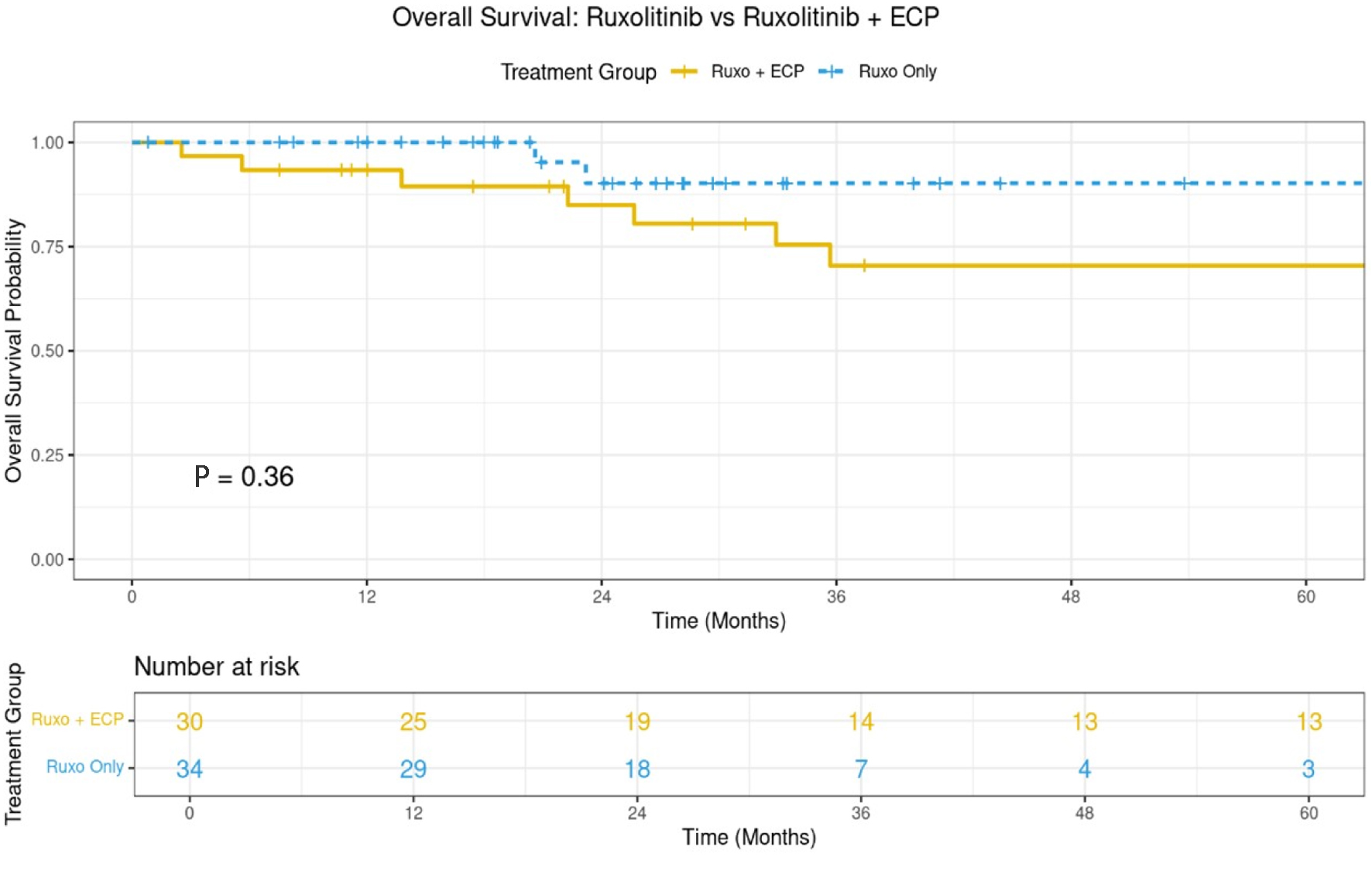
Figure 1. The 3-year overall survival for both arms (n = 68).
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 12, December 2025, pages 698-707
Ruxolitinib Plus Extracorporeal Photopheresis for Steroid-Refractory Acute and Chronic Graft-Versus-Host Disease
Figure

Tables
| Variable | Ruxo + ECP arm | Ruxo alone arm | P-value |
|---|---|---|---|
| AA: aplastic anemia; ATG: antithymoglobulin; BM: bone marrow; CB: cord blood; CNI: calcineurin; CR: complete remission; ECP: extracorporeal photopheresis; FA: Fanconi anemia; GvHD: graft-versus-host disease; HCT: hematopoietic cell transplantation; NoCR: non-remission; PBSCs: peripheral blood stem cells; PID: primary immunodeficiency; PTCy: post-transplant cyclophosphamide; RIC: reduced-intensity conditioning; RTC: reduced-toxicity conditioning; Ruxo: ruxolitinib. | |||
| Total patients (n, %) | 31 (100%) | 37 (100) | 0.086 |
| Age (years), median and range | 0.444 | ||
| Adult | 34 (18 - 62) | 28 (18 - 59) | |
| Pediatric | 13 (2 - 17) | 11.5 (6 - 17) | |
| Adult (n, %) | 22 (71%) | 25 (67%) | 0.760 |
| Pediatric (n, %) | 9 (29%) | 12 (33) | |
| Male (n, %) | 19 (61%) | 24 (65%) | 0.454 |
| Female (n, %) | 12 (39%) | 13 (35%) | |
| Primary disease, n (%) | 0.454 | ||
| Acute myeloid leukemia (AML) | 9 (29%) | 14 (38%) | |
| Acute lymphoid leukemia (ALL) | 9 (29%) | 8 (22%) | |
| Myelodysplastic syndrome (MDS) | 5 (16%) | 2 (5.5%) | |
| Myeloproliferative neoplasms (MPN) | 2 (7%) | 2 (5.5%) | |
| Lymphoma | 5 (16%) | 6 (16%) | |
| Nonmalignant (PID/AA/FA/thalassemia) | 1 (3%) | 5 (13%) | |
| CR of primary disease at HCT, n (%) | 23 (74%) | 30 (81%) | 0.454 |
| PR/VGPR, n (%) | 8 (26%) | 7 (19%) | |
| Allo-HCT first, n (%) | 31 (100%) | 37 (100%) | 0.454 |
| Donor type, n (%) | 0.454 | ||
| Matched related (MRD) | 22 (71%) | 27 (73%) | |
| Mismatch relative (MMR) | 9 (29%) | 9 (24%) | |
| CBT | 0 | 1 (3%) | |
| Graft source | 0.454 | ||
| PBSC | 31 (100%) | 34 (92%) | |
| BM | 0 | 2 (5.4%) | |
| CB | 0 | 1 (2.6%) | |
| Myeloablative | 18 | 23 | 0.358 |
| RIC-RTC | 13 | 14 | |
| GvHD prophylaxis therapy, n (%) | 0.098 | ||
| CNI-based +/-ATG | 29 (93%) | 37 (100%) | |
| PTCy-based+/-ATGATG | 4 (13%) | 0 | |
| Variable | Ruxo + ECP (n = 31) | Ruxo alone (n = 37) | P value |
|---|---|---|---|
| aGvHD: acute graft-versus-host disease; cGvHD: chronic graft-versus-host disease; ECP: extracorporeal photopheresis; GvHD: graft-versus-host disease; Ruxo: ruxolitinib; SR: steroid-refractory. | |||
| aGvHD | 27 (87%) | 27 (73%) | 0.229 |
| Grade II-IV | 25 (95.6%) | 22 (81.5%) | 0.420 |
| Grade III-IV | 18 (66.6%) | 5 (18.5%) | 0.007 |
| Skin | |||
| Grade I | 5 (18.5%) | 0 | 0.051 |
| Grade II | 4 (15%) | 8 (30%) | 0.202 |
| Grade III | 5 (18.5%) | 11 (41%) | 0.077 |
| Grade IV | 2 (7%) | 0 | 0.235 |
| Gut | |||
| Grade I | 1 (4%) | 0 | 0.051 |
| Grade II | 2 (7%) | 1 (4%) | 0.202 |
| Grade III | 10 (37%) | 4 (15%) | 0.077 |
| Grade IV | 4 (15%) | 2 (7%) | 0.490 |
| Liver | |||
| Grade I | 0 | 0 | 0.050 |
| Grade II | 3 (11%) | 1 (4%) | 0.202 |
| Grade III | 0 | 2 (7%) | 0.077 |
| Grade IV | 0 | 0 | 0.490 |
| cGvHD | 24 (77.4%) | 26 (70%) | 0.587 |
| NIH score I | 33% | 2 (8%) | 0.235 |
| NIH score II + III | 23 (96%) | 24 (92%) | 0.668 |
| Percentage of patients progressed to cGvHD | 20 (74%) (95% CI: 55-87%) | 16 (59%) (95% CI: 41-76%) | 0.387 |
| Skin and subcutaneous tissue | 8 (33%) | 19 (73%) | 0.002 |
| Liver | 11 (46%) | 7 (27%) | 0.386 |
| Eyes | 15 (63%) | 19 (73%) | 0.254 |
| Mouth | 17 (71%) | 20 (77%) | 0.371 |
| Lung | 10 (42%) | 3 (11.5%) | 0.053 |
| others | 2 (8%) | 1 (4%) | - |
| Duration of Ruxo continued treatment, median days | 222 (31 - 854) | 341 (17 - 1,274) | 0.046 |
| Time from Ruxo and start of ECP treatment, median days | 50 | ||
| Type of SR-aGvHD | |||
| Progressive after 3 days or no improvement after 7 days | 24 (77.5%) | 17 (65%) | 0.053 |
| Inability to taper steroids < 0.5 mg/kg | 7 (26%) | 11 (42%) | 0.2541 |
| Response category | Variable | Ruxo + ECP (n = 31) | Ruxo alone (n = 37) | P-value |
|---|---|---|---|---|
| aGvHD: acute GvHD; cGvHD: chronic GvHD; CI: cumulative incidence; CIR: cumulative incidence of relapse; CR: complete response; GFRS: graft-versus-host disease relapse-free survival; GvHD: graft-versus-host disease; NR: no response; NRM: non-relapse mortality; ORR: objective response; PR: partial response. | ||||
| aGvHD at last encounter | ORR | 18 (58%) | 18 (49%) | 0.02 |
| CR | 12 (39%) | 16 (43%) | 0.19 | |
| PR | 6 (19%) | 2 (6%) | 0.03 | |
| NR | 13 (42%) | 19 (51%) | 0.002 | |
| Steroid taper ≥ 50% | 87% | 93% | 0.69 | |
| Gut aGvHD (n = 23; 20 grade III-IV) at last encounter | 17 (74%) | 6 (26%) | 0.001 | |
| ORR (n, %) | 10 (59%) | 0 (0%) | 0.001 | |
| NR (n, %) | 7 (41%) | 6 (100%) | 0.506 | |
| cGvHD at last encounter | 12 months | 26% (95% CI: 22-64%) | 55% (95% CI: 41.9-69%) | 0.018 |
| 3-year GFRS | 100% | 97% | 0.481 | |
| Ongoing immunosuppression | 11 (35%) | 16 (43%) | 0.514 | |
| NRM | 19% (95% CI: 5-55%) | 0% | 0.006 | |
| CIR at 1 year | 6% (95% CI: 8-33%) | 2% (95% CI: 2-13%) | > 0.5 | |
| Median follow-up | 72 months (range: 54 - 71) | 72 months (range: 54 - 71) | - | |
| Primary disease status | CR | 94% | 97% | 0.588 |
| Patient status | Alive at last follow-up | 78% | 95% | 0.068 |
| Survival | 3-year survival | 70% (95% CI: 53-92%) | 80% (95% CI: 65-78%) | 0.327 |