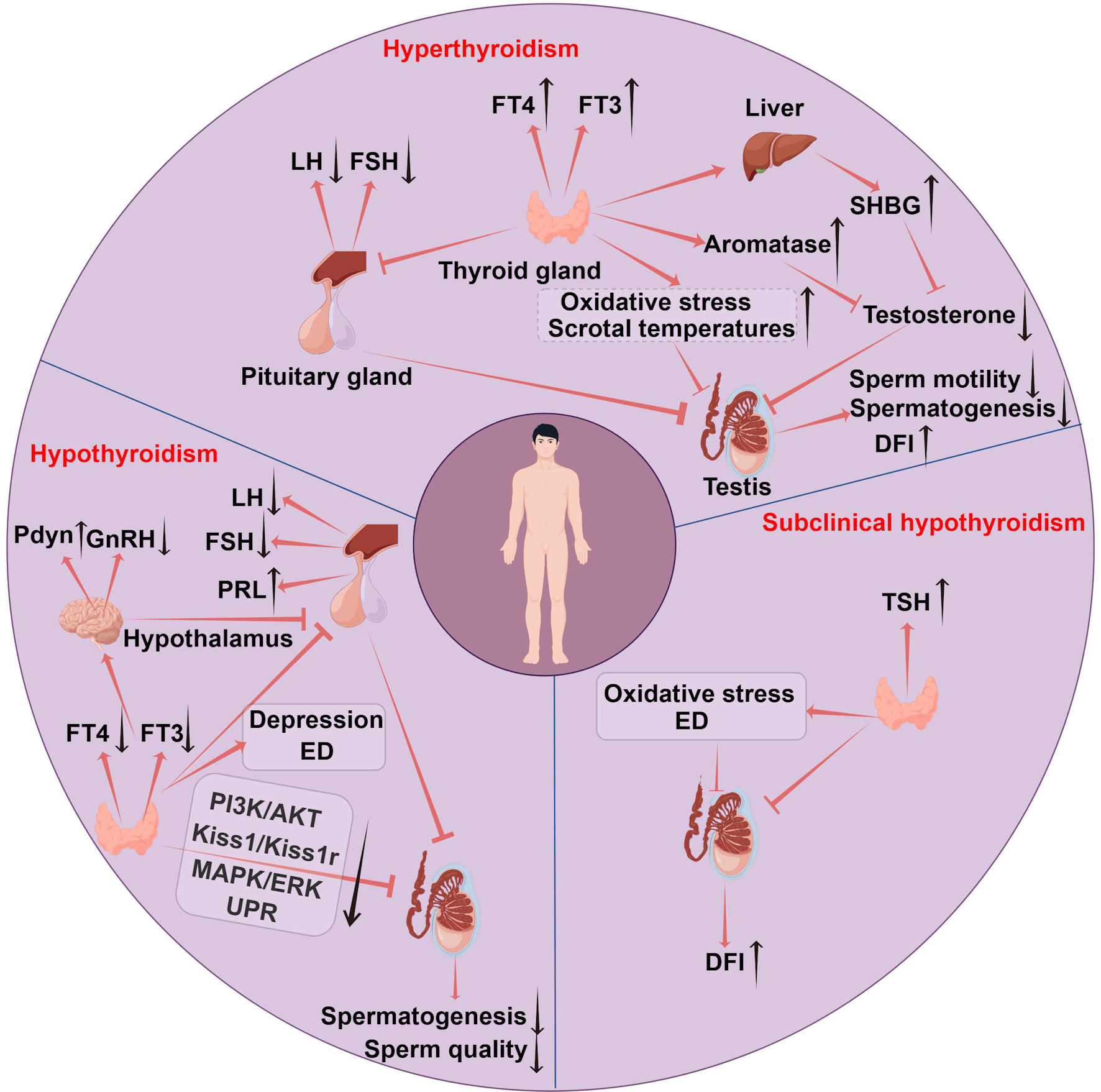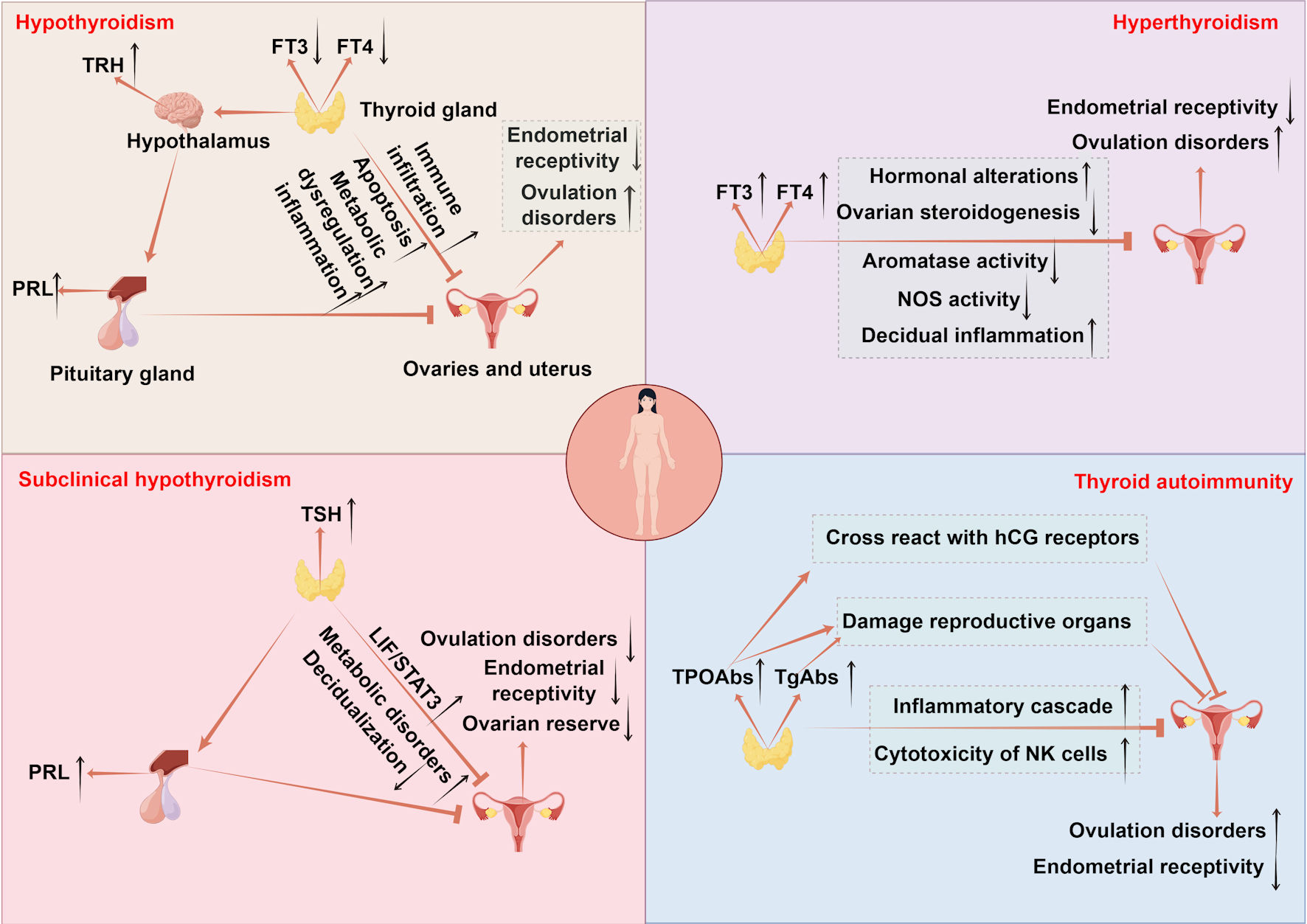
Figure 1. The mechanisms of thyroid disorders affecting female fertility. Hypothyroidism disrupts follicular development via CHOP and caspase3-mediated apoptosis and causes metabolic imbalance in lipids and glucose accompanied by immune infiltration, impairing ovarian and uterine function. Endometrial receptivity is diminished due to low E2 osteopontin and HOXA10, while uterine gland function is impaired with reduced integrins and LIF and increased MUC1. Uterine hyperplasia and inflammation occur due to elevated VEGF-A, alongside decreased PLINA, TAG and TC. Hyperprolactinemia from excessive TRH and menstrual irregularities further reduce fertility. Hyperthyroidism leads to infertility by elevating SHBG and estradiol which disrupts folliculogenesis through impaired antral follicle growth and dysregulated NOS expression. It also suppresses FSH stimulated aromatase activity in ovarian steroidogenesis and causes decidual imbalance with elevated DBA-positive uNK cells and abnormal cytokine production. SCH contributes to infertility by promoting metabolic dysregulation in lipids and glucose and hyperprolactinemia reducing ovarian reserve and upregulating LIF/STAT3 signaling, which collectively impair endometrial receptivity and decidualization. TAI drives follicular inflammation through IFNγ-induced CXCL9/10/11-mediated recruitment of CXCR3+ T cells and enhances NK cell cytotoxicity. Non organ specific antibodies cross react with trophoblasts and disrupt Th1/Th2 balance promoting implantation failure and pregnancy loss. TPOAbs cross reactivity with hCG receptors on the zona pellucida, direct damage by TPOAb and TgAb to reproductive tissues, and reduced ovarian reserve and embryo quality further contribute to reproductive impairment. TSH: thyroid-stimulating hormone; FT4: free thyroxine; FT3: free triiodothyronine; CHOP: C/EBP homologous protein; TAG: triacylglycerol; HOXA10: homeobox A10; LIF: leukemia inhibitory factor; MUC1: mucin 1; VEGF-A: vascular endothelial growth factor A; PLIN-A: perilipin A; DBA: dolichos biflorus agglutinin; uNK: uterine natural killer; IFNγ: interferon-γ; CXCL9/10/11: CXC chemokine ligands 9; CXCR3: CXC chemokine receptor 3; TRH: thyrotropin-releasing hormone; FSH: follicle-stimulating hormone; SHBG: sex hormone-binding globulin; NOS: nitric oxide synthase; PRL: prolactin; TPOAbs: thyroid peroxidase antibodies; TgAbs: thyroglobulin antibodies; hCG: human chorionic gonadotropin; SCH: subclinical hypothyroidism; TAI: thyroid autoimmunity.