RO, Medihoney, Manuka honey | 1:3 to 1:6,144 dilution | Stained with 1% w/v CV for 10 min. Two washes with PBS. Destaining with 70% ethanol. | MBIC, biofilm biomass | Honey dilution of 1:3 to 1:12 showed good results against P. aeruginosa biofilm (P < 0.05) |
| 4 | Lu et al, 2019 [13] | ATCC 15692, UCBPP-PA14 | Manuka, Medihoney, Clover honey | 1-80% w/v | Stained with 0.2% w/v CV for 60 min. Three washes with PBS. Destaining with 33% w/v acetic acid. | MIC, MBIC, biofilm biomass, viability | MBIC 16-32%; UCBPP-PA14 more susceptible than ATCC 15692; 64-80% honey eradicated biofilms |
| 5 | Maddocks et al, 2013 [29] | ATCC 9027, clinical isolate 867 | Medihoney | 0-60% w/v | Stained with 0.25% w/v CV for 10 min. Two washes with PBS. Destaining with 7% acetic acid. | Biofilm biomass, adhesion, invasion | Honey can reduce the amount of biofilm biomass to 33% (867) and 43% (ATCC 9027) |
| 6 | Morroni et al, 2018 [30] | Clinical isolate | Manuka honey, Kenya honey, Cuba honey | 2-22% v/v | Stained with 0.25% w/v CV for 10 min. Two washes with PBS. Destaining with 70% ethanol. | Antimicrobial activity, biofilm biomass, | Manuka honey minimal dilution for antimicrobial: 14%. Significant biomass reduction at ≥ 8% |
| No. | Criteria | Microbiological relevance | Camplin and Maddock, 2014 [10] | Cooper et al, 2014 [23] | Halstead et al, 2016 [24] | Lu et al, 2019 [27] | Maddocks et al, 2013 [25] | Morroni et al, 2018. [26] |
|---|---|---|---|---|---|---|---|---|
| ANOVA: analysis of variance; OD: optical density; QUIN: Quality Assessment Tool for In Vitro Studies. | ||||||||
| 1 | Clearly stated aims/objectives | The objectives are clearly stated. The specific strain of P. aeruginosa and type of medical-grade honey is defined. | 2 | 2 | 2 | 2 | 2 | 2 |
| 2 | Detailed explanation of sample size calculation | A sufficient number of replications had been performed for valid results. | 1 | 1 | 1 | 1 | 1 | 1 |
| 3 | Detailed explanation of sampling technique | The studies describe how the biofilm was harvested, how it was diluted, how the samples were processed to accurately measure the outcome. | 1 | 1 | 1 | 1 | 1 | 1 |
| 4 | Details of comparison group | The studies had defined the negative control and any positive controls. | 2 | 2 | 2 | 2 | 2 | 2 |
| 5 | Detailed explanation of methodology | The methodology of studies includes specific microbiological details: bacterial strain, culture conditions, the method used to grow the biofilm, the concentration and form of honey used, the exposure time. | 2 | 2 | 2 | 2 | 2 | 2 |
| 6 | Operator details | Operator details must meet standardized protocols. However, it is less critical for automated OD measurements. | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | Randomization | The studies did not provide details regarding sequence generation and allocation concealment. | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | Method of measurement of outcome | The method to measure outcome of biofilm biomass is crystal violet assay for optical density measurement. | 2 | 2 | 2 | 2 | 2 | 2 |
| 9 | Outcome assessor details | The studies did not state number of outcome assessors and details regarding training and calibration of assessor(s). | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | Blinding | No details regarding blinding of operator(s), outcome assessor(s), and statistician. | 0 | 0 | 0 | 0 | 0 | 0 |
| 11 | Statistical analysis | The studies use valid statistical tests (e.g., t-test, ANOVA). | 2 | 0 | 1 | 2 | 1 | 2 |
| 12 | Presentation of results | The studies provide summary statistics and visual representations. | 2 | 2 | 2 | 2 | 2 | 2 |