| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 16, Number 7-8, August 2024, pages 325-334
Impact of the First Twenty-Four-Hour Area Under the Concentration-Time Curve/Minimum Inhibitory Concentration of Vancomycin on Treatment Outcomes in Patients With Methicillin-Resistant Staphylococcus aureus Bacteremia
Mika Higashia, Takafumi Nakanoa, b, e, Keisuke Satoa, Yukiomi Eguchia, Norihiro Moriwakia, Mitsuhiro Kamadaa, Tadahiro Ikeuchia, Susumu Kaneshigea, Masanobu Uchiyamaa, b, Toshinobu Hayashic, Atsushi Togawad, Koichi Matsuoa, b, Hidetoshi Kamimuraa
aDepartment of Pharmacy, Fukuoka University Hospital, Jonan-ku, Fukuoka 814-0133, Japan
bDepartment of Oncology and Infectious Disease Pharmacy, Faculty of Pharmaceutical Sciences, Fukuoka University, Jonan-ku, Fukuoka 814-0180, Japan
cDepartment of Emergency and Disaster Medical Pharmacy, Faculty of Pharmaceutical Sciences, Fukuoka University, Jonan-ku, Fukuoka 814-0180, Japan
dDepartment of Medical Oncology, Hematology, and Infectious Diseases, Faculty of Medicine, Fukuoka University, Jonan-ku, Fukuoka, Japan
eCorresponding Author: Takafumi Nakano, Department of Pharmacy, Fukuoka University Hospital, Jonan-ku, Fukuoka 814-0133, Japan
Manuscript submitted June 17, 2024, accepted July 29, 2024, published online August 10, 2024
Short title: Impact of First 24-Hour AUC/MIC of VCM
doi: https://doi.org/10.14740/jocmr5238
| Abstract | ▴Top |
Background: Vancomycin regimens are designed to achieve an area under the concentration-time curve/minimum inhibitory concentration (AUC/MIC) ratio ranging between 400 and 600 µg·h/mL in the steady state. However, in cases of critical infections such as bacteremia requiring an early treatment approach, the clinical course may be affected by the AUC/MIC before reaching the steady state, that is, the AUC/MIC values 24 h after the first dose (first 24-h AUC/MIC). This study evaluated the relationship between the first 24-h AUC/MIC and the clinical course of methicillin-resistant Staphylococcus aureus (MRSA) infection.
Methods: We retrospectively reviewed the records of patients with MRSA bacteremia in a university hospital between 2015 and 2022. The first 24-h AUC/MIC cutoff was set at 300 µg·h/mL based on the results of early response, and eligible patients were divided into groups with a first 24-h AUC/MIC either < 300 µg·h/mL (< 300 group, n = 32) or ≥ 300 µg·h/mL (≥ 300 group, n = 38). The primary endpoint was the rate of treatment efficacy, and the secondary endpoints were time to clinical and bacteriological improvement and 30-day survival rate.
Results: Treatment efficacy and 30-day survival rates were not significantly different between the two groups (78.1% vs. 79.0%, P = 0.933 and 83.9% vs. 87.2%, P = 0.674, respectively). Among patients who showed treatment efficacy, the median time to clinical and bacteriological improvement was 11.5 days and 8.0 days in the < 300 and ≥ 300 groups, respectively; compared to the ≥ 300 group, the < 300 group had a significantly longer time to improvement (P = 0.001).
Conclusions: The first 24-h AUC/MIC had no effect on the treatment efficacy and 30-day survival rates. However, the time to clinical and bacteriological improvement was significantly prolonged in the < 300 group, indicating that the first 24-h AUC/MIC does not affect the rate of therapeutic efficacy but may affect the treatment period.
Keywords: First 24-h AUC/MIC; Vancomycin; Methicillin-resistant Staphylococcus aureus; Bacteremia
| Introduction | ▴Top |
Methicillin-resistant Staphylococcus aureus (MRSA) bacteremia is a critical and relatively frequent infection associated with significant morbidity and mortality. Bacteremia is associated with severe illnesses, including sepsis, infective endocarditis, and meningitis [1]. The 30-day all-cause mortality rate for patients with MRSA bacteremia is estimated to be 20%, and the infection-related mortality rate is approximately 10% [2, 3]. Moreover, a recent systematic review showed that while mortality associated with Staphylococcus aureus bacteremia (SAB), including MRSA, has decreased over the last three decades, more than one in four patients still succumb within 3 months [4]. Therefore, early initiation of antimicrobial chemotherapy and continuous care are important to increase the effectiveness of treatment for SAB.
Vancomycin (VCM), a glycopeptide antibiotic, has been recommended as the first-line agent for MRSA infections by guidelines and expert consensus worldwide [5-7]. The concentration of VCM is related to its efficacy and toxicity; however, its therapeutic range is narrower than that of other antimicrobial agents. Therefore, therapeutic drug monitoring (TDM) is recommended for VCM therapy to maximize its efficacy and minimize nephrotoxicity [8, 9]. Previous guidelines recommended using the VCM trough concentrations as a surrogate marker for the ratio of the area under the concentration-time curve/minimum inhibitory concentration (AUC/MIC) to facilitate clinical application, with target trough concentrations ranging from 10 to 20 µg/mL depending on the infection type [8, 10]. In 2020 and 2022, the revised consensus guidelines for TDM of VCM recommended a change from trough-based TDM to AUC/MIC-based TDM to optimize the efficacy of treatment and reduce the rate of nephrotoxicity [7, 11]. The target range of AUC/MIC is 400 - 600 µg·h/mL at the steady state, with an AUC/MIC ≥ 400 µg·h/mL essential for clinical efficacy and an AUC/MIC ≤ 600 µg·h/mL important for reduced nephrotoxicity [12-14]. Furthermore, the time to achieve a sufficient level of AUC/MIC is also regarded as an important factor in leading to therapeutic success. Administering an effective antimicrobial against Staphylococcus aureus within 48 h after collecting blood cultures is known to be the only significant predictor of survival in patients with SAB [15]. Therefore, there has been an increase in studies on pharmacokinetics/pharmacodynamics (PK/PD) evaluating the first 24 - 48 h AUC/MIC of VCM [15-18]. However, the relationship between early AUC levels and clinical efficacy has not yet been fully elucidated. Moreover, in patients with a critical infection that requires an early approach, such as MRSA bacteremia, the clinical course may be affected by the AUC/MIC before the steady state, specifically AUC/MIC values 24 h after the first dose (first 24-h AUC/MIC). Therefore, this study aimed to evaluate the relationship between the first 24-h AUC/MIC and the clinical course of MRSA infection using practical AUC-guided TDM for VCM (PAT) via Bayesian software [19], which is specialized in AUC calculations.
| Materials and Methods | ▴Top |
Study design and population
This retrospective, single-center, observational study was approved by the Medical Ethics Review Board of Fukuoka University (approval number: H23-05-010, May 25, 2023) and was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. The requirement for informed consent from participants was not necessary due to the retrospective nature of this study. We reviewed the records of patients who were admitted to our university hospital between 2015 and 2022 and diagnosed with MRSA bacteremia. Among the 137 patients diagnosed with MRSA bacteremia based on blood culture tests and clinical observations, 70 patients who received VCM therapy were included in this study (Fig. 1). The exclusion criteria were as follows: 1) age < 18 years; 2) duration of VCM therapy < 4 days; 3) missing data; 4) patients who underwent hemodialysis, renal replacement therapy, and plasma exchange. Moreover, this study excluded asymptomatic patients and those with contaminated blood cultures.
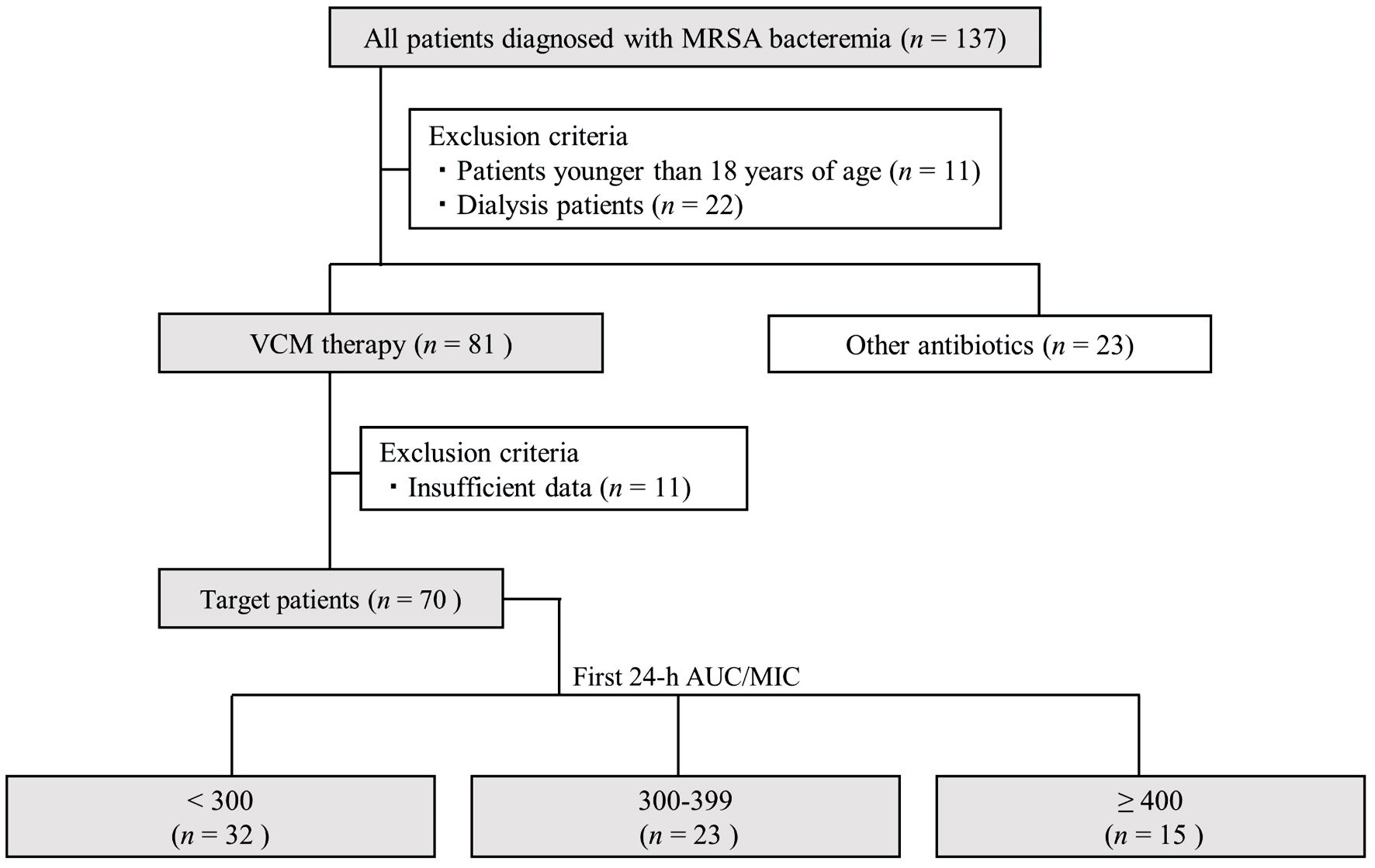 Click for large image | Figure 1. Flowchart depicting patient selection in this study. AUC/MIC: area under the concentration-time curve/minimum inhibitory concentration; VCM: vancomycin; MRSA: methicillin-resistant Staphylococcus aureus. |
Data collection and definitions
We evaluated the diagnosis, age, sex, body weight, patient background, medical history, source of infection, bacterial culture test results, blood laboratory results, and biochemical test results of each patient using our electronic medical record system. To assess the early clinical response at 72 h after the start of VCM therapy, we used the criteria from previous studies by Ueda et al [20, 21]. “Responder,” defined as an early positive response, was characterized by patients who demonstrated a ≥ 30% decrease in white blood cell (WBC) count or C-reactive protein (CRP) level, experienced a reduction in fever (defined as a daily maximum body temperature decrease of > 0.3 °C for at least 2 consecutive days in febrile patients), exhibited no worsening clinical features, and encountered no death within 96 h. To assess the incidence rate of acute kidney injury (AKI) associated with VCM therapy, we used the Kidney Disease: Improving Global Outcome (KDIGO) diagnostic criteria [22]. AKI was defined as an increase of ≥ 0.3 mg/dL or ≥ 1.5-fold from the baseline serum creatinine (SCr) level. The maximum SCr level during the VCM therapy was defined as the evaluation value, while the SCr measurement obtained before initiating VCM therapy was considered as the baseline SCr level. Creatinine clearance was estimated using the Cockcroft-Gault formula [23]. The MIC was determined using the broth microdilution method according to the Clinical and Laboratory Standard Institute manual.
Effectiveness of VCM therapy
The primary endpoint was the rate of treatment efficacy, and the secondary endpoints were the time to clinical and bacteriological improvement and the 30-day survival rate. Treatment efficacy was evaluated in terms of both bacteriological and clinical effects. Bacteriological efficacy was defined as negative blood cultures after initiating VCM therapy and was confirmed by repeating blood culture tests at 3 - 5-day intervals until negative results were obtained. Moreover, the clinical efficacy of VCM was evaluated on the final day of the VCM treatment period. The clinical efficacy was deemed effective if at least two of the following criteria were met (without any exacerbation observed in the unmet criteria): 1) body temperature below 37.0 °C (indicating a reduction from feverish levels); 2) a reduction in the WBC count to < 8,000/mm3 or to a standard value; 3) a decrease in the CRP level to ≤ 30% of the pre-dose value [24]. Ultimately, VCM therapy was considered effective when patients showed both bacteriological and clinical efficacy. These criteria were based on our previous studies on staphylococcal infections [25, 26]. The early response criteria [20] are also a useful indicator for simply and quickly examining the efficacy of treatment. However, because these criteria do not include factors such as bacteriological evaluation and reduction of fever to normal, we applied our own criteria to assess the overall treatment efficacy. The time to clinical and bacteriological improvement was defined as the first day these criteria for treatment efficacy were met in patients demonstrating a treatment response.
Calculation of the AUC/MIC values 24 h after the first dose (first 24-h AUC/MIC)
The Japanese Society of Chemotherapy developed the Bayesian software PAT [19] based on the population pharmacokinetic parameters reported by Yasuhara et al [27]. The PAT is a free specialized software for AUC calculations. The VCM trough concentration, administration interval, and implementation day of TDM were collected from the records of the TDM diary, and the first 24-h AUC/MIC was calculated using PAT ver. 3.0. Generally, the peak and trough concentrations are necessary to calculate the AUC. However, until the Japanese TDM guidelines were revised in 2022 [7], VCM blood samples were mostly collected only for trough concentrations in daily clinical practice in Japan. Therefore, there have been some previous studies where the AUC was calculated using PAT software, based on only trough concentrations [20, 28, 29]. In this study, because few patients had two-point samples, the first 24-h AUC/MIC was calculated using only the trough concentration within 3 - 5 days after initiating VCM therapy, according to previous studies. Before calculating the AUC, we confirmed that the patient parameters for both groups approximated those of the population [27], which was incorporated into the analysis software. The AUC at steady state (AUCss) was calculated using the VCM dosing data and trough concentrations after the implementation of TDM.
Statistical analysis
Sample sizes were predetermined using G*Power ver. 3.1.9.4 software with effect sizes based on our previous study [28]. We used a Chi-square goodness-of-fit test for contingency table analysis with input parameters of α = 0.05 and power (1 - β) = 0.8. Data are presented as median (interquartile range). We used the Mann-Whitney U test, Chi-square test, or Fischer’s exact test for between-group comparisons. Survival data were examined using Kaplan-Meier analysis, and comparisons between groups were performed using the log-rank test. A receiver operating characteristic (ROC) curve analysis was conducted on variables that demonstrated statistical significance in logistic regression to ascertain the cutoff value for the first 24-h AUC/MIC, aiming for an early positive response and the optimal dose of VCM required to achieve a first 24-h AUC/MIC ≥ 300 µg·h/mL. The cutoff values were determined using the Youden index. Confidence intervals (CIs) were calculated employing the DeLong method [30]. In all analyses, a P value < 0.05 was deemed to reflect statistical significance. All statistical evaluations were conducted using JMP version 12.0.1 (SAS Institute, Tokyo, Japan).
| Results | ▴Top |
Number of cases in each AUC/MIC range
The MIC was set to 1 µg/mL in all analyses because the MIC of VCM was ≤ 1 µg/mL in all patients. To evaluate the distribution of the first 24-h AUC/MIC, we categorized the first 24-h AUC/MIC as 100 - 199, 200 - 299, 300 - 399, and 400 - 499 based on previous studies [28, 31] (Table 1). The number of cases in each category was as follows: 100 - 199, two cases (2.9%); 200 - 299, 30 cases (42.9%); 300 - 399, 23 cases (32.9%); 400 - 499, 15 cases (21.4%). The first 24-h AUC/MIC was shown to be mostly in the range of 200 - 400 (75.7%).
 Click to view | Table 1. Number of Cases in Each AUC/MIC Range |
Early positive response rate and population in groups
We compared early positive response rates by dividing the groups into those with a first 24-h AUC/MIC < 400 µg·h/mL and those with a first 24-h AUC/MIC ≥ 400 µg·h/mL. Consequently, the early positive response showed 43.6% and 66.7% in the first 24-h AUC/MIC < 400 µg·h/mL and first 24-h AUC/MIC ≥ 400 µg·h/mL groups, respectively (P = 0.114) (Supplementary Material 1, www.jocmr.org). Moreover, we calculated the cutoff value of the first 24-h AUC/MIC to indicate an early positive response using ROC analysis. The analysis demonstrated that the cutoff value for the first 24-h AUC/MIC, indicative of an early positive response, was 309.7 µg·h/mL (AUC: 0.754, sensitivity: 76.5%, specificity: 69.4%, 95% CI: 0.640 - 0.869), as depicted in Figure 2a. Furthermore, when the cutoff was established at 300 µg·h/mL, the early positive response rate significantly increased in the ≥ 300 group compared to the < 300 group (25.0% vs. 68.4%, P = 0.001, as shown in Figure 2b). Based on these results, to evaluate the impacts on the first 24-h AUC/MIC, we defined the cutoff value of the first 24-h AUC/MIC as 300 µg·h/mL, and eligible patients were categorized into the following two groups: first 24-h AUC/MIC < 300 µg·h/mL (< 300 group, n = 32) and first 24-h AUC/MIC ≥ 300 µg·h/mL (≥ 300 group, n = 38).
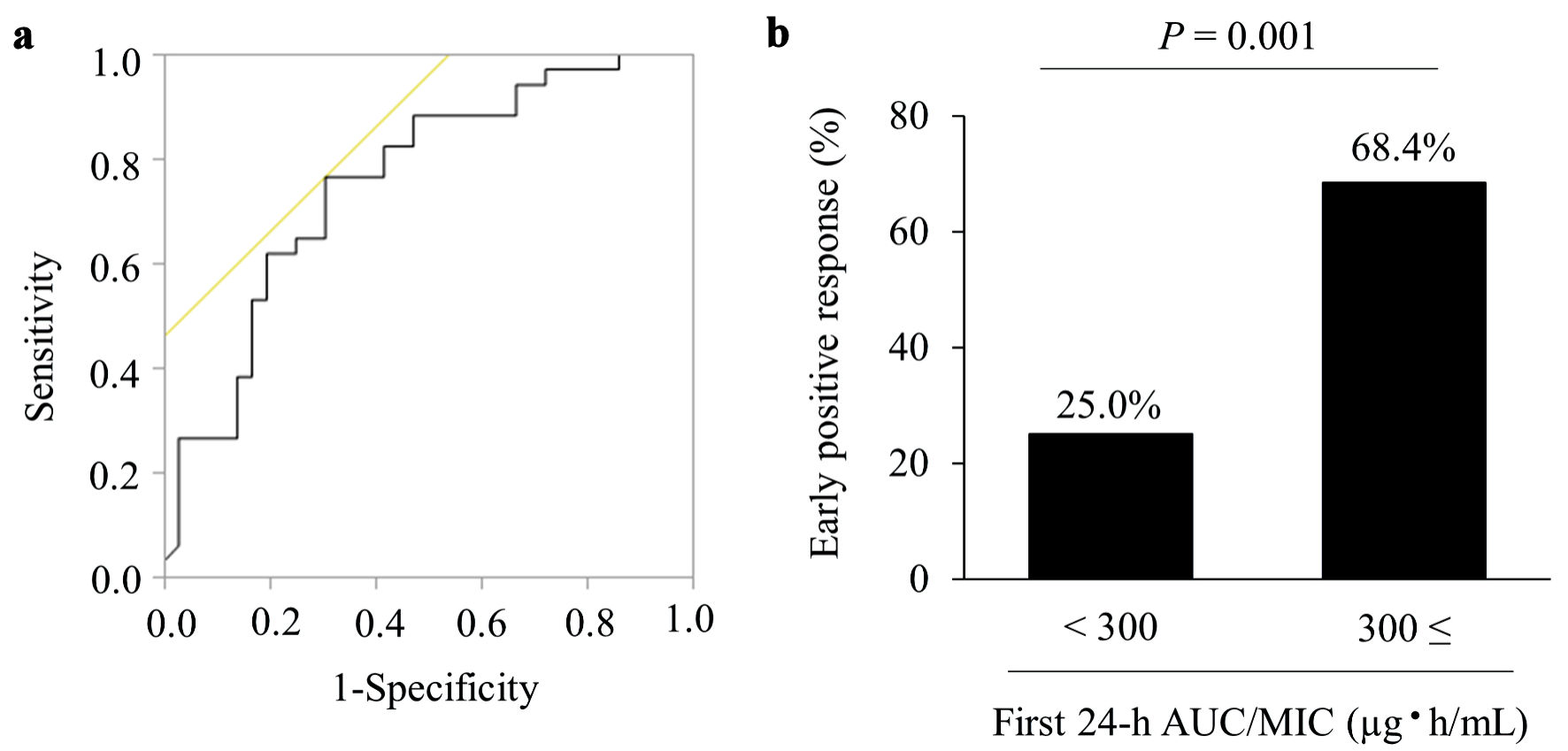 Click for large image | Figure 2. Receiver operating characteristic (ROC) curve and early positive response. (a) The ROC curve used to determine the cutoff value of the first 24-h AUC/MIC of vancomycin for early positive response. The analysis identified an AUC/MIC of 309.7 µg·h/mL, represented by an AUC value of 0.754, sensitivity of 76.5%, and specificity of 69.4%. (b)The early positive response rate in the < 300 and ≥ 300 groups. Values are presented as numbers (%). AUC/MIC: area under the concentration-time curve/minimum inhibitory concentration. |
The background characteristics of the patients in the two groups are presented in Table 2. Age, body weight, comorbidities, origin of infection, laboratory data, and concomitant antimicrobial agents were not significantly different between the two groups. Additionally, no serious diseases were found in either group, such as infectious endocarditis, immunodeficiency, or terminal cancer, which may affect the efficacy and time to improvement of VCM therapy. Moreover, more than 80% of patients with catheter-related bloodstream infections had their catheters removed within 3 days of positive blood culture tests, and all patients had their catheters removed within 10 days. The dose on the first day of VCM treatment and the initial trough concentration were significantly lower in the < 300 group than in the ≥ 300 group. Thus, the AUC values on days 1 (AUC0 - 24 h) and 2 (AUC24 - 48 h) were significantly different between the two groups, but the AUCss was not.
 Click to view | Table 2. Characteristics of the Patients Included in This Study |
Effectiveness of VCM therapy
Data for the effectiveness of VCM therapy are shown in Figure 3. The treatment efficacy rate, the primary endpoint, was 78.1% (25/32 cases) and 79.0% (30/38 cases) in the < 300 and ≥ 300 groups, respectively (Fig. 3a). Thus, the two groups showed no significant difference in treatment efficacy (P = 0.933). Moreover, the two groups showed no significant difference in 30-day survival (83.9% for the < 300 group vs. 87.2% for the ≥ 300 group; P = 0.674) (Fig. 3b). However, among patients who showed treatment efficacy, the median time to clinical and bacteriological improvement was 11.5 and 8.0 days in the < 300 (n = 25) and ≥ 300 (n = 30) groups, respectively. Therefore, the time to improvement was significantly longer in the < 300 group (P = 0.001) (Fig. 3c).
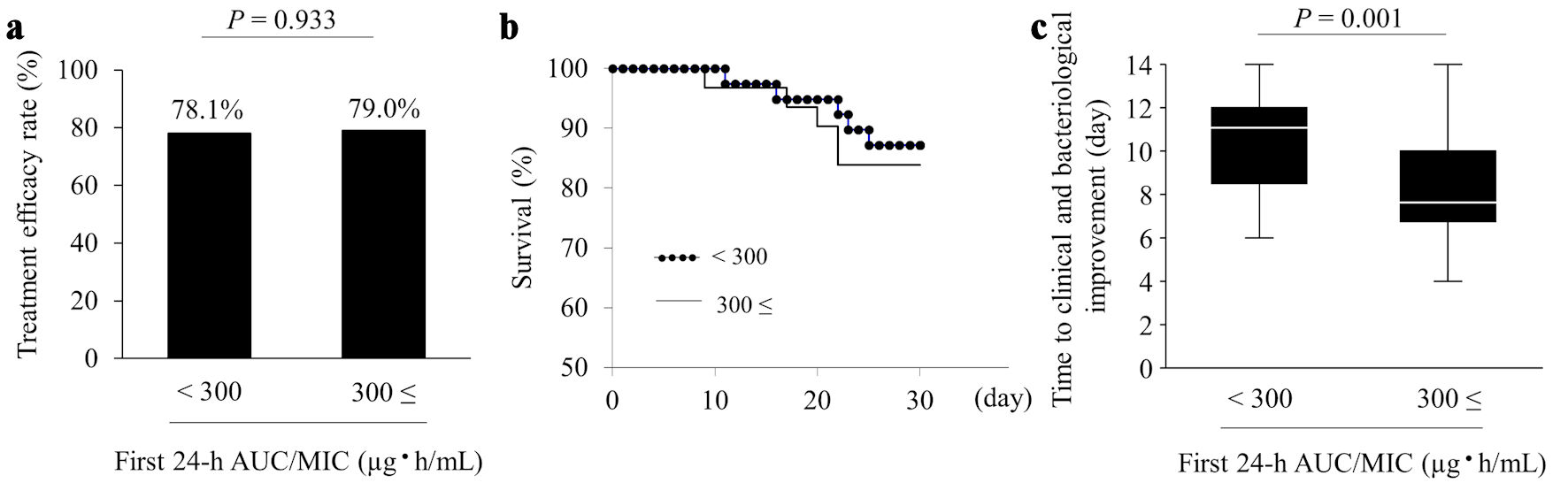 Click for large image | Figure 3. Effectiveness of VCM therapy. The panels show the treatment efficacy rate (a), 30-day survival (b), and time to clinical and bacteriological improvement (c). Values are presented as numbers (%), and time to clinical and bacteriological improvement is presented as median (interquartile range). AUC/MIC: area under the concentration-time curve/minimum inhibitory concentration; VCM: vancomycin. |
Frequency of AKI associated with VCM therapy
Ten patients met the definition of AKI during VCM therapy. The incidence of AKI associated with VCM therapy was 14.3% (10/70 cases). Three and seven cases of AKI were in the < 300 and ≥ 300 groups, respectively (9.4% vs. 18.4%, P = 0.281). When the ≥ 300 group was categorized into the 300 - 399 (n = 23) and ≥ 400 (n = 15) groups, the number of AKI cases was three and four, respectively (13.0% vs. 26.7%, P = 0.290). Although the rate of AKI tended to be higher in the ≥ 400 group, the three groups showed no significant difference (P = 0.344).
Optimal dose of VCM to achieve a first 24-h AUC/MIC value ≥ 300 µg·h/mL
An ROC curve analysis was performed to identify the optimal dose of VCM to achieve a first 24-h AUC/MIC value ≥ 300 µg·h/mL. The analysis revealed that a VCM dose of 27.8 mg/kg was the threshold to achieve a first 24-h AUC/MIC value ≥ 300 µg·h/mL (AUC: 0.735, sensitivity: 68.4%, specificity: 87.5%, 95% CI: 0.610 - 0.859), as shown in Figure 4.
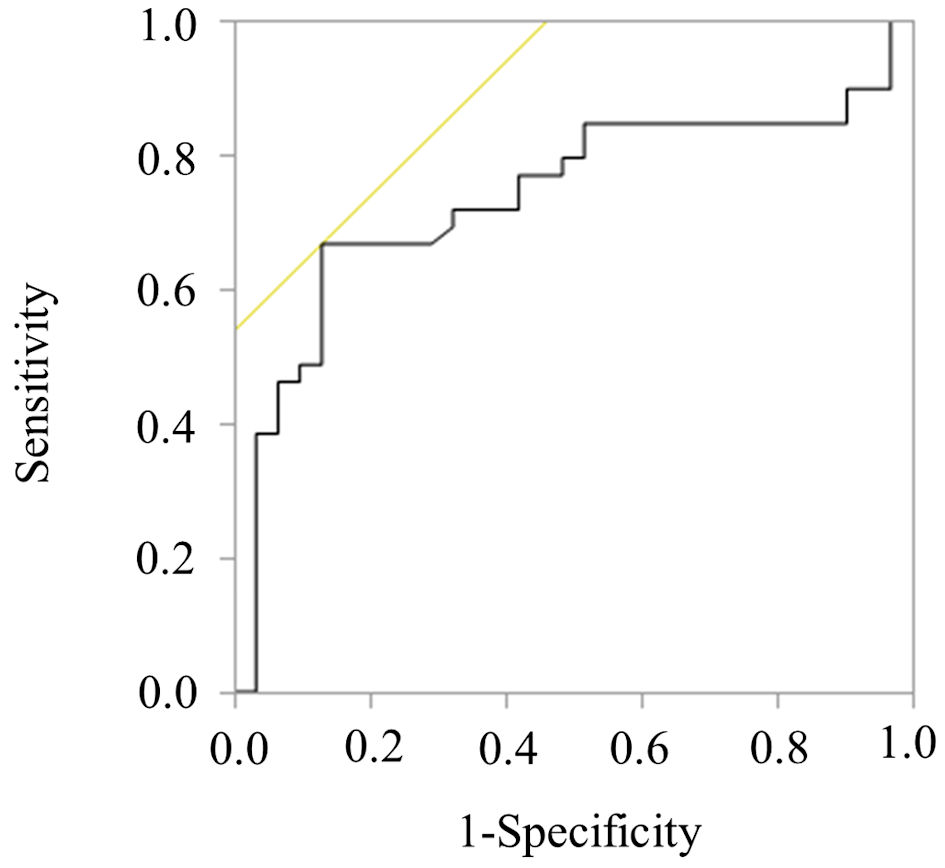 Click for large image | Figure 4. ROC curve for achieving first 24-h AUC/MIC ≥ 300 µg·h/mL. The figure presents the ROC curve used to determine the optimal dose of VCM to achieve a first 24-h AUC/MIC ≥ 300 µg·h/mL. The analysis identified an optimal VCM dose of 27.8 mg/kg, represented by an AUC value of 0.735, sensitivity of 68.4%, and specificity of 87.5%. VCM: vancomycin; AUC/MIC: area under the concentration-time curve/minimum inhibitory concentration; ROC: receiver operating characteristic. |
| Discussion | ▴Top |
In this study, we focused on the early AUC levels and evaluated the impact of the first 24-h AUC/MIC of VCM on the clinical outcomes of patients with MRSA bacteremia. AUC estimations of VCM can be calculated using Bayesian modeling software or first-order pharmacokinetic equations. The Bayesian method can estimate the AUC with a minimum of one concentration value in patients receiving VCM, potentially reducing the resources needed for AUC monitoring. In contrast, the first-order pharmacokinetic equations require two concentration values of VCM obtained at appropriate times. Although both methods have their strengths and limitations [32], the revised consensus guidelines for the TDM of VCM published in Clinical Infectious Diseases recommend the use of Bayesian-derived AUC/MIC values to accurately achieve targets for clinical efficacy while improving patient safety [11]. Therefore, in this study, we calculated the AUC using the Bayesian software and evaluated endpoints. Consequently, we found that the first 24-h AUC/MIC does not affect the overall therapeutic efficacy rate but may affect the time to improvement. This is the first report to show the relationship between the first 24-h AUC/MIC and the clinical course of MRSA infection. We believe that our study contributes significantly to the development of MRSA bacteremia treatment strategy because information regarding the influence of the first 24-h AUC/MIC on the treatment parameters and outcomes is currently limited.
First, we compared the early positive response rate between the first 24-h AUC/MIC < 400 µg·h/mL and ≥ 400 µg·h/mL groups. The results showed that the early positive response rate was 43.6% and 66.7% in the first 24-h AUC/MIC < 400 µg·h/mL and ≥ 400 µg·h/mL groups, respectively. These values were similar to those of a previous study by Ueda et al [20]; however, our results showed no significant difference between the two groups. Therefore, we considered the cutoff value indicating an early positive response to be < 400 µg·h/mL and evaluated it using ROC analysis. As the result was 309.7 µg·h/mL, we categorized eligible patients into the < 300 and ≥ 300 groups and reanalyzed the early positive response rate. Consequently, the early positive response rate significantly increased in the ≥ 300 group than in the < 300 group. Therefore, we hypothesized that the first 24-h AUC/MIC being above or below 300 µg·h/mL might influence treatment outcomes. Thus, we decided to compare the treatment efficacy rate of VCM between the < 300 and ≥ 300 groups.
The treatment efficacy rates in the < 300 and ≥ 300 groups were 78.1% and 79.0%, respectively. The 30-day survival rates in the two groups were 83.9% and 87.2%, respectively. Thus, the overall efficacy against MRSA bacteremia did not differ between the two groups. Claeys et al reported that the rate of clinical success in patients with MRSA bacteremia who received VCM therapy was 71.8%, and the all-cause 30-day survival rate was 84.7% [33]. A systematic review and meta-analysis also described that the all-cause survival rate extracted from randomized controlled trials for patients treated with VCM therapy was 63.4% [34]. Therefore, our results were consistent with those of previous studies on the treatment of MRSA bacteremia, which reported VCM treatment success rates of 55-75% [33-35]. Particularly, overall treatment efficacy rates were obtained not only in the ≥ 300 group but also in the < 300 group. We attribute this result to the fact that the AUCss/MIC was regulated to ≥400 µg·h/mL by implementing TDM after the blood collection point. Thus, this result indicates that the first 24-h AUC/MIC may not affect the final efficacy against MRSA bacteremia as long as the AUCss/MIC reaches ≥ 400 µg·h/mL. In contrast, the time to clinical and bacteriological improvement was significantly longer in the < 300 group than in the ≥ 300 group. Most patients in the ≥ 300 group required only 1 - 2 days to reach AUCss/MIC ≥ 400 µg·h/mL, whereas those in the < 300 group required approximately 4 - 7 days to stabilize AUCss/MIC ≥ 400 µg·h/mL owing to dose adjustment. This factor may have influenced the time to improvement. Moreover, this study indicated that when the first 24-h AUC/MIC was < 300 µg·h/mL, AUC24 - 48 h was 366.6 (315.1 - 406.5). However, when the first 24-h AUC/MIC was ≥ 300 µg·h/mL, AUC24 - 48 h was 509.0 (388.1 - 588.7). Previous studies evaluating early AUC/MIC have reported that to enhance the efficacy of VCM therapy, the AUC/MIC should be 400 - 600 µg·h/mL at least during the first 24 - 48 h [15-18]. Therefore, our findings that the first 24-h AUC/MIC requires ≥ 300 µg·h/mL are consistent with those of previous studies. Based on these results, our study showed that the first 24-h AUC/MIC affected the time required to reach AUCss/MIC ≥ 400 µg·h/mL, indicating that inadequate AUC/MIC levels for the first 24 h may prolong the therapeutic period.
We considered that the factor most influencing the first 24-h AUC/MIC levels was the initial loading dose. Our study demonstrated that the initial loading dose in the ≥ 300 group was 30.4 mg/kg, while that in the < 300 group was 22.4 mg/kg. Recently updated consensus guidelines on VCM state that a loading dose of 20 - 35 mg/kg body weight (not exceeding 3,000 mg) is required for rapid attainment of targeted concentrations in critical patients with MRSA infections [11]. Moreover, the TDM guideline for VCM in Japan recommends an initial dose of 25 - 30 mg/kg to achieve the therapeutic range of AUC/MIC earlier [7]. Thus, the ≥ 300 group in this study received treatment within the guideline-recommended dose ranges and may have achieved AUCss/MIC ≥ 400 µg·h/mL sooner, potentially leading to a shorter treatment duration. In contrast, the < 300 group in this study received less than the recommended dose of 25 - 30 mg/kg required to achieve the target range of AUC/MIC earlier. Specifically, an initial loading dose of < 25 mg/kg may not achieve the first 24-h AUC/MIC ≥ 300 µg·h/mL and may result in a longer treatment period. Therefore, ROC analysis in this study showed that the high efficacy of VCM required the first 24-h AUC/MIC ≥ 300 µg·h/mL, and an initial loading dose of approximately 28 mg/kg was needed to achieve this range. These findings support the Japanese guidelines, which recommend an initial dose of 25 - 30 mg/kg. However, further studies are necessary to ascertain whether an initial loading dose of 20 - 25 mg/kg suffices. Importantly, this study revealed that the AUC/MIC in the first 24 h may influence the duration of treatment. Specifically, we identified that the cutoff value for the initial loading dose to achieve the first 24-h AUC/MIC ≥ 300 µg·h/mL was 27.8 mg/kg.
We also assessed the impact of the first 24-h AUC/MIC on the incidence of AKI. No significant difference was found in the incidence of AKI between the two groups. Wagner et al and Hodiamont et al reported that a weight-based initial loading dose of VCM led to significantly more patients achieving AUCss/MIC ≥ 400 µg·h/mL without increasing the risk of AKI [36, 37]. However, the risk of AKI was significantly higher in patients who achieved an AUCss/MIC ≥ 600 µg·h/mL [7, 11]. Higher VCM exposure is associated with an increased risk of AKI. However, previous studies have not clarified whether the initial loading dose of VCM directly poses an additional risk of AKI. This is because the maximum daily dose of VCM is set, and it is considered difficult to exceed AUCss/MIC ≥ 600 µg·h/mL with only the initial loading dose. However, if the first 24-h AUC/MIC is high, the maintenance dose should be carefully adjusted through TDM because continued high exposure increases the risk of AKI. In fact, our results showed that although the three groups (≥ 300, 300 - 399, and ≥ 400 groups) showed no significant difference, the rate of AKI tended to be higher in the ≥ 400 group. Critically, if the first 24-h AUC/MIC surpasses 400 µg·h/mL, the likelihood of achieving AUCss/MIC ≥ 600 µg·h/mL escalates unless the maintenance dose is meticulously adjusted. Moreover, the association between the first 24-h AUC/MIC and AKI risk warrants further exploration in future studies.
Our study has some limitations that require consideration. First, this was a retrospective, single-center, observational study involving a small number of patients. The study also had several confounding factors, including the patients’ underlying diseases, comorbidities, the severity of infection symptoms, the use of concomitant medications, and multiple infections. We believe that a multivariate analysis is essential to remove confounding factors; however, this was impossible in this study because of the small number of cases. Similarly, while an evaluation of the relationship between the PK/PD parameters and each primary lesion of bacteremia was necessary, it was impossible because of the small number of cases. Second, because the AUC was calculated using only trough concentrations in all cases, there remains an issue with the accuracy of AUC estimation. Oda et al indicated that the use of trough-only sampling data would produce more bias in the calculation of AUC/MIC data [19]. Future studies should analyze AUC based on a two-point sample to obtain less biased estimates. Third, the diagnosis of AKI was based only on the KDIGO diagnostic criteria using SCr values because evaluation by urine volume could not be performed. Moreover, the number of individuals experiencing AKI within the entire study cohort was small, potentially making it difficult to determine statistical significance. Although our results were not statistically significant, it should be noted that the incidence of AKI doubled in the ≥ 300 µg·h /mL group compared with that in the < 300 µg·h/mL group. Multicenter prospective observational studies are necessary to overcome these limitations. Moreover, recent evidence has provided information on dosing to achieve an AUC/MIC ≥ 400 µg·h/mL within the first 24 h [38, 39]. In the future, it may be necessary to evaluate the utility at a larger level of the first 24-h AUC/MIC while ensuring the safety and economical aspects of VCM therapy.
Conclusions
This retrospective, single-center study could not yield definitive conclusions. However, when the cutoff value for the first 24-h AUC/MIC was set at 300 µg·h/mL, the levels of AUC/MIC showed no effect on the rate of treatment efficacy between the < 300 and ≥ 300 groups. This may be owing to the fact that the AUCss/MIC of all patients was ≥ 400 µg·h/mL. In contrast, the time to clinical and bacteriological improvement was significantly shorter in the ≥ 300 group than in the < 300 group. Our results indicate that the first 24-h AUC/MIC does not affect the overall efficacy rate but may affect the time to improvement. Additionally, future studies of a cost-benefit analysis of the faster time to improvement with higher initial dosing versus the potential increased risk of AKI would further strengthen conclusions regarding the importance of the first 24-h AUC/MIC.
| Supplementary Material | ▴Top |
Suppl 1. The early positive response in the < 400 and ≥ 400 groups.
Acknowledgments
None to declare.
Financial Disclosure
No funding was received for this report.
Conflict of Interest
The authors declare that they have no competing interest.
Informed Consent
As a retrospective study, the need for individual written informed consent was waived.
Author Contributions
MH, TN, and KS contributed to the concept and design of the study. MH and TN drafted the manuscript. YE, NM, MK, TI, SK MU and TH contributed to data and statistical analysis. AT advised on the interpretation of the therapeutic course and revised the manuscript. KM and HK supervised the writing of the manuscript. All authors read and approved the final manuscript.
Data Availability
Data are available from the authors upon reasonable request and with permission of the Medical Ethics Review Board of Fukuoka University.
Abbreviations
AKI: acute kidney injury; AUC/MIC: area under the blood concentration-time curve/minimum inhibitory concentration; CI: confidence interval; CRP: C-reactive protein; Ccr: creatinine clearance; KDIGO: kidney disease improving global outcome; MRSA: methicillin-resistant Staphylococcus aureus; PK/PD: pharmacokinetics/pharmacodynamics; PAT: practical AUC-guided TDM for VCM; ROC: receiver operating characteristic; SAB: Staphylococcus aureus bacteremia; SCr: serum creatinine; TDM: therapeutic drug monitoring; VCM: vancomycin; WBC: white blood cell
| References | ▴Top |
- Broadley SP, Plaumann A, Coletti R, Lehmann C, Wanisch A, Seidlmeier A, Esser K, et al. Dual-track clearance of circulating bacteria balances rapid restoration of blood sterility with induction of adaptive immunity. Cell Host Microbe. 2016;20(1):36-48.
doi pubmed - Turnidge JD, Kotsanas D, Munckhof W, Roberts S, Bennett CM, Nimmo GR, Coombs GW, et al. Staphylococcus aureus bacteraemia: a major cause of mortality in Australia and New Zealand. Med J Aust. 2009;191(7):368-373.
doi pubmed - Lesens O, Methlin C, Hansmann Y, Remy V, Martinot M, Bergin C, Meyer P, et al. Role of comorbidity in mortality related to Staphylococcus aureus bacteremia: a prospective study using the Charlson weighted index of comorbidity. Infect Control Hosp Epidemiol. 2003;24(12):890-896.
doi pubmed - Bai AD, Lo CKL, Komorowski AS, Suresh M, Guo K, Garg A, Tandon P, et al. Staphylococcus aureus bacteraemia mortality: a systematic review and meta-analysis. Clin Microbiol Infect. 2022;28(8):1076-1084.
doi pubmed - Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285-292.
doi pubmed - Brown NM, Goodman AL, Horner C, Jenkins A, Brown EM. Treatment of methicillin-resistant Staphylococcus aureus (MRSA): updated guidelines from the UK. JAC Antimicrob Resist. 2021;3(1):dlaa114.
doi pubmed pmc - Matsumoto K, Oda K, Shoji K, Hanai Y, Takahashi Y, Fujii S, Hamada Y, et al. Clinical practice guidelines for therapeutic drug monitoring of vancomycin in the framework of model-informed precision dosing: a consensus review by the Japanese society of chemotherapy and the Japanese society of therapeutic drug monitoring. Pharmaceutics. 2022;14(3):489.
doi pubmed pmc - Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Jr., Craig W, Billeter M, Dalovisio JR, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98.
doi pubmed - Bradley N, Ng K. Evaluation of real-world vancomycin dosing and attainment of therapeutic drug monitoring targets. Pharmacy (Basel). 2023;11(3):95.
doi pubmed pmc - Matsumoto K, Takesue Y, Ohmagari N, Mochizuki T, Mikamo H, Seki M, Takakura S, et al. Practice guidelines for therapeutic drug monitoring of vancomycin: a consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J Infect Chemother. 2013;19(3):365-380.
doi pubmed - Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, Mueller BA, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-system Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2020;71(6):1361-1364.
doi pubmed - Tsutsuura M, Moriyama H, Kojima N, Mizukami Y, Tashiro S, Osa S, Enoki Y, et al. The monitoring of vancomycin: a systematic review and meta-analyses of area under the concentration-time curve-guided dosing and trough-guided dosing. BMC Infect Dis. 2021;21(1):153.
doi pubmed pmc - Abdelmessih E, Patel N, Vekaria J, Crovetto B, SanFilippo S, Adams C, Brunetti L. Vancomycin area under the curve versus trough only guided dosing and the risk of acute kidney injury: Systematic review and meta-analysis. Pharmacotherapy. 2022;42(9):741-753.
doi pubmed pmc - Oda K, Jono H, Nosaka K, Saito H. Reduced nephrotoxicity with vancomycin therapeutic drug monitoring guided by area under the concentration-time curve against a trough 15-20 mug/mL concentration. Int J Antimicrob Agents. 2020;56(4):106109.
doi pubmed - Shime N, Kosaka T, Fujita N. The importance of a judicious and early empiric choice of antimicrobial for methicillin-resistant Staphylococcus aureus bacteraemia. Eur J Clin Microbiol Infect Dis. 2010;29(12):1475-1479.
doi pubmed - Aljefri DM, Avedissian SN, Rhodes NJ, Postelnick MJ, Nguyen K, Scheetz MH. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis. 2019;69(11):1881-1887.
doi pubmed pmc - Oda K, Yamada T, Matsumoto K, Hanai Y, Ueda T, Samura M, Shigemi A, et al. Model-informed precision dosing of vancomycin for rapid achievement of target area under the concentration-time curve: A simulation study. Clin Transl Sci. 2023;16(11):2265-2275.
doi pubmed pmc - Pongchaidecha M, Changpradub D, Bannalung K, Seejuntra K, Thongmee S, Unnual A, Santimaleeworagun W. Vancomycin area under the curve and pharmacokinetic parameters during the first 24 hours of treatment in critically ill patients using Bayesian forecasting. Infect Chemother. 2020;52(4):573-582.
doi pubmed pmc - Oda K, Hashiguchi Y, Kimura T, Tsuji Y, Shoji K, Takahashi Y, Matsumoto K, et al. Performance of area under the concentration-time curve estimations of vancomycin with limited sampling by a newly developed web application. Pharm Res. 2021;38(4):637-646.
doi pubmed - Ueda T, Takesue Y, Nakajima K, Ichiki K, Ishikawa K, Yamada K, Tsuchida T, et al. Validation of vancomycin area under the concentration-time curve estimation by the bayesian approach using one-point samples for predicting clinical outcomes in patients with methicillin-resistant staphylococcus aureus infections. Antibiotics (Basel). 2022;11(1):96.
doi pubmed pmc - Ueda T, Takesue Y, Nakajima K, Ichiki K, Doita A, Wada Y, Tsuchida T, et al. Enhanced loading regimen of teicoplanin is necessary to achieve therapeutic pharmacokinetics levels for the improvement of clinical outcomes in patients with renal dysfunction. Eur J Clin Microbiol Infect Dis. 2016;35(9):1501-1509.
doi pubmed - Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179-184.
doi pubmed - Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31-41.
doi pubmed - The Japanese Society of Chemotherapy. A clinical evaluation method for new antimicrobial agents in respiratory infection. Jpn J Chemother. 1997;45(9):762-778.
- Nakano T, Miyazaki M, Togawa A, Takata T, Mishima K, Iwasaki K, Futagami K. Evaluation of the efficacy and safety of intravenous daptomycin on infection with gram-positive cocci. Iryo Yakugaku (Jpn J Pharm Health Care Sci). 2014;40(3):147-153.
- Nakano T, Mizuno Y, Kawahira T, Ueda Y, Ikeuchi T, Kaneshige S, Ogata K, et al. Influence of empirical therapy utilizing vancomycin in patients with suspected bacterial meningitis: a single-center retrospective study. BPB Reports. 2020;3(5):150-156.
- Yasuhara M, Iga T, Zenda H, Okumura K, Oguma T, Yano Y, Hori R. Population pharmacokinetics of vancomycin in Japanese adult patients. Ther Drug Monit. 1998;20(2):139-148.
doi pubmed - Shimizu S, Nakano T, Eguchi Y, Moriwaki N, Ikeuchi T, Togawa A, Kaneshige S. Investigating the relationship between pharmacokinetics/pharmacodynamics of vancomycin and clinical outcome for Enterococcus faecium bloodstream infection: a single-center retrospective study. Iryo Yakugaku (Jpn J Pharm Health Care Sci). 2023;49(4):143-152.
- Yamada Y, Niwa T, Ono Y, Yamada S, Niwa K, Yasue M, Yamamoto T, et al. Comparison of the incidence of vancomycin-associated nephrotoxicity following the change from trough-guided dosing to AUC-guided doing using trough-only data. J Antimicrob Chemother. 2023;78(12):2933-2937.
doi pubmed - DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845.
pubmed - Nham E, Huh K, Sohn YM, Park HJ, Kim H, Woo SY, Ko JH, et al. Pharmacokinetic/pharmacodynamic parameters of vancomycin for predicting clinical outcome of enterococcal bacteremia. BMC Infect Dis. 2022;22(1):686.
doi pubmed pmc - Alsowaida YS, Kubiak DW, Dionne B, Kovacevic MP, Pearson JC. Vancomycin area under the concentration-time curve estimation using bayesian modeling versus first-order pharmacokinetic equations: a quasi-experimental study. Antibiotics (Basel). 2022;11(9):1239.
doi pubmed pmc - Claeys KC, Zasowski EJ, Casapao AM, Lagnf AM, Nagel JL, Nguyen CT, Hallesy JA, et al. Daptomycin improves outcomes regardless of vancomycin MIC in a propensity-matched analysis of methicillin-resistant staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother. 2016;60(10):5841-5848.
doi pubmed pmc - Kawasuji H, Nagaoka K, Tsuji Y, Kimoto K, Takegoshi Y, Kaneda M, Murai Y, et al. Effectiveness and safety of linezolid versus vancomycin, teicoplanin, or daptomycin against methicillin-resistant staphylococcus aureus bacteremia: a systematic review and meta-analysis. Antibiotics (Basel). 2023;12(4):697.
doi pubmed pmc - Moore CL, Osaki-Kiyan P, Haque NZ, Perri MB, Donabedian S, Zervos MJ. Daptomycin versus vancomycin for bloodstream infections due to methicillin-resistant Staphylococcus aureus with a high vancomycin minimum inhibitory concentration: a case-control study. Clin Infect Dis. 2012;54(1):51-58.
doi pubmed - Wagner P, Arnold J, Sheridan K. Vancomycin loading doses and nephrotoxicity on medicine teaching services. Int J Gen Med. 2022;15:7685-7692.
doi pubmed pmc - Hodiamont CJ, Juffermans NP, Berends SE, van Vessem DJ, Hakkens N, Mathot RAA, de Jong MD, et al. Impact of a vancomycin loading dose on the achievement of target vancomycin exposure in the first 24 h and on the accompanying risk of nephrotoxicity in critically ill patients. J Antimicrob Chemother. 2021;76(11):2941-2949.
doi pubmed pmc - Yang S, Antoniello A, Smoke S. Impact of a 20 mg/kg vancomycin loading dose on early AUC target attainment. Diagn Microbiol Infect Dis. 2024;109(4):116355.
doi pubmed - Ishigo T, Matsumoto K, Yoshida H, Tanaka H, Ibe Y, Fujii S, Fukudo M, et al. Relationship between nephrotoxicity and area under the concentration-time curve of vancomycin in critically ill patients: a multicenter retrospective study. Microbiol Spectr. 2024;12(7):e0373923.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.