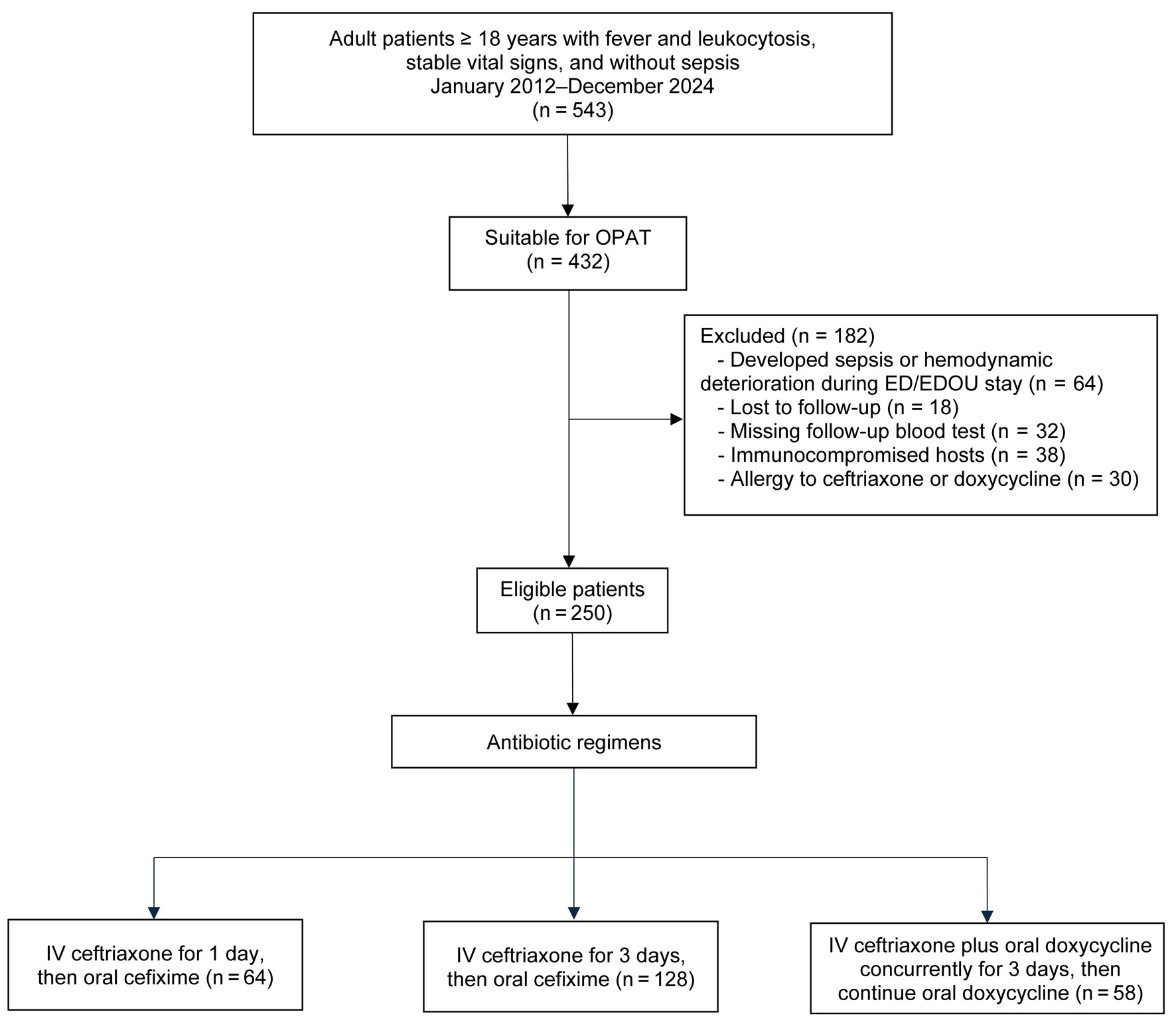
Figure 1. Study flow diagram.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 9, October 2025, pages 499-506
Effectiveness of Antibiotic Regimens in Reducing White Blood Cell Count Within Three to Five Days in Febrile Leukocytosis Treated With Ambulatory Therapy
Figures

Tables
| Baseline characteristics | Missing | Regimen A (n = 64), n (%) | Regimen B (n = 128), n (%) | Regimen C (n = 58), n (%) | P value |
|---|---|---|---|---|---|
| Regimen A: IV ceftriaxone for 1 day, then oral cefixime; Regimen B: IV ceftriaxone for 3 days, then oral cefixime; Regimen C: IV ceftriaxone plus oral doxycycline concurrently for 3 days, then continue oral doxycycline. IQR: interquartile range; PMN: polymorphonuclear neutrophils; SD: standard deviation; Tx: treatment; WBC: white blood cell. | |||||
| Female | 0 | 47 (73.4) | 80 (62.5) | 31 (53.4) | 0.075 |
| Age (years), mean ± SD | 0 | 44.5 ± 21.4 | 52.3 ± 21.4 | 48.8 ± 21.1 | 0.057 |
| Body mass index (kg/m2), mean ± SD | 0 | 23.1 ± 4.5 | 23.2 ± 5.1 | 23.5 ± 6.0 | 0.909 |
| Temperature (°C), mean ± SD | 0 | 38.5 ± 0.6 | 38.5 ± 0.5 | 38.6 ± 0.6 | 0.793 |
| Symptoms | |||||
| Respiratory | 0 | 6 (9.4) | 10 (7.8) | 2 (3.5) | 0.692 |
| Gastrointestinal | 0 | 8 (12.5) | 13 (10.2) | 10 (17.2) | |
| Genitourinary | 0 | 6 (9.4) | 14 (10.9) | 4 (6.9) | |
| Non-specific | 0 | 44 (68.7) | 91 (71.1) | 42 (72.4) | |
| Comorbidity | |||||
| Diabetes Mellitus | 0 | 15 (23.4) | 30 (23.4) | 19 (32.8) | 0.379 |
| Hypertension | 0 | 16 (25.0) | 43 (33.6) | 15 (25.9) | 0.378 |
| Dyslipidemia | 0 | 10 (15.6) | 21 (16.4) | 13 (22.4) | 0.564 |
| Chronic kidney disease | 0 | 3 (4.7) | 12 (9.4) | 9 (15.5) | 0.126 |
| Pre-Tx WBC (× 103/µL), median (IQR) | 0 | 15.4 (13.5 - 17.5) | 15.8 (13.6 - 18.1) | 15.4 (13.7 - 18.1) | 0.664 |
| Pre-Tx PMN (%), mean ± SD | 0 | 82.0 ± 10.1 | 82.7 ± 9.5 | 80.9 ± 9.6 | 0.361 |
| Time to follow-up (h), mean ± SD | 0 | 73.0 ± 12.2 | 71.9 ± 11.4 | 70.6 ± 10.6 | 0.562 |
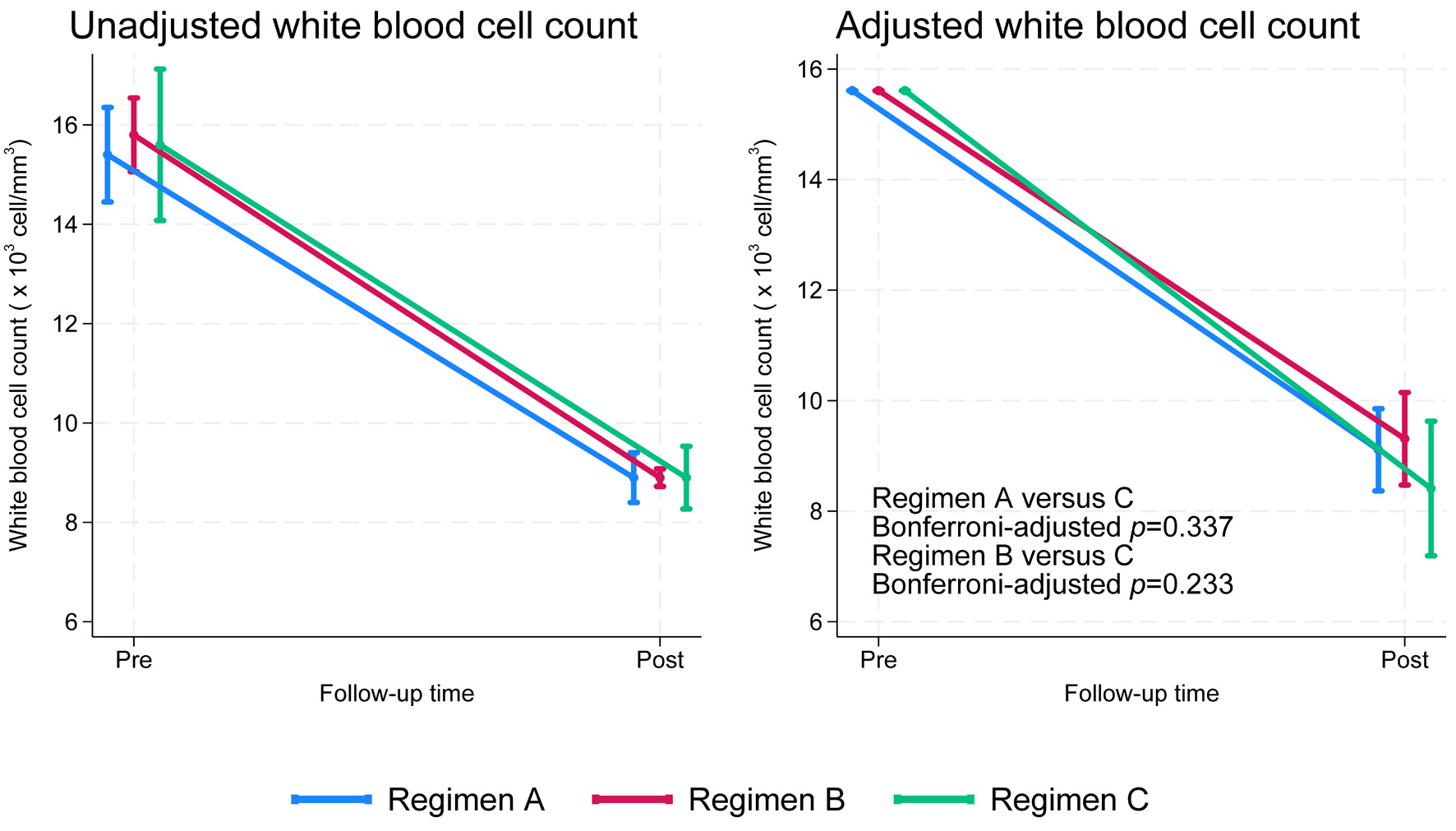
| Regimens | Pre-treatment WBC (× 103 cells/mm3), median (p25, p75) | P value | Post-treatment WBC (× 103 cells/mm3), median (p25, p75) | P value |
|---|---|---|---|---|
| Values are presented as median (25th percentile, 75th percentile). WBC: white blood cell count; Regimen A: IV ceftriaxone for 1 day, then oral cefixime; Regimen B: IV ceftriaxone for 3 days, then oral cefixime; Regimen C: IV ceftriaxone plus oral doxycycline concurrently for 3 days, then continue oral doxycycline; IV: intravenous. | ||||
| Regimen A | 15.4 (13.5, 17.5) | 0.664 | 8.7 (7.2, 9.6) | 0.612 |
| Regimen B | 15.8 (13.6, 18.1) | 8.9 (7.9, 9.6) | ||
| Regimen C | 15.4 (13.7, 18.1) | 8.9 (6.9, 9.4) | ||
| Regimens | WBC reduction (× 103 cells/mm3) | 95% confidence interval | P valuea |
|---|---|---|---|
| aOverall P value derived from a Wald test. WBC: white blood cell count; Regimen A: IV ceftriaxone for 1 day, then oral cefixime; Regimen B: IV ceftriaxone for 3 days, then oral cefixime; Regimen C: IV ceftriaxone plus oral doxycycline concurrently for 3 days, then continue oral doxycycline; IV: intravenous. | |||
| Regimen A | -6.6 | -7.0 to -6.2 | 0.484 |
| Regimen B | -6.8 | -7.2 to -6.5 | |
| Regimen C | -6.9 | -7.5 to -6.3 | |
| Regimens | A | B | C |
|---|---|---|---|
| Regimen A: IV ceftriaxone for 1 day, then oral cefixime; Regimen B: IV ceftriaxone for 3 days, then oral cefixime; Regimen C: IV ceftriaxone plus oral doxycycline concurrently for 3 days, then continue oral doxycycline; IV: intravenous. | |||
| A | - | - | - |
| B | 0.727 | - | |
| C | 0.337 | 0.233 | - |
| Regimens | A (n = 64), n (%) | B (n = 128), n (%) | C (n = 58), n (%) | P value |
|---|---|---|---|---|
| Regimen A: IV ceftriaxone for 1 day, then oral cefixime; Regimen B: IV ceftriaxone for 3 days, then oral cefixime; Regimen C: IV ceftriaxone plus oral doxycycline concurrently for 3 days, then continue oral doxycycline; IV: intravenous. | ||||
| Improved | 51 (79.7) | 110 (85.9) | 52 (89.7) | 0.283 |
| Continued or switched IV antibiotics | 13 (20.3) | 18 (14.1) | 6 (10.3) | |
| Admitted | 0 (0) | 0 (0) | 0 (0) | |