| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 16, Number 10, October 2024, pages 465-471
High Blood Glucose After Starch Loading in Young Women With Small Increase in Salivary Amylase: Another Crucial Role of Postprandial Salivary Amylase
Airi Sekinea , Kei Nakajimaa, b, c
aFood and Nutrition, Faculty of Human Sciences and Design, Japan Women’s University, Bunkyo-ku, Tokyo 112-8681, Japan
bSaitama Medical Center, Department of Endocrinology and Diabetes, Saitama Medical University, Kawagoe 350-8550, Japan
cCorresponding Author: Kei Nakajima, Food and Nutrition, Faculty of Human Sciences and Design, Japan Women’s University, Bunkyo-ku, Tokyo 112-8681, Japan
Manuscript submitted September 4, 2024, accepted October 14, 2024, published online October 17, 2024
Short title: Salivary Amylase and BG After Starch Loading
doi: https://doi.org/10.14740/jocmr6057
| Abstract | ▴Top |
Background: Salivary α-amylase plays a crucial role in the glucose metabolism. However, postprandial salivary α-amylase activity (SAA) and its relationship with blood glucose (BG) are poorly understood. Therefore, we investigated SAA and BG after starch loading in healthy young women.
Methods: In 60 healthy non-obese young women, we investigated SAA, BG, and blood 3-hydroxybutyrate (3HB) after the consumption of 150 g rice (starch 48.8 g). Participants were classified into two groups based on the changes (Δ) in SAA from baseline at 60 min: small- and large-increase in ΔSAA groups (SI-ΔSAA and LI-ΔSAA).
Results: BG levels were significantly higher at 60, 90, and 120 min in participants with SI-ΔSAA (n = 31) than LI-ΔSAA (n = 29). Baseline 3HB concentration was also higher in participants with SI-ΔSAA. ΔSAA at 60 min was most closely and inversely correlated with BG and ΔBG at 90 min (r = -0.53 and -0.50, both P < 0.0001). Generalized linear model analysis also indicated that ΔSAA at 60 min was the most predictive of ΔBG at 90 min.
Conclusions: Higher levels of BG and ΔBG were observed after starch loading in healthy young women with smaller increase in salivary amylase, suggesting another crucial role of postprandial salivary amylase for the postprandial glucose metabolism.
Keywords: Salivary amylase; Blood glucose; Starch loading; 3-hydroxybutyrate
| Introduction | ▴Top |
Salivary α-amylase (EC 3.2.1.1), a major enzyme in the oral cavity in man, facilitates carbohydrate digestion; its activity is of high inter-individual and intra-individual variability [1]. Although the physiological functions of salivary α-amylase have not been fully explored, salivary α-amylase activity (SAA) affects glucose metabolism and consequently contributes to the incidents of type 2 diabetes and obesity [2, 3]. However, there are conflicting results about the relationship between fasting SAA and glucose metabolism [4, 5]. We previously [6] found no relationship between fasting oral SAA and blood glucose (BG), along with their changes from baseline, after starch loading. Many studies have investigated exclusively fasting but not postprandial levels of SAA and serum α-amylase activity [4, 5, 7, 8].
Meanwhile, hyperglycemia 1 h after the meal is commonly observed in individuals with impaired glucose metabolism and early type 2 diabetes [9, 10]. Nevertheless, postprandial oral SAA, for example 1 h after starch ingestion, and its relationship with BG has not been investigated [2, 4, 5, 6], although there is barely any starch left in the oral cavity 1 h after meal consumption. Based on these backgrounds, we investigated the relationship between SAA, BG, and their changes from baseline after starch loading in healthy young women, which can elucidate clinical relevance of postprandial SAA. From the perspective of energy metabolism, we also investigated 3-hydroxybutyrate (3HB), a ketone body endogenously yield from fatty acid substrates in the liver, and respiratory quotient (RQ) because 3HB is often observed during glucose starvation [11] and RQ is commonly used to speculate which substrates are oxidized for the energy [12].
| Materials and Methods | ▴Top |
We recruited 60 young, healthy, Japanese female university students who had undergone regularly a checkup aged 20 - 23 years in the first half of 2023; all participants were non-smokers, non-heavy drinkers, nonpregnant, and had a normal body mass index (BMI) (< 25.0 kg/m2). Participants with metabolic disorders including diabetes, prediabetes, and dyslipidemia, regardless of primary or secondary, were not included, which was individually confirmed by face-to-face interview.
In the morning following a 12-h overnight fast, anthropometric and laboratory measurements were performed after the rest sitting for 5 min in our laboratory of university. Participants were free to drink water until 30 min before the measurements. Before and after starch loading with a 150 g ordinary white rice (energy 209 kcal, starch 48.8 g, protein 2.9 g, fat 0 g) prepared using cooked rice pack [13], saliva samples (0, 60, and 120 min) were collected using an oral swab (SalivaBio Oral Swabs, Salimetrics, Carlsbad, USA).
The swab was centrifuged for 15 min at 1,500 × g to extract saliva. SAA was duplicately measured using a kinetic reaction assay (Salivary alpha-Amylase Assay Kit, Salimetrics) exclusively for salivary amylase with the assay range of 2.0 to 400 U/mL [14]. This method utilizes a chromogenic substrate, 2-chloro-p-nitrophenol linked with maltotriose. The enzymatic action of SAA yields 2-chloro-p-nitrophenol, which responds to spectrophotometrically measured at 405 nm. The reaction was read in a 96-well microtiter plate with controls provided in the kit. SAA was calculated using the following equation [14].
(ΔAbs./min: absorbance difference per minute; TV: total assay volume (0.328 mL); DF: dilution factor; MMA: millimolar absorptivity of 2-chloro-p-nitrophenol (12.9); SV: sample volume (0.008 mL); LP: light path = 0.97 (specific to plate received with kit).
Saliva total protein was measured using a bicinchoninic acid assay protein assay (Takara BCA Protein Assay Kit, Takara Bio Inc., Shiga, Japan). BG (0, 30, 60, 90, and 120 min) and blood 3HB concentration (0 and 120 min) were measured in finger-prick blood samples using a StatStrip Xpress2 Glucose/Ketone Meter (Nova Biomedical Corporation, Waltham, USA). Dietary intake was estimated using designated computer software (FFQNEXT, Kenpakusha, Tokyo, Japan) based on the Standard Tables of Food Composition [15].
To estimate RQ, respiratory monitoring for 5 min at rest was conducted after the adequate stabilization using an AR-1 portable gas monitor (ARCO SYSTEM Inc., Japan).
For each sample, SAA was adjusted for salivary protein concentration. From measurements taken at different time points, changes in SAA and BG from baseline were calculated as delta (Δ) values, and areas under the curve (AUC) for BGs were calculated using the trapezoid rule [16].
Participants were classified into two groups based on protein-adjusted SAA changes from baseline (ΔSAA) at 60 min: small- and large-increase in ΔSAA groups (SI-ΔSAA and LI-ΔSAA), considering that maximum BG often occurs 1 h after glucose or starch load in healthy people [9, 10].
The differences in BGs, AUCs, and 3HB between these two groups were tested by t-test or non-parametric test. Relationships between variables were assessed by Spearman’s correlation analysis because of the high likelihood of non-parametric distributions in the parameters.
A generalized linear model controlling for BMI, baseline SAA, BG, and 3HB was used to analyze the associations for ΔBG. In our study, the sample size was calculated using Easy R software [17], based on the assumption of mean and standard deviation (SD) of BG at 60 min, α, and effect as 20 mg/dL, 25 mg/dL, 5%, and 80%, respectively, to test the difference in postprandial BG at 60 min between SI-ΔSAA and LI-ΔSAA groups. Then, a total of around 25 subjects were needed for each group. Statistical analyses were performed using SAS-Enterprise Guide in the SAS system, version 9.4 (SAS Institute, Cary, USA). A two-tailed P value < 0.05 was considered significant.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the Ethics Committee of Japan Women’s University (ID number 575).
| Results | ▴Top |
The characteristics of the participants are shown in Table 1; all of the parameters were within normal ranges. SAA levels at 60 and 120 min were higher than baseline (both P < 0.0001), but no difference was observed between at 60 and 120 min. The mean BMI (19.8 kg/m2), energy intake (1,666 ± 33 kcal/day), and carbohydrate intake (59.7±0.7% of energy intake) were relatively low.
 Click to view | Table 1. Characteristics of Participants |
Participants were classified into two groups: LI-ΔSAA (≥ 28 U/mL, n = 29) and SI-ΔSAA (< 28 U/mL, n = 31) considering the value of ΔSAA at 60 min (median (interquartile range (IQR)): 27.8 (17.1 - 41.8) U/mL). No difference was observed in parameters listed in Table 1 except for SAA at 60 and 120 min and BG at 60, 90, and 120 min, AUC and 3HB between LI-ΔSAA and SI-ΔSAA groups.
Although AUCs was higher in SI-ΔSAA group, statistical significance was not marginally found (P = 0.051). Baseline 3HB was also higher in SI-ΔSAA group (median (IQR): 0.20 (0.0 - 0.40)) than LI-ΔSAA (0.0 (0.0 - 0.10), P < 0.001).
The levels of SAA increased over time in both LI-ΔSAA and SI-ΔSAA groups (Fig. 1) (analysis of variance, P < 0.0001 and P = 0.003). BG levels at 60, 90, and 120 min were significantly higher in SI-ΔSAA group than LI-ΔSAA group (Fig. 2).
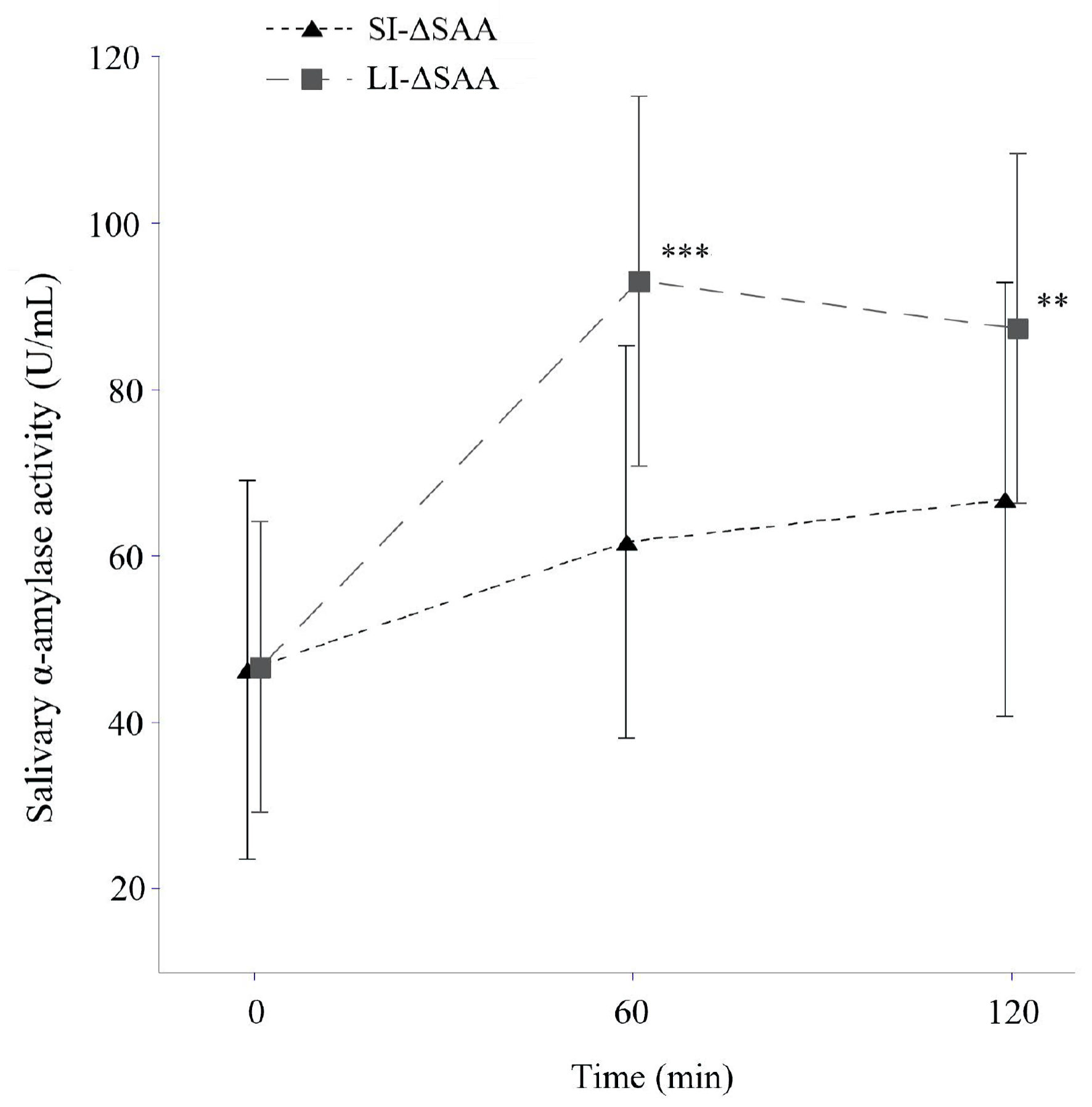 Click for large image | Figure 1. Salivary amylase levels after starch loading according to SI- and LI-ΔSAA (means ± standard deviation). **P < 0.001, ***P < 0.0001 vs. SI-ΔSAA. LI-ΔSAA: ΔSAA at 60 min ≥ 28 IU/L (n = 29); SI-ΔSAA: ΔSAA at 60 min < 28 IU/L (n = 31). SAA: salivary α-amylase activity; LI-ΔSAA: large-increase in ΔSAA; SI-ΔSAA: small-increase in ΔSAA. |
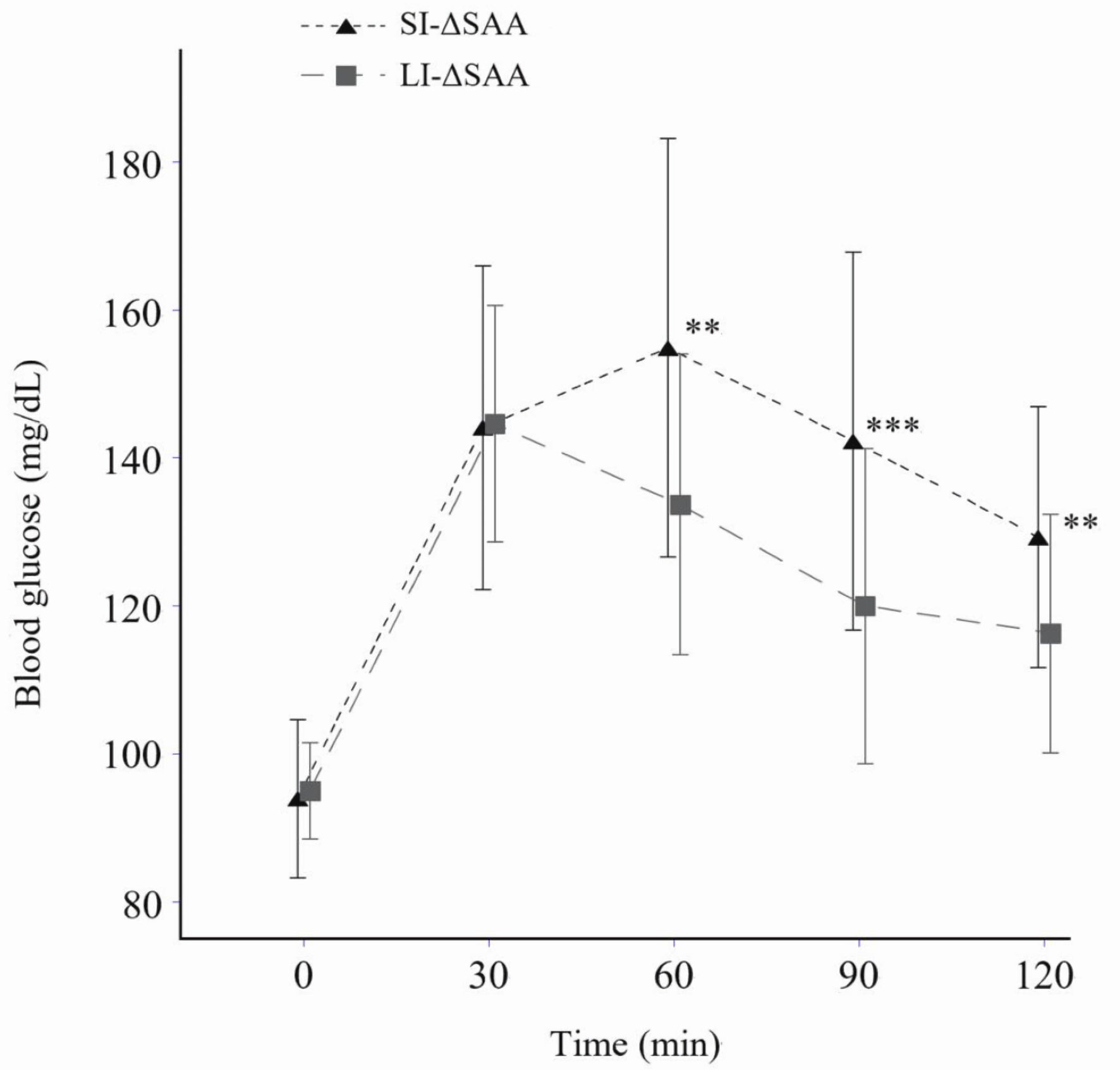 Click for large image | Figure 2. Blood glucose levels after starch loading according to SI- and LI-ΔSAA (means ± standard deviation). **P < 0.01, ***P < 0.001 vs. LI-ΔSAA. No significant difference in areas under the curve was observed between SI-ΔSAA (mean ± standard deviation: 53.1 ± 23.3 mg·min/dL) and LI-ΔSAA (43.5 ± 11.6 mg·min/dL) (P = 0.051). LI-ΔSAA: ΔSAA at 60 min ≥ 28 IU/L (n = 29); SI-ΔSAA: ΔSAA at 60 min < 28 IU/L (n = 31). SAA: salivary α-amylase activity; LI-ΔSAA: large-increase in ΔSAA; SI-ΔSAA: small-increase in ΔSAA. |
ΔSAAs were inversely correlated with BG at 60, 120, and particularly 90 min (r = -0.53 for ΔSAA60 and r = -0.46 for ΔSAA120), and also with ΔBG at 60, 120, and particularly 90 min (r = -0.50 for ΔSAA60 and r = -0.43 for ΔSAA120) (Table 2). Baseline 3HB was inversely correlated with SAA and ΔSAA at 60 min. Furthermore, 3HB at 120 min was inversely correlated with baseline SAA (r = -0.34).
 Click to view | Table 2. Correlations Between SAA, BG Concentrations, Their Changes From Baseline, and Blood 3HB Levels Across Participants |
Although baseline SAA was significantly correlated with SAA at 60 and 120 min (r = 0.69 and 0.63, both P < 0.0001), it was not correlated with ΔSAA at 60 and 120 min (r = -0.05 and -0.11, data not shown). Consistent with this, baseline SAA was not significantly correlated with BG or ΔBG at any timepoint (Table 2). Although RQ at baseline was inversely correlated with 3HB at baseline (r = -0.37, P = 0.004), RQ was not correlated with BG and SAA at any time points (data not shown).
The results of generalized linear model analysis showed that ΔSAA at 60 min was significantly inversely associated with ΔBG at 60 and 90 min (Table 3) and had the strongest predictive value (largest β regression coefficient (β = -0.31 and -0.35) and Wald Chi-square value (6.78 and 8.98)) among relevant variables.
 Click to view | Table 3. Generalized Liner Model of Variables for ΔBGs |
| Discussion | ▴Top |
Our study is the first to show higher BG levels after starch loading in healthy young women with smaller increase in salivary amylase, suggesting another crucial role of postprandial salivary amylase for the postprandial glucose metabolism.
Results of postprandial glucose metabolism according to low and high SAA were shown in previous study, although postprandial SAA was not measured [2]. The previous study also showed “cephalic phase insulin response” in high SAA participants, which could prevent postprandial high BGs. Unfortunately, insulin measurement was not conducted in this study. Therefore, further studies including hormone measurements are needed to elucidate the underlying mechanism.
Alternatively, higher 3HB at baseline, but not at 120 min, in SI-ΔSAA participants may suggest that lipids utility for the energy was increased by nature and was not prepared for glucose usage in individuals with smaller increase in salivary amylase, linking to subsequent high BGs, which is consistent with our previous study [18]. Furthermore, inverse correlation between baseline SAA and 3HB at 120 min suggests that individuals with high SAA at baseline utilize much glucose for the energy and produce less ketone during the starch loading.
Interestingly, no difference was observed in baseline SAA between LI- and SI-ΔSAA groups. In addition, baseline SAA was not correlated with ΔSAA at 60 and 120 min, suggesting that baseline SAA is a poor marker for subsequent glucose metabolism compared with postprandial SAA.
In general, maximum BG occurs around 1 h post-glucose or starch ingestion in healthy people and those with early type 2 diabetes [9, 10], which is consistent with current results.
However, in our study, SAA and ΔSAA at 60 min were most inversely correlated with BG and ΔBG at 90 min, but not at 60 min, suggesting that SAA 1 h postprandially can relate with the postprandial glucose metabolism probably with some time lag. Unfortunately, the underlying mechanism of SAA 1 h postprandially, when the oral cavity is empty, on BG metabolism was unclear.
Several limitations should be mentioned in this study. First, all participants were apparently healthy university students who underwent regular checkups including urinalysis, but not blood test for biochemical parameters. Therefore, individuals with latent endocrine disease were not ruled out completely in this study. Second, it is unclear whether participants under menstruation were included in this study. Third, hormones such as insulin, glucagon, glucagon like peptide 1 were not measured in this study, which does not allow us to speculate the underlying mechanism. Finally, different results may be observed in patients with type 2 diabetes, whose maximum BG occurs later (around 2 h postprandially) [10, 19], because participants’ BMI and energy intake were relatively low in this study, albeit these values were within normal ranges for young Japanese women [20].
In conclusion, we found higher BG levels after starch loading in healthy young women with smaller increase in oral salivary amylase, suggesting another crucial role of postprandial salivary amylase for the postprandial glucose metabolism.
Acknowledgments
We thank eight of our university students for helping the measurements for BG and 3HB, and for research on dietary intake.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Written informed consent was obtained from each participant.
Author Contributions
AS measured salivary amylase activity and protein concentrations. KN and AS contributed to interpretation of the initial analysis and discussion of the literature. KN prepared the first draft of the manuscript. All authors read and approved the final version of the manuscript and agreed to the published version.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
AUC: areas under the curve; SAA: salivary α-amylase activity; BG: blood glucose; BMI: body mass index; ΔSAA: SAA changes from baseline (protein-adjusted); 3HB: 3-hydroxybutyrate; LI-ΔSAA: large-increase in ΔSAA; SI-ΔSAA: small-increase in ΔSAA; RQ: respiratory quotient
| References | ▴Top |
- Boehlke C, Zierau O, Hannig C. Salivary amylase - The enzyme of unspecialized euryphagous animals. Arch Oral Biol. 2015;60(8):1162-1176.
doi pubmed - Mandel AL, Breslin PA. High endogenous salivary amylase activity is associated with improved glycemic homeostasis following starch ingestion in adults. J Nutr. 2012;142(5):853-858.
doi pubmed - Elder PJD, Ramsden DB, Burnett D, Weickert MO, Barber TM. Human amylase gene copy number variation as a determinant of metabolic state. Expert Rev Endocrinol Metab. 2018;13(4):193-205.
doi pubmed - Ko J, Cho J, Petrov MS. Low serum amylase, lipase, and trypsin as biomarkers of metabolic disorders: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2020;159:107974.
doi pubmed - Perez-Ros P, Navarro-Flores E, Julian-Rochina I, Martinez-Arnau FM, Cauli O. Changes in salivary amylase and glucose in diabetes: a scoping review. Diagnostics (Basel). 2021;11(3):453.
doi pubmed - Higuchi R, Iwane T, Iida A, Nakajima K. Copy number variation of the salivary amylase gene and glucose metabolism in healthy young Japanese women. J Clin Med Res. 2020;12(3):184-189.
doi pubmed - Ittichaicharoen J, Phrommintikul A, Chattipakorn N, Chattipakorn S. Reduced salivary amylase activity in metabolic syndrome patients with obesity could be improved by treatment with a dipeptidyl peptidase IV inhibitor. Clin Oral Investig. 2018;22(9):3113-3120.
doi pubmed - Jalal M, Gbadegesin SA, Tehami N, Nakajima K. What is the clinical significance of low serum amylase? Systematic review of the conditions associated with low serum amylase. Frontline Gastroenterol. 2024;15(2):154-161.
doi pubmed - Trico D, Mengozzi A, Frascerra S, Scozzaro MT, Mari A, Natali A. Intestinal glucose absorption is a key determinant of 1-hour postload plasma glucose levels in nondiabetic subjects. J Clin Endocrinol Metab. 2019;104(6):2131-2139.
doi pubmed - Bergman M, Manco M, Satman I, Chan J, Schmidt MI, Sesti G, Vanessa Fiorentino T, et al. International Diabetes Federation Position Statement on the 1-hour post-load plasma glucose for the diagnosis of intermediate hyperglycaemia and type 2 diabetes. Diabetes Res Clin Pract. 2024;209:111589.
doi pubmed - Newman JC, Verdin E. beta-Hydroxybutyrate: a signaling metabolite. Annu Rev Nutr. 2017;37:51-76.
doi pubmed - Price ER, Mager EM. Respiratory quotient: effects of fatty acid composition. J Exp Zool A Ecol Integr Physiol. 2020;333(9):613-618.
doi pubmed - Available at: https://www.satosyokuhin.co.jp/en/en_rice_1044.html. July 12th, 2024.
- Available at: https://salimetrics.com/wp-content/uploads/2018/03/alpha-amylase-saliva-elisa-kit.pdf. July 12th, 2024.
- Yokoyama Y, Takachi R, Ishihara J, Ishii Y, Sasazuki S, Sawada N, Shinozawa Y, et al. Validity of short and long self-administered food frequency questionnaires in ranking dietary intake in middle-aged and elderly Japanese in the Japan Public Health Center-Based Prospective Study for the Next Generation (JPHC-NEXT) protocol area. J Epidemiol. 2016;26(8):420-432.
doi pubmed - Wolever TM, Jenkins DJ. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr. 1986;43(1):167-172.
doi pubmed - Kanda Y. [Statistical analysis using freely-available "EZR (Easy R)" software]. Rinsho Ketsueki. 2015;56(10):2258-2266.
doi pubmed - Nakajima K, Higuchi R, Iwane T, Iida A. The association of low serum salivary and pancreatic amylases with the increased use of lipids as an energy source in non-obese healthy women. BMC Res Notes. 2020;13(1):237.
doi pubmed - Wang X, Zhao X, Zhou R, Gu Y, Zhu X, Tang Z, Yuan X, et al. Delay in glucose peak time during the oral glucose tolerance test as an indicator of insulin resistance and insulin secretion in type 2 diabetes patients. J Diabetes Investig. 2018;9(6):1288-1295.
doi pubmed - Ministry of Health, Labour and Welfare: Results of the National Health and Nutrition Survey Japan 2019. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/eiyou/r1-houkoku_00002.html. Available on May15th, 2024.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.