| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 16, Number 11, December 2024, pages 527-535
Direct Comparison of Treatment Outcome Between the Botulinum Toxin and Calcitonin Gene-Related Peptide Monoclonal Antibody in Migraine Patients
Majed Mohammad Alabdalia, Nazish Rafiqueb, g , Deena A. AlDossaryc, Rahaf S. Alalloushc, Haya A. AlHemlic, Mohammad Zeerakd, Rabia Latifb, Lubna Ibrahim Al-Asoomb, Ahmed Abdulrahman AlSunnib, Ayad Mohammed Salemb, Mohammed Alshurema, Dana Aljaafaria, Shumaila Obaide, Aseel Alabdulhadif
aDepartment of Neurology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
bDepartment of Physiology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
cCollege of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
dKarlsruhe University of applied sciences, Karlsruhe, Germany
eFoundation University Medical College, FUIC, Islamabad
fCollege of Clinical Pharmacy, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
gCorresponding Author: Nazish Rafique, Department of Physiology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam 31441, Saudi Arabia
Manuscript submitted September 3, 2024, accepted November 13, 2024, published online November 30, 2024
Short title: BoNT and CGRP Monoclonal Antibody for Migraine
doi: https://doi.org/10.14740/jocmr6054
| Abstract | ▴Top |
Background: Migraine is a genetic disorder characterized by recurrent episodes of headache that are throbbing in nature. The objective of this study was to directly compare the efficacy and safety of anti-calcitonin gene-related peptide (anti-CGRP) and botulinum neurotoxin (BoNT) for the preventive treatment of chronic migraine.
Methods: This quasi-experimental comparative study was conducted on 80 “chronic migraine patients” at King Fahad University Hospital, Dammam, KSA. Chronic migraineurs were divided into two groups (40 patients/group) and were treated with the standard doses of BoNT (group I) and anti-CGRP (group II). All the patients filled out the migraine pain scale, migraine disability assessment score, headache impact test (HIT-6), and adverse drug event questionnaire before the start and at the end of 9 months of treatment.
Results: Most of the patients were females (76.3% vs. 23.8%) and were suffering from migraine for more than 24 months (66%). The mean age of the participants was 39.07 ± 10.01 years. Both BoNT and anti-CGRP groups showed a statistically significant decrease in mean HIT-6 and pain scores after 9 months of intervention. A direct comparison between the two treatment groups showed that the anti-CGRP drug caused a higher decrease in HIT-6 and pain scores as compared to the botulinum drug, but the difference was not statistically significant (P = 0.075 and 0.07, respectively). The most common adverse effect was “headache”, reported by 45% and 40% of patients, followed by “pain at the site of injection” reported by 27.5% and 32.5% of BoNT and anti-CGRP groups, respectively. The two groups did not differ significantly in the frequency of adverse effects such as nausea, vomiting, visual problems, etc., except “joint stiffness”. A significantly higher number of anti-CGRP patients experienced joint stiffness as compared to the BoNT group (17.5% vs. 0%, P = 0.006).
Conclusion: A direct comparison between the two treatments indicated that neither of the two interventions is statistically superior to the other in terms of efficacy and both are equally effective in the management of migraine. However, BoNT can be preferred over anti-CGRP because of its cost-effectiveness.
Keywords: Migraine; Botulinum neurotoxin; Calcitonin gene-related peptide inhibitors
| Introduction | ▴Top |
Migraine is a genetic disorder characterized by recurrent episodes of headache that is throbbing in nature. Attacks are usually provoked by light, unpleasant sounds, or certain stimuli. Patients complain of associated symptoms such as nausea, anorexia, and a sense of unsteadiness [1]. The pathophysiology of migraine is based on three observations: cortical spreading depression, activation of the trigeminal-vascular system, and peripheral and central sensitization [2]. Hormonal, biological, and genetic factors initiate the primary pathophysiology behind the aura of migraine by an initial wave of neuronal depolarization which leads to cortical activity changes and reduced blood flow. As a result, activation of the trigemino-vascular system occurs leading to the release of calcitonin gene-related peptide (CGRP) from trigeminal ganglia nerves followed by central and peripheral sensitization that is manifested by headache pain. According to the Global Burden of Disease (GBD), a study conducted in 2019 stated that the prevalence of migraine was 1.1 billion cases. It is seen more in females than males [3]. The prevalence of headaches ranged from 8% to 12% in Saudi Arabia, 72.5% in Qatar, and 83.6% in Oman [4].
Available treatment options for migraine differ depending on the severity of the attack. The keystone treatment for migraine is based on abortive and prophylactic treatment. Abortive therapy varies from simple analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) to triptans, antiemetics, CGRP inhibitors (anti-CGRP), and dihydroergotamine [5].
On the other hand, botulinum neurotoxin (BoNT) is the recommended choice for prophylactic therapy. BoNT is a protein complex extracted from a gram-positive, anaerobic bacterium called Clostridium botulinum. The mechanism of action starts after an intramuscular or subcutaneous injection. It then gets internalized by SV2 binding protein into the peripheral motor neurons and enzymatically breaks down 25 kDa synaptosomal-associated protein (SNAP-25), which is a protein that mediates the fusion of neurotransmitter-containing vesicles with the cell membrane. By this mechanism, neurotransmitter release from presynaptic nerve endings is inhibited. In addition, recent studies have also observed the role of BoNT in modifying other neurotransmitters release such as substance P and CGRP which are responsible for the transduction of pain. Thus, peripheral sensitization inhibition results in the indirect inhibition of central sensitization showing the efficacy of BoNT in treating chronic pain [6].
CGRP monoclonal antibody is a newly introduced class of drug used as a prophylactic and in the acute and chronic management of migraine. CGRP is a neuropeptide present in both peripheral and central nervous systems in two isoforms alpha and beta [7]. CGRP exerts its role in migraine causation by vasodilation of the cerebral artery and mast cell degranulation in the trigeminal neurons leading to headache pain [7]. Previously done clinical trials have proven that CGRP levels are raised in patients with migraine and, migraine attacks were evoked with exogenous administration of CGRP. The mechanism of action of CGRP inhibitors is either by antagonizing the CGRP molecules with monoclonal antibodies or, by antagonizing the CGRP receptor, thus, preventing the CGRP vasodilator and inflammatory effect and stopping the headache pain [8]. Although many aspects of CGRP inhibitors are still undiscovered in terms of their specific physiology and pharmacology, yet they showed high levels of improvement in the outcome of migraine patients. In 2019, a systemic review was conducted on 16 synthesized clinical trials to assess the effect of CGRP inhibitors on migraine patients. The study results showed a marked decrease in migraine episodes as compared to the placebo group [9].
Although there are international studies that indicate that both CGRP monoclonal antibody and BoNT are effective in the management of migraine, which of the two drugs is superior in terms of efficacy and safety still needs to be established. The currently available “Comparison of treatment outcome between botulinum toxin and CGRP monoclonal antibody in migraine patients “includes only the systematic reviews that provide indirect comparisons between these two treatment modalities. This inconclusive and inadequate data led us to design this study in which we did a direct comparison of the efficacy and safety between CGRP monoclonal antibody and BoNT for the preventive treatment of chronic migraine.
| Materials and Methods | ▴Top |
This quasi-experimental comparative study was conducted on 80 “chronic migraine patients” at King Fahad University Hospital, and College of Health Sciences, Imam Abdulrahman Bin Faisal University (IAU) Dammam, KSA. The average age of the patients was 39.07 ± 10.010 years, out of which 23.8% were males and 76.3% were females.
The sample size was calculated by using EpiTools epidemiological calculator [10, 11].
CGRP monoclonal antibody and BoNT are approved drugs, that are already being used at King Fahad University Hospital for the treatment of migraine. Therefore, a total of 80 chronic migraine patients were recruited from the Headache Clinic of the Neurology Department.
Inclusion criteria were: chronic migraine patients who were visiting the Headache Clinic of Kind Fahad Hospital and were not getting relief from their current medication and were willing to participate in the study.
Exclusion criteria were: subjects diagnosed with primary headache disorder (tension and cluster headaches), other than migraine. Moreover, the patients with migraine pain scale questionnaire (MPSQ) score less than 7, headache impact test (HIT-6) score less than 50 and migraine disability assessment (MIDAS) score less than 6 were also excluded from the study.
The patients fulfilling the inclusion criteria were subdivided into two main groups: group I (BoNT group) included 40 patients with chronic migraine who were treated with a Saudi Food and Drug Authority (SFDA)-approved standard dose of BoNT (200 units/session, session repeated after 3 months); group II (anti-CGRP group) included 40 patients with chronic migraine who were treated with an SFDA-approved standard dose of anti-CGRP (240 mg loading dose followed by 120 mg once monthly).
Before the commencement of the treatment, all the patients filled out the following three “validated standard pain questionnaires”: 1) MPSQ; 2) MIDAS; 3) HIT-6.
The MIDAS questionnaire is used to measure the impact of headaches on the lives of patients. Following is the four-point grading system for the MIDAS questionnaire: grade 1 (scores ranging from 0 to 5) as little or no disability; grade 2 (score ranging from 6 to 10) as mild disability; grade 3 (scores ranging from 11 to 20) moderate disability and finally, grade 4 (≥ 21) as severe disability [12]. The score ranges from 1 to 21, and any patient with an MIDAS score of less than 6 was excluded from the study.
HIT is a tool that helps patients to describe and communicate the way they feel, and what they cannot do because of headaches. The HIT-6 consists of six items: pain, social functioning, role functioning, vitality, cognitive functioning, and psychological distress. These responses are summed to produce a total HIT-6 score that ranges from 36 to 78, where a higher score indicates a greater impact of headache on the daily life of the respondent [13]. Any patient with a HIT-6 score of less than 50 was excluded from the study.
MPSQ is an 11-point pain scale (0 = no pain, 10 = pain as bad as it could be), which is used to measure the intensity of migraine headaches. The score ranges from 1 to 10 [14]. Any patient with an MPSQ score of less than 7 was excluded from the study.
Both the anti-CGRP and BoNT groups were treated with the approved standard doses of BoNT (200 units/session, session repeated after 3 months) and CGRP monoclonal antibody (240 mg loading dose followed by 120 mg once monthly) for 9 months.
Compliance with the medication was assured through phone calls.
After 9 months of treatment, all the patients in both groups refilled the MPSQ, MIDAS, and HIT-6 questionnaires.
The pre- and post-treatment scores of MPSQ, MIDAS, and HIT-6 questionnaires were analyzed to compare the efficiency between CGRP monoclonal antibody and BoNT.
Moreover, the patients also filled out a patient-reported adverse drug event questionnaire (PRADQ) related to the possible adverse effects of CGRP monoclonal antibody and BoNT treatment [15, 16]. This questionnaire was filled out during every follow-up visit of the patient.
All the patients signed an informed consent form, and the ethical approval was taken from the Institutional Review Board of IAU (IRB-2024-01-120). This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Data analysis
Statistical analysis was performed by Statistical Package for the Social Sciences software (IBM SPSS Statistics for Windows, Version 27, IBM Corporation, Armonk, NY, USA). Data normality was checked by the Shapiro-Wilk test. The frequencies and distributions were calculated by descriptive statistics. Comparison of continuous and categorical variables between the two groups was done by independent sample t-test and Chi-square test, respectively. Within-the-group comparison of pre- and post-treatment scores of pain scale, MIDAS scores, and HIT-6 scores was done by paired sample t-test. A P-value of < 0.05 was considered “significant” in all tests.
| Results | ▴Top |
The demographic characteristics of all study participants (N = 80) are shown in Table 1. Most of the patients were females (76.3% vs. 23.8%) and were suffering from migraine for more than 24 months (66%). The mean age of the participants was 39.07 ± 10.01 years and their migraine headache pain intensity (on a scale of 0 - 10) was 8.72 ± 1.387. The participants’ MIDAS total scores and HIT-6 total scores were 45.3 ± 46.641 and 65.30 ± 6.846, respectively. Most of the participants (85%) used various medications (other than botulinum and anti-CGRP) for an average of about 2.26 ± 2.014 years.
 Click to view | Table 1. General Characteristics of the Pain in Migraineurs (N = 80) |
Table 2 shows the comparison of demographic characteristics between the two groups before the start of the intervention (i.e., baseline). No significant difference was observed between the two groups regarding gender, age, migraine duration, pain intensity, HIT-6 scores, and previous or current use of medications. In comparison to the botulinum group, the anti-CGRP group had significantly higher MIDAS scores and more days of affected productivity due to migraine headaches (P = 0.000 and 0.015, respectively) at the baseline.
 Click to view | Table 2. Comparison of the Demographic Characteristics of Migraineurs Between the Botulinum and Anti-CGRP Groups |
Table 3 shows the results of the paired t-test (within-the-group comparison). Both groups showed a statistically significant decrease in mean HIT-6 and pain scores after 9 months of intervention (P = 0.005 and 0.000 in the botulinum group vs. 0.000 and 0.000 in the anti-CGRP group).
 Click to view | Table 3. Comparison of the MIDAS, HIT-6, and Pain Scale Scores Between the Botulinum and Anti-CGRP Groups Before and After Treatment |
Table 4 shows “between-the-group comparisons” of mean differences (pre-intervention value minus post-intervention value) and all three P-values were statistically insignificant. Although the anti-CGRP drug caused a higher decrease in HIT-6 and pain scores as compared to the botulinum drug, the difference was statistically insignificant (Fig. 1). This revealed that neither of the two interventions was statistically superior to the other in terms of efficacy and both were equally effective.
 Click to view | Table 4. Comparison Between Two Groups for Effectiveness of the Intervention |
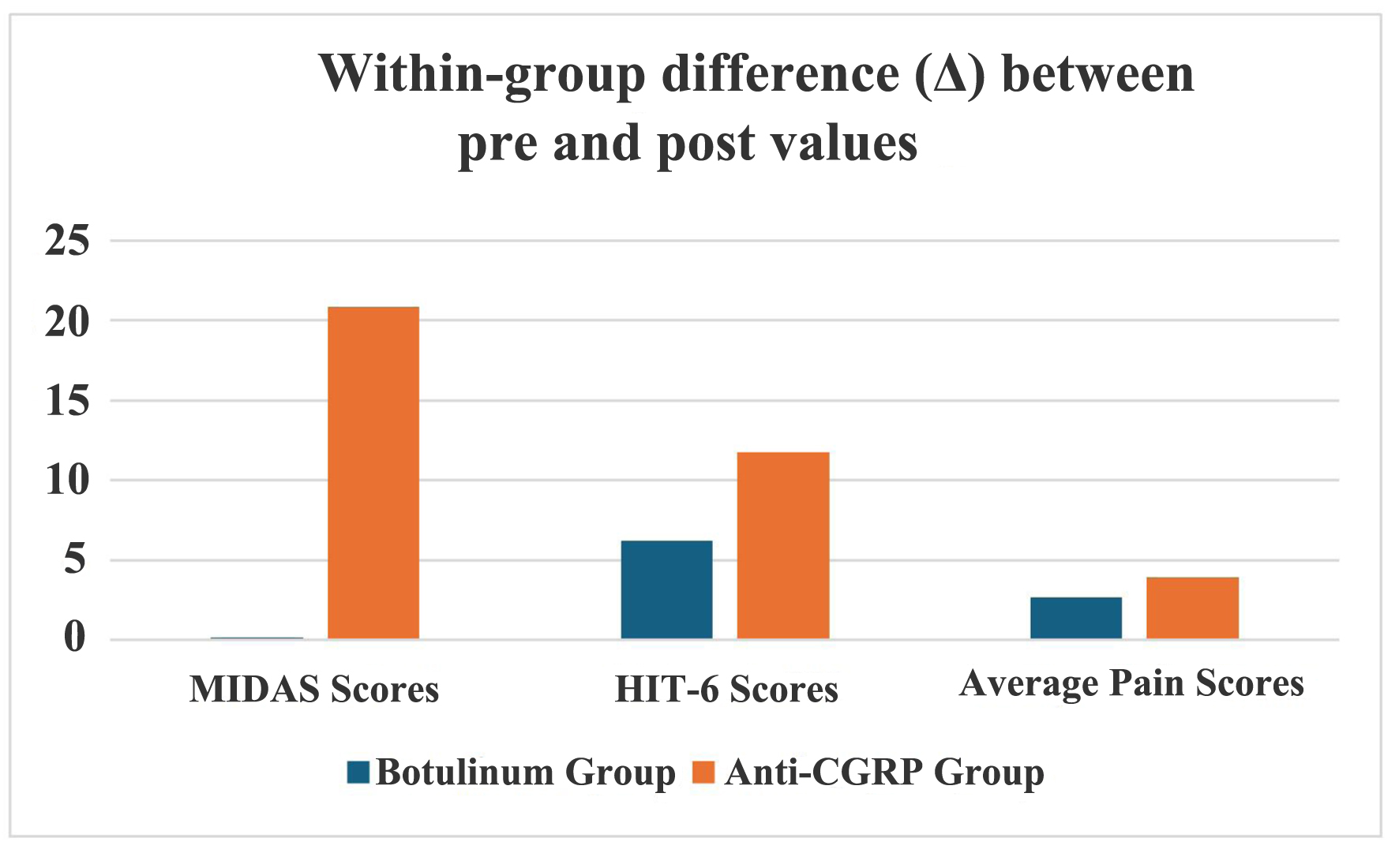 Click for large image | Figure 1. Comparison between two groups for the effectiveness of the intervention. CGRP: calcitonin gene-related peptide; HIT-6: headache impact test; MIDAS: migraine disability assessment. |
Table 5 shows the comparison between two groups of patient-reported “drug adverse effects”. The most reported adverse effect was “headache”, reported by 45% and 40% of patients in BoNT and anti-CGRP groups, respectively. This was followed by the adverse effect of “pain at the injection site” reported by 27.5% and 32.5% of patients, respectively. The two groups did not differ significantly in the frequency of patients reporting adverse effects such as nausea, vomiting, headache, visual problems, etc., except “joint stiffness”. In the anti-CGRP group, 17.5% of patients experienced “joint stiffness” in comparison to 0% of the patients in the BoNT group (Fig. 2).
 Click to view | Table 5. Comparison of Patient-Reported “Adverse Drug Reactions” Between the Botulinum and Anti-CGRP Groups |
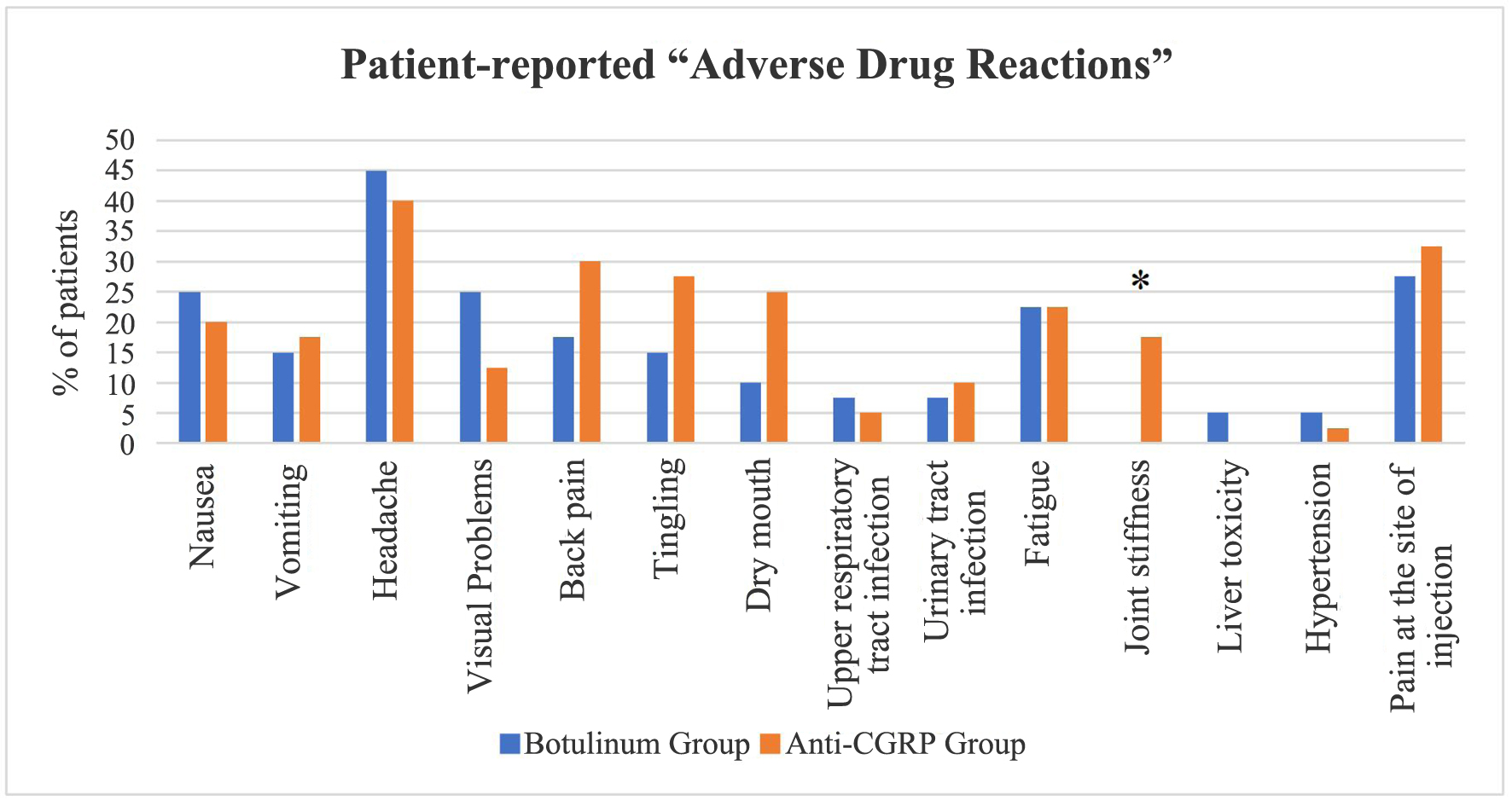 Click for large image | Figure 2. Comparison of patient-reported “adverse drug reactions” between the botulinum and anti-CGRP groups. *P-value: 0.006. CGRP: calcitonin gene-related peptide. |
| Discussion | ▴Top |
Our study results indicated that 76.3% of the participants were females which corroborates with prior research indicating that migraine headaches primarily affect. The greater frequency of migraines in females may be explained by a combination of factors, including life events, brain structure, hormones, and genetics [17, 18].
Most of our study participants (85%) used various medications (other than BoNT and anti-CGRP) for an average of about 2 years. This indicates that patients usually go to the option of BoNT or anti-CGRP after the other medications have failed. The American Headache Society released a statement in 2021 recommending the use of anti-CGRP treatments in cases where patients with high-frequency episodic migraines did not show improvement after trying two or more oral migraine prevention medications [19].
Our study results showed that both BoNT and anti-CGRP groups had a statistically significant decrease in mean HIT-6 and pain scores after 9 months of intervention, thereby indicating that both treatments are significantly effective in migraine management. Similar findings were reported in the systematic reviews by Shaterian and Muddam et al indicating that BoNT decreases the frequency of migraine episodes and enhances the quality of life of the patients [20, 21]. With regard to anti-CGRP, our study findings were supported by a review highlighting that anti-CGRP is an effective treatment in preventing and decreasing the severity of migraines [9]. Moreover, Scuteri et al also investigated the impact of anti-CGRP on 667 migraineurs revealing that anti-CGRP treatment is very effective in controlling migraine headaches [22]. Few studies have also documented that combination therapy with anti-CGRP and BoNT can provide clinically significant synergistic effects in the management of migraine [23]. But the guidelines regarding dual therapy do not currently exist for the treatment of chronic migraine. Nevertheless, monotherapy must be ruled in as a first-line prevention [24].
With regard to monotherapy, limited data are available about the “Direct comparison of treatment outcome between BoNT and CGRP monoclonal antibody in migraine patients”. In addition, the available comparisons are only the systematic reviews that provide indirect comparisons between the two treatments. Therefore, one of the major strengths of our study was that we performed a direct comparison of the mean differences between MIDAS, HIT-6 and average pain scores (pre-intervention value minus post-intervention value) between BoNT and anti-CGRP groups. Our findings revealed that, although anti-CGRP caused a higher decrease in HIT-6 and pain scores as compared to the botulinum drug, the difference was not statistically significant, thereby indicating that neither of the two interventions is statistically superior to the other in terms of efficacy and both are equally effective in the management of migraine. Our results are in line with the findings of the review article of Villarta et al where they did an indirect comparison between anti-CGRP and BoNT-A, and found that both treatments had similar effects in the prevention of migraine [10].
In contrast to our results, a systematic review by Siddiqui et al summarized that anti-CGRP was slightly superior to BoNT in terms of efficacy. However, this systematic review was based on an indirect comparison, and the authors themselves recommended direct and head-to-head trials for the exact results [25]. To date we found only one comparative study documenting the direct comparison of treatment outcomes between anti-CGRP and BoNT in migraine patients. The results of this study showed that CGRP monoclonal antibody was slightly better than BoNT in terms of efficacy and safety. But it was also documented that BoNT was cost-effective which makes it a better option in the treatment of migraine in comparison to CGRP monoclonal antibody [26].
We also compared the two groups with regard to “drug adverse effects”. The most reported adverse effect was “headache” reported by 45% and 40% of patients in the BoNT and anti-CGRP groups, respectively. Our findings are supported by other studies which indicate that headache is one of the most common side effects experienced after anti-CGRP and BoNT treatment [27]. The possible explanation for this headache can be the initial muscular spasm of the bacterial toxin, the needle effect on the periosteum or the injection stress [28].
Moreover, a majority of the patients (27.5%) (BoNT) and 32.5% (anti-CGRP) also reported having “pain at the injection site”. This finding is in accordance with the FDA Adverse Event Reporting System (FAERS) database, which highlights that among the adverse effects reported, injection site pain constitutes 14.08% of the side effects reported with CGRP and 24.37% with galcanezumab, and 13.10% with fremanezumab. The pain is usually mild and is due to the needle puncture effect [29].
Another important finding of our study was that 17.5% of anti-CGRP patients experienced “joint stiffness” in comparison to 0% of the patients in BoNT group. One of the possible explanations for this finding may be that CGRP has an anti-inflammatory/immunoregulatory role in body parts, and inhibition of the normal action of this peptide may have promoted a pro-inflammatory response leading to stiffness in the joints [30]. Further studies are recommended to find out the underlying pathophysiological reason for this finding.
To conclude, most of the patients in both our study groups were satisfied with the treatment and did not experience any major side effects, so we conclude that both treatments are safe and have minimum side effects.
To our knowledge, this is the first study in the Kingdom of Saudi Arabia that provides a direct comparison of treatment outcomes between BoNT and CGRP monoclonal antibody in migraine patients by using standard questionnaires and strict follow-up. However, the major limitation of this study was a small sample size because of the poor follow-up of the patients, we therefore recommend future studies with larger sample size, for the direct comparison between these two drugs.
Conclusion
BoNT and anti-CGRP caused a statistically significant decrease in mean HIT-6 and pain scores, thereby indicating that both treatments are significantly effective in migraine management.
A direct comparison between the two treatments indicated that neither of the two interventions is statistically superior to the other in terms of efficacy and both are equally effective in the management of migraine.
Regarding side effects, both anti-CGRP and BoNT were well tolerated by the patients and showed mild short-term adverse effects; however, 17% of the anti-CGRP group experienced joint stiffness that was not seen in the botulinum group.
Therefore, we conclude that both treatments are equally effective, and demonstrate overall safety. But BoNT can be preferred over anti-CGRP because of its cost-effectiveness.
Acknowledgments
The author is thankful to Noha Abdul Kareem, Lab technician Physiology Department at IAU, for her technical assistance during anthropometric measurements.
Financial Disclosure
It was nonfunded research.
Conflict of Interest
The authors declare no conflict of interest.
Informed Content
Complete written informed content was taken.
Author Contributions
Conceptualization: MM, NR, SO, and DA; methodology: MM, NR, RA, and HA; software: MZ and RL; validation: RL, MZ, LI, and AAA; formal analyses: AA, AS, and MZ; investigation: LI, AS, MA, DA, and AA; data curation: MZ, RA, HA, AS, NR, LI, DA, AA, MA, RL, and MM; writing - original draft preparation: MM, RA, RL, HA, and MA; writing - review and editing: MM, NR, and DA, LI; writing - literacy search: AS, MM, and AAA; supervision: NR, MM, and AAA; project administration: DA. All the authors have critically revised the manuscript and have given the final approval for this manuscript for publication. All the authors agree to be accountable for all the aspects of this work.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
Anti-CGRP: anti-calcitonin gene-related peptide; BoNT: botulinum neurotoxin; GBD: Global Burden of Disease; NSAIDs: nonsteroidal anti-inflammatory drugs; MIDAS: migraine disability assessment; HIT-6: headache impact test; MPSQ: migraine pain scale questionnaire; PRADQ: patient-reported adverse drug event questionnaire
| References | ▴Top |
- Rafique N, Al-Asoom LI, Latif R, Alsunni AA, Salem AM, Alkhalifa ZH, Almaharfi RM, et al. Prevalence of migraine and its relationship with psychological stress and sleep quality in female university students in Saudi Arabia. J Pain Res. 2020;13:2423-2430.
doi pubmed - Khan J, Asoom LIA, Sunni AA, Rafique N, Latif R, Saif SA, Almandil NB, et al. Genetics, pathophysiology, diagnosis, treatment, management, and prevention of migraine. Biomed Pharmacother. 2021;139:111557.
doi pubmed - Amiri P, Kazeminasab S, Nejadghaderi SA, Mohammadinasab R, Pourfathi H, Araj-Khodaei M, Sullman MJM, et al. Migraine: A Review on Its History, Global Epidemiology, Risk Factors, and Comorbidities. Front Neurol. 2021;12:800605.
doi pubmed - Benamer HT, Deleu D, Grosset D. Epidemiology of headache in Arab countries. J Headache Pain. 2010;11(1):1-3.
doi pubmed - Eigenbrodt AK, Ashina H, Khan S, Diener HC, Mitsikostas DD, Sinclair AJ, Pozo-Rosich P, et al. Diagnosis and management of migraine in ten steps. Nat Rev Neurol. 2021;17(8):501-514.
doi pubmed - Escher CM, Paracka L, Dressler D, Kollewe K. Botulinum toxin in the management of chronic migraine: clinical evidence and experience. Ther Adv Neurol Disord. 2017;10(2):127-135.
doi pubmed - Rashid A, Manghi A. Calcitonin gene-related peptide receptor. In: StatPearls. Treasure Island (FL). 2024.
pubmed - Mohanty D, Lippmann S. CGRP Inhibitors for Migraine. Innov Clin Neurosci. 2020;17(4-6):39-40.
pubmed - Vandervorst F, Van Deun L, Van Dycke A, Paemeleire K, Reuter U, Schoenen J, Versijpt J. CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain. 2021;22(1):128.
doi pubmed - Villarta RL Jr., Asaad AS. Sample size determination in an epidemiologic study using the EpiTools web-based calculator. Acta Medica Philippina. 2014:48(3):42-47.
- http://epitools.ausvet.com.au/content.php?page=1ProportionandProportion.
- Stewart WF, Lipton RB, Kolodner KB, Sawyer J, Lee C, Liberman JN. Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain. 2000;88(1):41-52.
doi pubmed - Yang M, Rendas-Baum R, Varon SF, Kosinski M. Validation of the Headache Impact Test (HIT-6) across episodic and chronic migraine. Cephalalgia. 2011;31(3):357-367.
doi pubmed - Kwong WJ, Pathak DS. Validation of the eleven-point pain scale in the measurement of migraine headache pain. Cephalalgia. 2007;27(4):336-342.
doi pubmed - Deen M, Correnti E, Kamm K, Kelderman T, Papetti L, Rubio-Beltran E, Vigneri S, et al. Blocking CGRP in migraine patients - a review of pros and cons. J Headache Pain. 2017;18(1):96.
doi pubmed - Binder WJ, Brin MF, Blitzer A, Schoenrock LD, Pogoda JM. Botulinum toxin type A (BOTOX) for treatment of migraine headaches: an open-label study. Otolaryngol Head Neck Surg. 2000;123(6):669-676.
doi pubmed - Al Asoom LI, Alajmi MS, Alsudairi RR, AlShamlan AA, Almomaten AA, Alqarni AA, Alshammari MH, et al. Association between sex hormones and migraine in young Saudi females. Saudi Med J. 2021;42(7):793-797.
doi pubmed - Ailani J, Burch RC, Robbins MS, Board of Directors of the American Headache S. The American headache society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021-1039.
doi pubmed - Herd CP, Tomlinson CL, Rick C, Scotton WJ, Edwards J, Ives N, Clarke CE, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database Syst Rev. 2018;6(6):CD011616.
doi pubmed - Shaterian N, Shaterian N, Ghanaatpisheh A, Abbasi F, Daniali S, Jahromi MJ, Sanie MS, et al. Botox (OnabotulinumtoxinA) for treatment of migraine symptoms: a systematic review. Pain Res Manag. 2022;2022:3284446.
doi pubmed - Muddam MR, Obajeun OA, Abaza A, Jaramillo AP, Sid Idris F, Anis Shaikh H, Vahora I, et al. Efficacy and safety of anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies in preventing migraines: a systematic review. Cureus. 2023;15(9):e45560.
doi pubmed - Scuteri D, Tonin P, Nicotera P, Vulnera M, Altieri GC, Tarsitano A, Bagetta G, et al. Pooled analysis of real-world evidence supports anti-CGRP mAbs and OnabotulinumtoxinA combined trial in chronic migraine. Toxins (Basel). 2022;14(8):529.
doi pubmed - Pellesi L, Do TP, Ashina H, Ashina M, Burstein R. Dual therapy with anti-CGRP monoclonal antibodies and botulinum toxin for migraine prevention: is there a rationale? Headache. 2020;60(6):1056-1065.
doi pubmed - Lu J, Zhang Q, Guo X, Liu W, Xu C, Hu X, Ni J, et al. Calcitonin gene-related peptide monoclonal antibody versus botulinum toxin for the preventive treatment of chronic migraine: evidence from indirect treatment comparison. Front Pharmacol. 2021;12:631204.
doi pubmed - Siddiqui M, Shah PV, Balani P, Lopez AR, Nobleza CMN, Khan S. Comparing the Efficacy, Safety, and Superiority of Calcitonin Gene-Related Peptide Monoclonal Antibodies and Botox in Preventing and Treating Migraines. Cureus. 2021;13(1):e13002.
doi pubmed - Alam M, Arndt KA, Dover JS. Severe, intractable headache after injection with botulinum a exotoxin: report of 5 cases. J Am Acad Dermatol. 2002;46(1):62-65.
doi pubmed - Witmanowski H, Blochowiak K. The whole truth about botulinum toxin - a review. Postepy Dermatol Alergol. 2020;37(6):853-861.
doi pubmed - Becker WJ. Botulinum Toxin in the Treatment of Headache. Toxins (Basel). 2020;12(12):803.
doi pubmed - Sun W, Li Y, Xia B, Chen J, Liu Y, Pang J, Liu F, et al. Adverse event reporting of four anti-Calcitonin gene-related peptide monoclonal antibodies for migraine prevention: a real-world study based on the FDA adverse event reporting system. Front Pharmacol. 2023;14:1257282.
doi pubmed - Ray JC, Allen P, Bacsi A, Bosco JJ, Chen L, Eller M, Kua H, et al. Inflammatory complications of CGRP monoclonal antibodies: a case series. J Headache Pain. 2021;22(1):121.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.