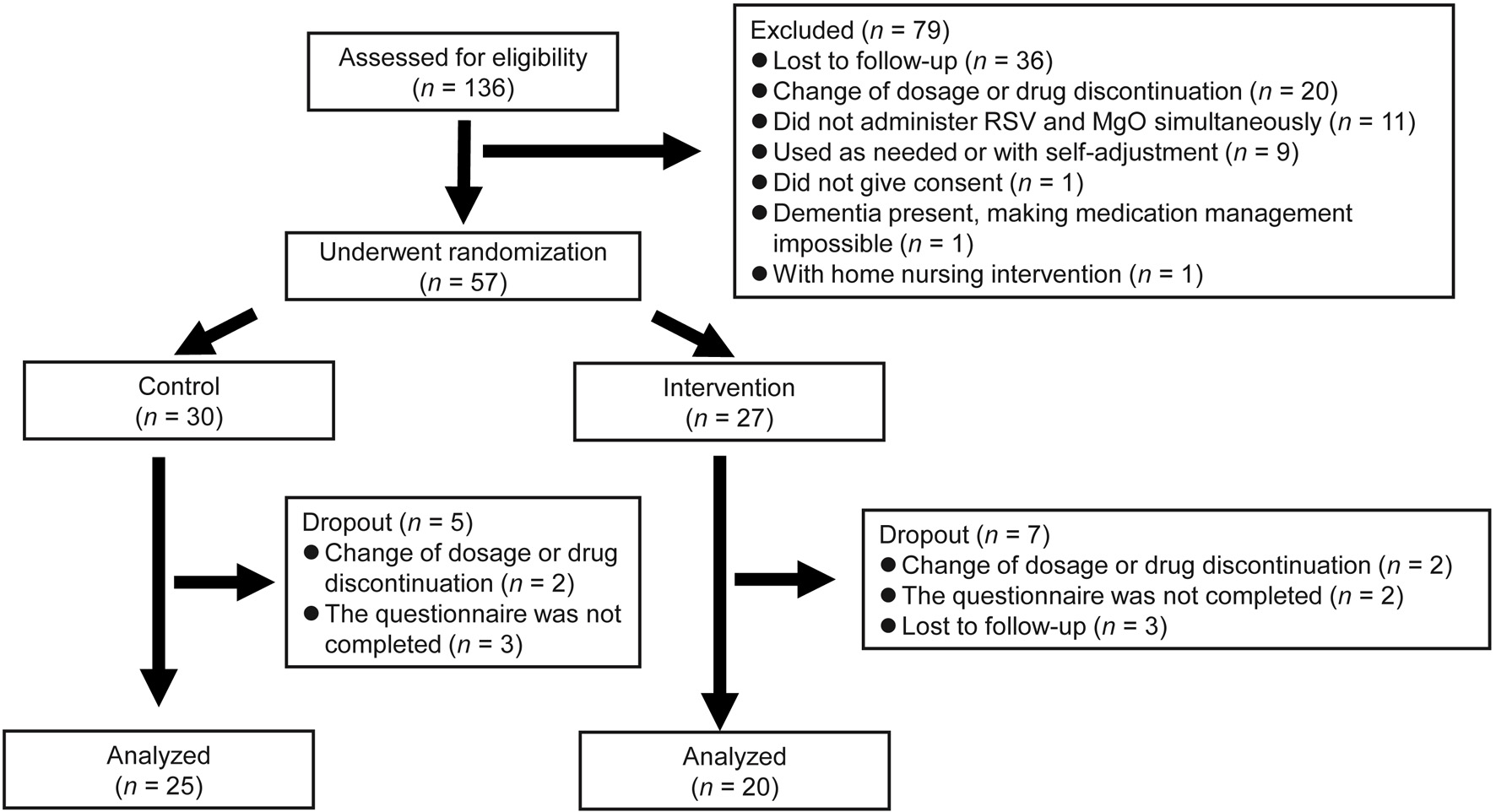
Figure 1. Study flowchart.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 10, October 2025, pages 550-555
Clinical Effects of the Concurrent Ingestion of Rosuvastatin and Magnesium Oxide: A Multicenter, Randomized, Parallel-Group Trial
Figures

Table
| Control (n = 25) | Intervention (n = 20) | P value | |
|---|---|---|---|
| Missing data: an = 1, bn = 9, cn = 4, dn = 1, en = 2, fn = 1, gn = 2. BMI: body mass index; HDL-C: high-density lipoprotein cholesterol; IQR: interquartile range; LDL-C: low-density lipoprotein cholesterol; MgO: magnesium oxide; RSV: rosuvastatin; T-Cho: total cholesterol; TG: triglycerides. | |||
| Sex | 1.0000 | ||
| Male, n (%) | 18 (72.0) | 14 (70.0) | |
| Female, n (%) | 7 (28.0) | 6 (30.0) | |
| Age (years), median (IQR) | 78 (69.0 - 82.0) | 76 (74.3 - 82.5) | 0.7058 |
| BMI (kg/m2), median (IQR) | 23.7 (22.1 - 26.4)a | 23.9 (21.2 - 27.4) | 1.0000 |
| Medication possession ratio (%), median (IQR) | 100 (97.70 - 100) | 100 (99.25 - 100) | 0.8949 |
| RSV dose | 0.7482 | ||
| 2.5 mg, n (%) | 15 (60.0) | 11 (55.0) | |
| 5 mg, n (%) | 10 (40.0) | 8 (40.0) | |
| 10 mg, n (%) | 0 (0) | 1 (5.0) | |
| MgO dose | 0.4199 | ||
| 330 mg, n (%) | 18 (72.0) | 15 (75.0) | |
| 500 mg, n (%) | 5 (20.0) | 2 (10.0) | |
| 660 mg, n (%) | 1 (4.0) | 3 (15.0) | |
| 1,000 mg, n (%) | 1 (4.0) | 0 (0) | |
| Administration timing | 0.3792 | ||
| After breakfast, n (%) | 16 (64.0) | 10 (50.0) | |
| After dinner, n (%) | 9 (36.0) | 10 (50.0) | |
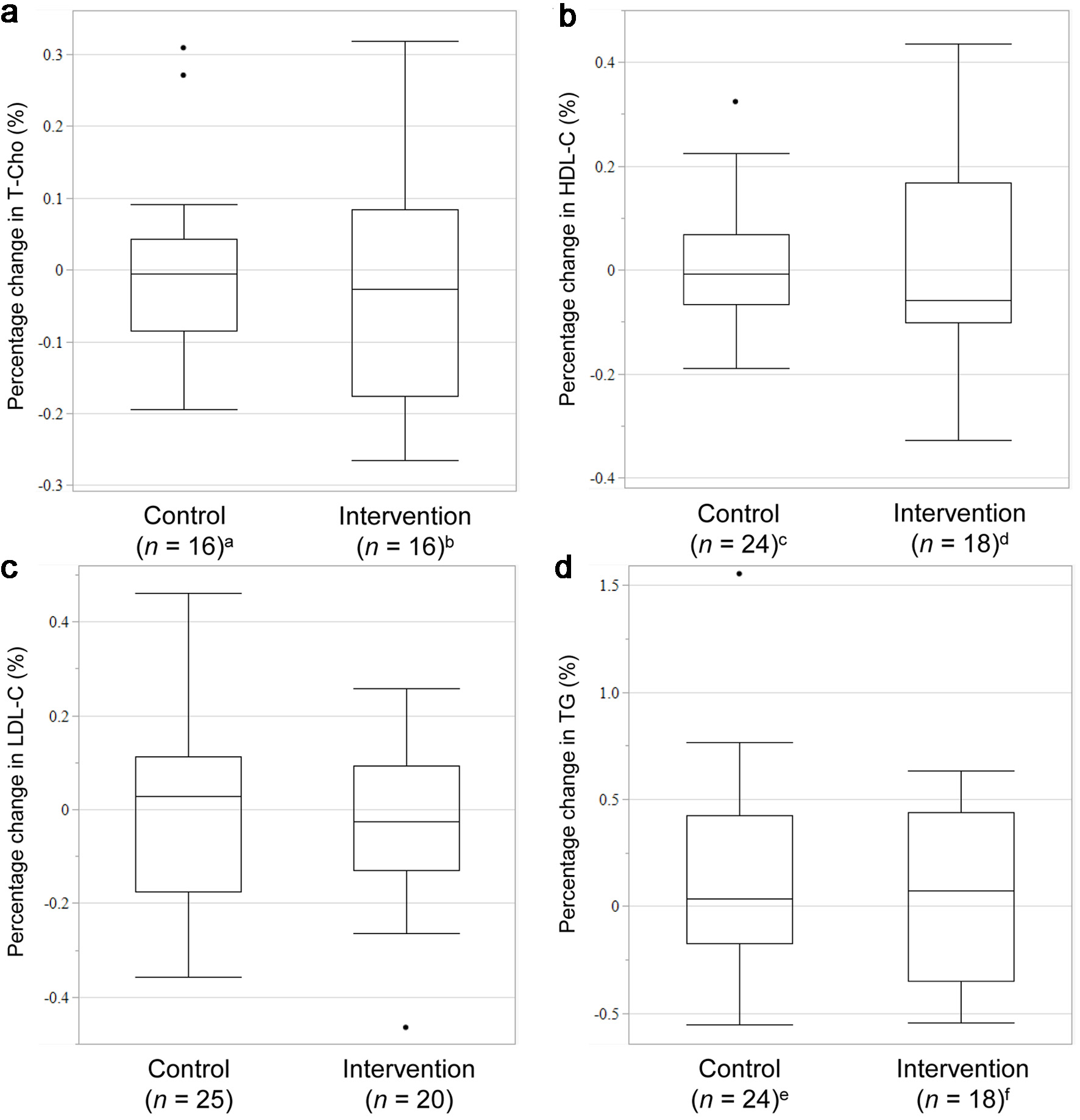
| T-Cho (mg/dL), median (IQR) | 159.5 (153.3 - 169.5)b | 152 (134.3 - 165.8)c | 0.2423 |
| HDL-C (mg/dL), median (IQR) | 54.5 (48.3 - 62.8)d | 49 (43.0 - 57.3)e | 0.2681 |
| LDL-C (mg/dL), median (IQR) | 77 (62.5 - 93.5) | 77.5 (65.0 - 94.5) | 0.7751 |
| TG (mg/dL), median (IQR) | 113 (84.5 - 154.3)f | 105 (80.3 - 175.8)g | 0.8688 |