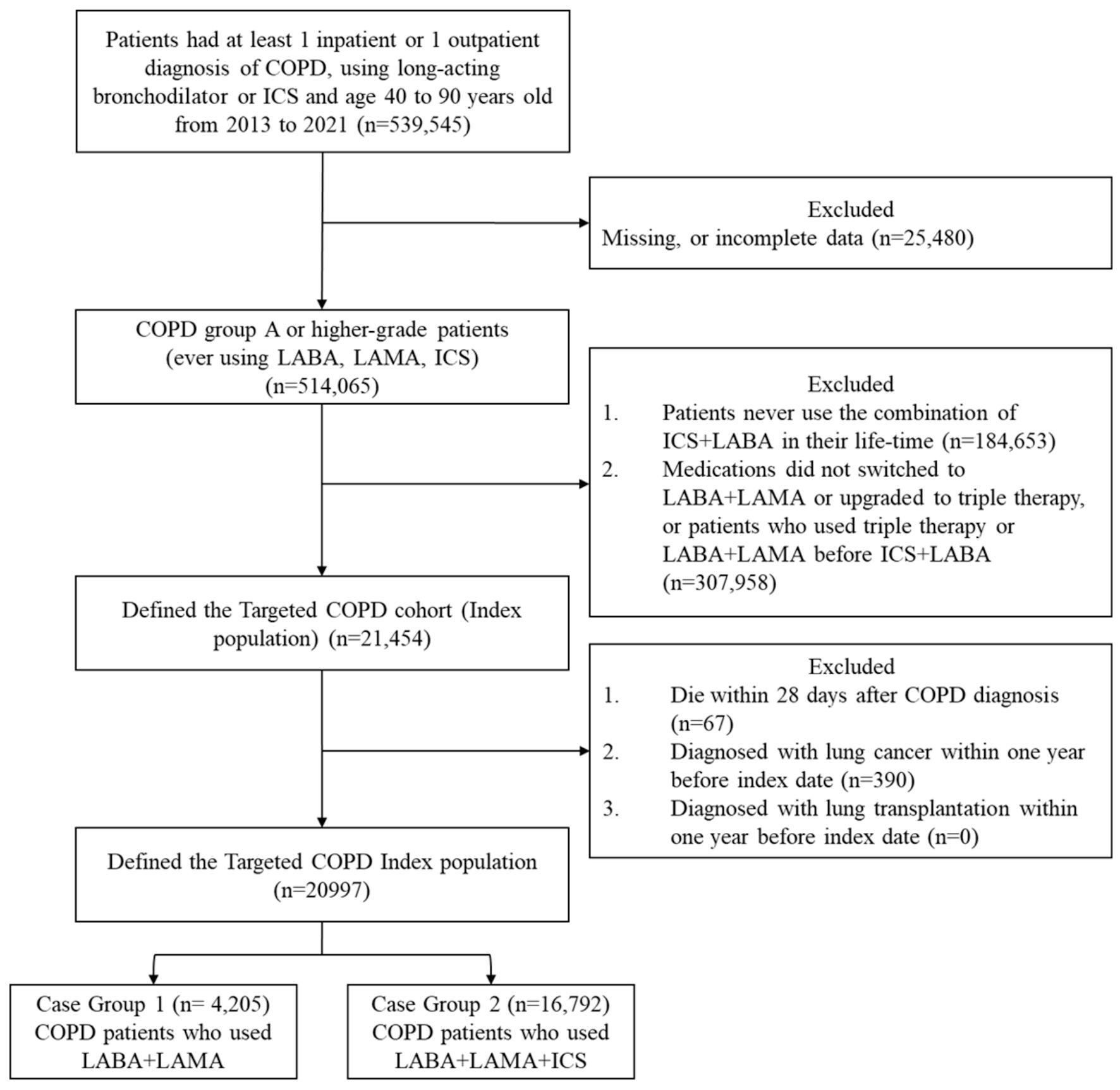
Figure 1. Study flowchart for the composite outcome. COPD: chronic obstructive pulmonary disease; LABA: long-acting beta2-agonist; LAMA: long-acting muscarinic antagonist; ICSs: inhaled corticosteroids.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 9, October 2025, pages 507-517
Evaluating the Effectiveness of Triple Therapy in Chronic Obstructive Pulmonary Disease Patients: An Asian Population-Based Survey
Figure

Tables
| Variables | Triple therapy (n = 16,792) | LABA + LAMA (n = 4,205) | Total (n = 20,997) | P value |
|---|---|---|---|---|
| ACEI: angiotensin-converting enzyme inhibitor; ACOs: asthma-COPD overlap syndrome; ARB: angiotensin II receptor blockers; BZD: benzodiazepine; CCB: calcium channel blocker; GERD: gastroesophageal reflux disease; LABA: long-acting beta2-agonists; LAMA: long-acting muscarinic antagonists; NSAID: non-steroid anti-inflammatory drug; PPI: proton-pump inhibitor; SABA: short-acting beta-agonists; SAMA: short-acting muscarinic antagonist; SCC: systematic corticosteroid. | ||||
| Age, years old, mean (SD) | 67.19 (10.72) | 65.77 (11.73) | 66.06 (11.54) | < 0.0001 |
| Age group | < 0.0001 | |||
| < 65yeras old | 7,905 (47.08) | 1,764 (41.95) | 9,669 (46.05) | |
| ≥ 65 years old | 8,887 (52.92) | 2,441 (58.05) | 11,328 (53.95) | |
| Gender, n (%) | < 0.0001 | |||
| Male | 9,877 (58.79) | 3,100 (73.70) | 12,977 (61.80) | |
| Female | 6,915 (41.21) | 1,105 (26.30) | 8,020 (38.20) | |
| Geographic area, n (%) | 0.4935 | |||
| North | 9,246 (55.06) | 2,129 (50.63) | 11,375 (54.17) | |
| Middle | 2,656 (15.82) | 741 (17.62) | 3,397 (16.18) | |
| South | 4,513 (26.88) | 1,184 (28.16) | 5,697 (27.13) | |
| East | 377 (2.24) | 151 (3.59) | 528 (2.52) | |
| COPD exacerbation (moderate or severe) | 0.7758 | |||
| 0 | 13,213 (78.70) | 3,325 (79.12) | 16,538 (78.78) | |
| 1 | 1,625 (9.74) | 392 (9.28) | 2,017 (9.61) | |
| ≥ 2 | 1,954 (11.56) | 488 (11.60) | 24,42 (11.61) | |
| Disease duration | 207.8 (277.3) | 183.9 (266) | 203.1 (275.3) | < 0.0001 |
| Urbanization, n (%) | 0.023 | |||
| Urban | 9,583 (57.07) | 2,303 (54.77) | 11,886 (56.60) | |
| Suburban | 5,257 (31.31) | 1,348 (32.06) | 6,605 (31.46) | |
| Rural | 1,870 (11.13) | 527 (12.53) | 2,397 (11.42) | |
| Unknown | 82 (0.49) | 27 (0.64) | 109 (0.52) | |
| Insurance premium, n (%) | 0.4935 | |||
| Less than minimum monthly wage | 4,161 (24.77) | 1,064 (25.30) | 5,225 (24.88) | |
| More than minimum monthly wage | 12,631 (75.23) | 3,141 (74.70) | 15,772 (75.12) | |
| Comorbidity, n (%) | ||||
| Hypertension | 5,348 (31.80) | 1,504 (35.80) | 6,852 (32.63) | < 0.0001 |
| Atrial fibrillation | 925 (5.50) | 242 (5.80) | 1,167 (5.56) | 0.5327 |
| Ischemic stroke | 884 (5.26) | 243 (5.78) | 1,127 (5.37) | 0.1856 |
| Hemorrhagic stroke | 93 (0.55) | 28 (0.67) | 121 (0.58) | 0.3907 |
| Heart failure | 1,045 (6.22) | 297 (7.06) | 1,342 (6.39) | 0.0465 |
| Dyslipidemia | 2,730 (16.25) | 743 (17.67) | 3,473 (16.54) | 0.0276 |
| Cardiac arrhythmia | 1,144 (6.81) | 334 (7.84) | 1,478 (7.04) | 0.0104 |
| Peripheral vascular disease | 121 (0.72) | 31 (0.74) | 152 (0.72) | 0.9094 |
| Rheumatic heart disease | 143 (0.85) | 35 (0.83) | 178 (0.85) | 0.9031 |
| ACOs | 2,248 (13.39) | 267 (6.35) | 2,515(11.98) | < 0.0001 |
| Pneumonia | 2,237 (13.32) | 645 (15.34) | 2,882 (13.73) | 0.0007 |
| Acute bronchitis | 2,647 (15.76) | 689 (16.4) | 3,336 (15.89) | 0.324 |
| Influenza | 335 (1.99) | 89 (2.12) | 424 (2.02) | 0.6163 |
| Dementia | 371 (2.21) | 100 (2.38) | 471 (2.24) | 0.5088 |
| Depression | 605 (3.60) | 123 (2.93) | 728 (4.37) | 0.0317 |
| Parkinsonism | 241 (1.44) | 50 (1.19) | 291 (1.39) | 0.2221 |
| Diabetes mellitus | 2,394 (14.26) | 674 (16.03) | 3,068 (14.61) | 0.0036 |
| Chronic kidney disease | 651 (3.88) | 180 (4.28) | 831 (3.96) | 0.2298 |
| Chronic liver disease | 625 (3.72) | 167 (3.97) | 792 (3.77) | 0.4477 |
| Coronary artery disease | 2,040 (12.15) | 612 (14.55) | 2,652 (12.63) | < 0.0001 |
| GERD | 2,222 (13.23) | 434 (10.32) | 2,656 (12.65) | < 0.0001 |
| Malignancy | 2,021 (12.04) | 525 (12.49) | 2,546 (12.13) | 0.4244 |
| Sepsis | 371 (2.21) | 105 (2.50) | 476 (2.27) | 0.2624 |
| Co-medication, n (%) | ||||
| Antiplatelet agents | 262 (1.56) | 78 (1.85) | 340 (1.62) | 0.1758 |
| Anticoagulants | 4,900 (29.18) | 1,128 (26.83) | 6,028 (28.71) | 0.0025 |
| Lipid-lowering drugs | 219 (1.30) | 70 (1.66) | 289 (1.38) | 0.0728 |
| PPI | 729 (4.34) | 152 (3.61) | 881 (4.20) | 0.0356 |
| H2-blocker | 220 (1.31) | 53 (1.26) | 273 (1.30) | 0.799 |
| BZD drugs or Z-drugs | 133 (0.79) | 45 (1.07) | 178 (0.85) | 0.0786 |
| Antipsychotics | 635 (3.78) | 143 (3.40) | 778 (3.71) | 0.2423 |
| Opioids | 1,629 (9.70) | 377 (8.97) | 2,006 (9.55) | 0.1468 |
| Systematic corticosteroids | 395 (2.35) | 95 (2.26) | 490 (2.33) | 0.7206 |
| NSAID | 206 (1.23) | 44 (1.05) | 250 (1.19) | 0.3348 |
| CCB | 267 (1.59) | 92 (2.19) | 359 (1.71) | 0.0075 |
| Diuretics | 621 (3.70) | 162 (3.85) | 783 (3.73) | 0.6366 |
| ACEI | 2,798 (16.66) | 667 (15.86) | 3,465 (16.50) | 0.211 |
| ARB | 352 (2.09) | 110 (2.62) | 20,535 (97.8) | 0.0399 |
| Beta-blocker | 408 (2.43) | 80 (1.90) | 488 (2.32) | 0.0086 |
| Digoxin | 25 (0.15) | 7 (0.17) | 32 (0.15) | 0.7937 |
| Antiarrhythmics | 3,123 (18.60) | 758 (18.03) | 3,881 (18.48) | 0.3928 |
| Nitrates | 1,567 (9.23) | 368 (8.75) | 1,935 (9.22) | 0.2353 |
| Influenza vaccine | 1,635 (9.74) | 465 (11.06) | 2,100 (10.00) | 0.0106 |
| Pneumococcal vaccine | 3,196 (19.03) | 882 (20.98) | 4,078 (19.42) | 0.0044 |
| Observation period (days) | Event number | Crude HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|---|---|
| COPD: chronic obstructive pulmonary disease; LABA: long-acting beta2-agonist; LAMA: long-acting muscarinic antagonist; HR: hazard ratio; CI: confidence interval. | ||||
| Primary outcomes | ||||
| Primary outcome 1 | ||||
| LABA + LAMA | 2,490,676 | 1,534 | 1 (reference) | |
| Triple therapy | 7,355,285 | 5,105 | 1.081 (1.020 - 1.145) | 1.162 (1.098 - 1.230) |
| Primary outcome 2 | ||||
| LABA + LAMA | 2,490,676 | 1,475 | 1 (reference) | |
| Triple therapy | 7,355,285 | 4,982 | 1.097 (1.034 - 1.164) | 1.171 (1.105 - 1.241) |
| Secondary outcomes | ||||
| All-cause mortality | ||||
| LABA + LAMA | 3,676,382 | 779 | 1 (reference) | |
| Triple therapy | 10,547,405 | 1,902 | 0.842 (0.779 - 0.910) | 1.074 (0.994 - 1.161) |
| Respiratory-related mortality | ||||
| LABA + LAMA | 3,676,405 | 524 | 1 (reference) | |
| Triple therapy | 10,547,405 | 1,292 | 0.846 (0.770 - 0.930) | 1.089 (0.991 - 1.196) |
| Moderate COPD exacerbation | ||||
| LABA + LAMA | 3,146,834 | 494 | 1 (reference) | |
| Triple therapy | 9,247,962 | 1,688 | 1.033 (0.934 - 1.143) | 1.027 (0.931 - 1.134) |
| Sever COPD exacerbation | ||||
| LABA + LAMA | 3,572,160 | 88 | 1 (reference) | |
| Triple therapy | 10,221,161 | 445 | 1.335 (1.059 - 1.683) | 1.346 (1.078 - 1.682) |
| Acute respiratory failure | ||||
| LABA + LAMA | 3,628,098 | 95 | 1 (reference) | |
| Triple therapy | 10,425,265 | 323 | 1.132 (1.061 - 1.207) | 1.315 (1.047 - 1.653) |
| Pneumonia | ||||
| LABA + LAMA | 3,325,536 | 539 | 1 (reference) | |
| Triple therapy | 9,563,094 | 1,715 | 1.149 (1.043 - 1.266) | 1.221 (1.109 - 1.344) |
| Respiratory-related admission | ||||
| LABA + LAMA | 2,748,346 | 614 | 1 (reference) | |
| Triple therapy | 7,986,776 | 2,163 | 1.189 (1.087 - 1.301) | 1.264 (1.157 - 1.382) |
| Observation period (days) | Event number | Crude HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|---|---|
| The multivariable models were adjusted for baseline characteristics, comorbidities, and concomitant medications. COPD: chronic obstructive pulmonary disease; LABA: long-acting beta2-agonist; LAMA: long-acting muscarinic antagonist; HR: hazard ratio; CI: confidence interval. | ||||
| Primary outcomes | ||||
| Primary outcome 1 | ||||
| LABA + LAMA | 2,490,676 | 1,534 | 1 (reference) | |
| Triple therapy | 7,355,285 | 5,105 | 0.978 (0.930 - 1.030) | 1.004 (0.954 - 1.056) |
| Primary outcome 2 | ||||
| LABA + LAMA | 2,490,676 | 1,475 | 1 (reference) | |
| Triple therapy | 7,355,285 | 4,982 | 0.988 (0.938 - 1.041) | 1.013 (0.961 - 1.067) |
| Secondary outcomes | ||||
| All-cause mortality | ||||
| LABA + LAMA | 3,676,382 | 779 | 1 (reference) | |
| Triple therapy | 10,547,405 | 1,902 | 0.989 (0.916 - 1.068) | 1.110 (1.011 - 1.219) |
| Respiratory-related mortality | ||||
| LABA + LAMA | 3,676,405 | 524 | 1 (reference) | |
| Triple therapy | 10,547,405 | 1,292 | 1.032 (0.936 - 1.137) | 1.179 (1.046 - 1.329) |
| Moderate COPD exacerbation | ||||
| LABA + LAMA | 3,146,834 | 494 | 1 (reference) | |
| Triple therapy | 9,247,962 | 1,688 | 1.159 (1.058 - 1.271) | 1.134 (1.034 - 1.244) |
| Sever COPD exacerbation | ||||
| LABA + LAMA | 3,572,160 | 88 | 1 (reference) | |
| Triple therapy | 10,221,161 | 445 | 0.333 (0.300 - 0.371) | 0.323 (0.291 - 0.361) |
| Acute respiratory failure | ||||
| LABA + LAMA | 3,628,098 | 95 | 1 (reference) | |
| Triple therapy | 10,425,265 | 323 | 1.313 (1.041 - 1.658) | 1.406 (1.112 - 1.780) |
| Pneumonia | ||||
| LABA + LAMA | 3,325,536 | 539 | 1 (reference) | |
| Triple therapy | 9,563,094 | 1,715 | 1.007 (0.919 - 1.105) | 1.040 (0.948 - 1.141) |
| Respiratory-related admission | ||||
| LABA + LAMA | 2,748,346 | 614 | 1 (reference) | |
| Triple therapy | 7,986,776 | 2,163 | 1.168 (1.072 - 1.273) | 1.200 (1.101 - 1.308) |
| Observation period (days) | Event number | Crude HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|---|---|
| The multivariable models were adjusted for baseline characteristics, comorbidities, and concomitant medications. COPD: chronic obstructive pulmonary disease; LABA: long-acting beta2-agonist; LAMA: long-acting muscarinic antagonist; HR: hazard ratio; CI: confidence interval. | ||||
| Primary outcomes | ||||
| Primary outcome 1 | ||||
| LABA + LAMA | 2,442,682 | 1,490 | 1 (reference) | |
| Triple therapy | 6,333,410 | 4,473 | 1.117 (1.053 - 1.185) | 1.174 (1.107 - 1.246) |
| Primary outcome 2 | ||||
| LABA + LAMA | 2,442,682 | 1,434 | 1 (reference) | |
| Triple therapy | 6,333,410 | 4,359 | 1.130 (1.064 - 1.200) | 1.187 (1.117 - 1.261) |
| Secondary outcomes | ||||
| All-cause mortality | ||||
| LABA + LAMA | 3,616,963 | 868 | 1 (reference) | |
| Triple therapy | 9,158,924 | 1,988 | 0.905 (0.835 - 0.980) | 1.017 (0.938 - 1.102) |
| Respiratory-related mortality | ||||
| LABA + LAMA | 3,616,986 | 590 | 1 (reference) | |
| Triple therapy | 9,158,924 | 1,352 | 0.902 (0.819 - 0.994) | 1.027 (0.932 - 1.132) |
| Moderate COPD exacerbation | ||||
| LABA + LAMA | 3,068,886 | 491 | 1 (reference) | |
| Triple therapy | 7,999,751 | 1,493 | 1.031 (0.930 - 1.143) | 1.064 (0.959 - 1.180) |
| Sever COPD exacerbation | ||||
| LABA + LAMA | 3,521,047 | 89 | 1 (reference) | |
| Triple therapy | 8,863,890 | 387 | 1.312 (1.039 - 1.657) | 1.370 (1.084 - 1.732) |
| Acute respiratory failure | ||||
| LABA + LAMA | 3,201,942 | 81 | 1 (reference) | |
| Triple therapy | 8,157,867 | 259 | 1.227 (0.955 - 1.577) | 1.375 (1.066 - 1.772) |
| Pneumonia | ||||
| LABA + LAMA | 3,303,350 | 492 | 1 (reference) | |
| Triple therapy | 8,449,309 | 1,283 | 1.064 (0.959 - 1.181) | 1.162 (1.046 - 1.291) |
| Respiratory-related admission | ||||
| LABA + LAMA | 3,201,942 | 569 | 1 (reference) | |
| Triple therapy | 8,157,867 | 1,681 | 1.122 (1.020 - 1.235) | 1.216 (1.104 - 1.338) |