| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Case Report
Volume 16, Number 9, September 2024, pages 440-448
An Autopsy Case of Renal-Limited Granulomatosis With Polyangiitis Presenting With Acute Renal Failure and Initial Delirium
Syuichi Tetsukaa, c , Tomohiro Suzukia, Tomoko Ogawaa, Yoh Dobashib, Ritsuo Hashimotoa
aDepartment of Neurology, International University of Health and Welfare Hospital, 537-3, Iguchi, Nasushiobara, Tochigi 329-2763, Japan
bDepartment of Pathology, International University of Health and Welfare Hospital, 537-3, Iguchi, Nasushiobara, Tochigi 329-2763, Japan
cCorresponding Author: Syuichi Tetsuka, Department of Neurology, International University of Health and Welfare Hospital, 537-3, Iguchi, Nasushiobara, Tochigi 329-2763, Japan
Manuscript submitted July 22, 2024, accepted August 29, 2024, published online September 4, 2024
Short title: Autopsy of Granulomatosis With Polyangiitis
doi: https://doi.org/10.14740/jocmr5273
| Abstract | ▴Top |
Granulomatosis with polyangiitis (GPA) has three clinicopathological features, namely, necrotizing granulomatosis of the upper respiratory tract and lungs, focal segmental necrotizing glomerulonephritis of the kidney, and necrotizing vasculitis of small vessels throughout the body. A 92-year-old man with clinically diagnosed probable Alzheimer’s disease (AD) exhibited subacute deterioration in cognitive function. On admission, he was diagnosed with acute renal failure with an elevated creatinine level (5.48 mg/dL) as well as severe disturbance of consciousness. Antineutrophil cytoplasmic antibodies (ANCAs) directed against proteinase 3 (PR3-ANCA) were highly positive with ≥ 350 U/mL. The patient was diagnosed with GPA and was managed with steroid pulse therapy. However, he died without any improvement in renal function. As a result of the autopsy, the patient was diagnosed with definite AD, and his impaired consciousness was found not to be caused by central nervous system involvement due to GPA. As necrotizing crescentic glomerulonephritis was observed, the cause of the acute progressive renal failure was found to be PR3-ANCA-positive GPA. The autopsy revealed no GPA-related lesions in other parts of the body aside from the kidneys. It is rare to encounter cases of PR3-ANCA-positive GPA with renal-limited vasculitis and acute renal failure as the initial manifestation, as in the present case. Making an accurate clinical diagnosis of older patients suffering from various diseases in multiple organs is challenging. Although autopsy has the limitation of a terminal image, it is extremely useful in elucidating the pathophysiology of the older patient in this case.
Keywords: Alzheimer’s disease; Delirium; Granulomatosis with polyangiitis; Renal-limited; PR3-ANCA
| Introduction | ▴Top |
Granulomatosis with polyangiitis (GPA) is a type of vasculitis syndrome in which inflammation occurs in the walls of small vessels. It is classified as an antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. This disease was first reported by the German pathologist Friedrich Wegener in 1938, hence the former name Wegener’s granulomatosis. However, at the 2012 Chapel Hill Consensus Conference, the disease was renamed GPA due to the new international classification criteria based on pathogenesis [1]. GPA is a refractory vasculitis with three clinicopathological features: 1) necrotizing granulomatosis of the upper respiratory tract (nose, pharynx, and sinuses) and lungs, 2) focal segmental necrotizing glomerulonephritis of the kidney, and 3) necrotizing vasculitis of small vessels throughout the body. ANCAs directed against proteinase 3 (PR3-ANCA) is a disease-labeling antibody for ANCA-associated vasculitis. PR3-ANCA is present in the majority of patients with GPA and appears to be extremely specific [2, 3]. The titer of PR3-ANCA easily parallels GPA severity and is a predictor of relapse and disease severity [2]. The involvement of the central nervous system (CNS) due to systemic secondary CNS vasculitis, such as cognitive impairment, consciousness disturbance, headache, and visual disturbance, may not be a major symptom of ANCA-associated vasculitis [4, 5]. However, the frequency of CNS involvement in all cases of ANCA-associated vasculitis is reportedly 11 times higher than that in the general population [6].
We have recently experienced an autopsy case of an older patient with Alzheimer’s disease (AD) who initially presented with cognitive impairment and later developed rapidly progressive glomerulonephritis with highly elevated PR3-ANCA. Thus, we herein report the clinical course and autopsy results of this patient.
| Case Report | ▴Top |
Approximately 2 months before admission, a 92-year-old man with a history of chronic heart failure and hypertension presented to our hospital due to a gradual progressive deterioration of cognitive dysfunction, such as recent memory impairment and disorientation. There was no history of smoking or alcohol consumption, and no notable family history of hereditary diseases. Comprehensive neuropsychological tests were conducted to assess his cognitive function. A decline of 17/30 points was observed in the mini-mental state examination. The clinical dementia rating was 2.0, and the functional assessment staging of AD was stage 5. On the fluid-attenuated inversion recovery (FLAIR) images of brain magnetic resonance imaging (MRI), significant brain atrophy was detected predominantly in the hippocampus and temporal lobes, suggesting AD (Fig. 1a). The patient’s blood thyroid hormone and vitamin B levels were within the normal range. He was diagnosed with probable AD, meeting the clinical diagnostic criteria, and denying any organic disease that would accompany other dementias. His laboratory results are shown in Table 1. Blood examination at this time revealed a serum creatinine (Cr) level of 1.34 mg/dL, suggesting mild renal dysfunction, but hematuria was not observed.
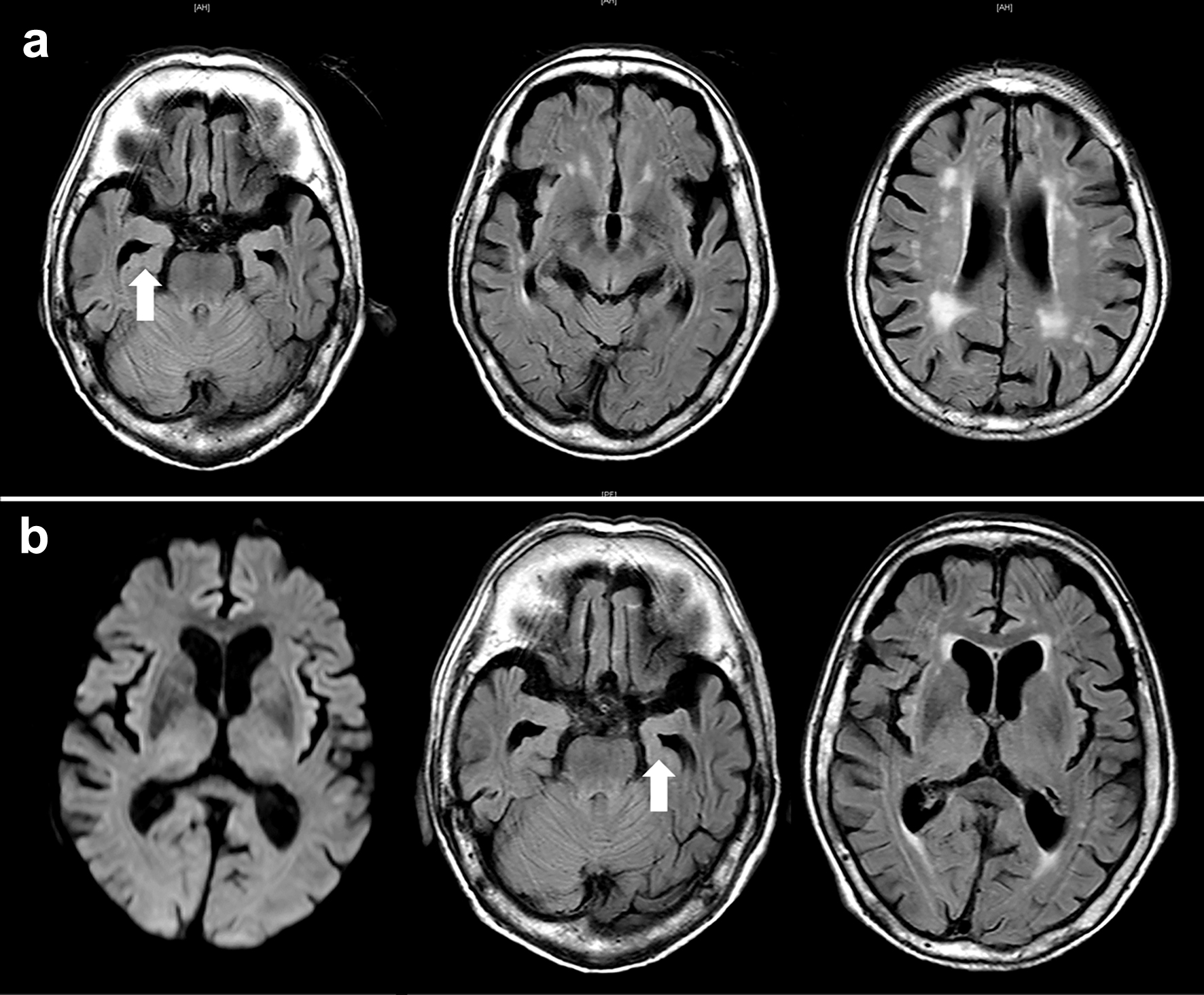 Click for large image | Figure 1. Axial view of brain magnetic resonance imaging (MRI). (a) Approximately 2 months before admission, fluid-attenuated inversion recovery images show atrophy of the brain cortex with significant hippocampal atrophy in the medial temporal lobe, indicating Alzheimer’s disease. Mild periventricular white matter lesions are observed. (b) MRI image of the brain on admission. Diffusion-weighted imaging shows no apparent abnormalities. FLAIR images also show findings similar to the previous ones. No lesions in the brain are observed that would cause impaired consciousness. The arrows indicate hippocampal atrophy. |
 Click to view | Table 1. Laboratory Data |
Approximately 2 weeks before admission, he began showing symptoms consistent with delirium, including incoherence, hallucination, and disorientation. These symptoms also seemed to indicate behavioral and psychological symptoms of dementia (BPSD). The patient stopped eating. Eventually, he developed a worsening consciousness disorder and was urgently admitted to our hospital. His level of consciousness was E1V1M2 on the Glasgow coma scale, indicating severe consciousness impairment. His initial vital signs were as follows: heart rate, 63 beats/min; respiratory rate, 24 breaths/min; blood pressure, 156/77 mm Hg; body temperature, 36.7 °C; and oxygen saturation, 97% with room air. He had a height of 147.3 cm, a body weight of 45.6 kg, and a body mass index of 21.0 kg/m2. Furthermore, gross hematuria and anemia in the palpebral conjunctiva were observed. Diffusion-weighted and FLAIR images of MRI depicted no apparent abnormalities (Fig. 1b). Blood counts showed a decrease in hemoglobin to 6.8 g/dL, possibly due to hematuria. Renal dysfunction was detected with elevated levels of serum Cr (5.48 mg/dL) and blood urea nitrogen (BUN, 73.8 mg/dL) (Table 1). In electrolyte concentrations, serum potassium was elevated at 5.5 mmol/L. Based on the presence of renal dysfunction and heart failure (N-terminal pro-B-type natriuretic peptide 1,052 pg/mL) detected through laboratory investigations on admission, a thoracic abdominal computed tomography (CT) scan was performed (Fig. 2). A chest CT scan revealed no sign of lesions in the lung, but an enlarged left atrium and congestion were found in the heart (Fig. 2a, b). On abdominal CT scan, no obvious abnormal lesions in the kidneys or urinary system organs causing postrenal failure were observed (Fig. 2c, d). Initially, the patient was managed with fluid therapy, considering the possibility of prerenal renal failure due to dehydration. However, his condition did not improve. On laboratory investigations 1 week after admission, serological tests revealed elevated PR3-ANCA levels (≥ 350 U/mL; normal, < 3.5 U/mL) with negative myeloperoxidase (MPO)-ANCA (Table 1). Based on these findings, the patient was diagnosed with probable GPA. However, his renal function acutely deteriorated within 1 week, with Cr of 9.82 mg/dL and BUN of 126.0 mg/dL (Table 1). Subsequently, the treatment was commenced for possible GPA with a high-dose intravenous methylprednisolone (1,000 mg/day) for 3 days as steroid pulse therapy. As aforementioned, the patient was extremely old and suffering from moderate AD. Thus, hemodialysis therapy could not be performed. The family also did not wish for further treatment and decided to follow up with the best support care. Approximately 10 days after admission, the patient became anuric, went into cardiac arrest, and died. The timeline of this case is shown in Figure 3.
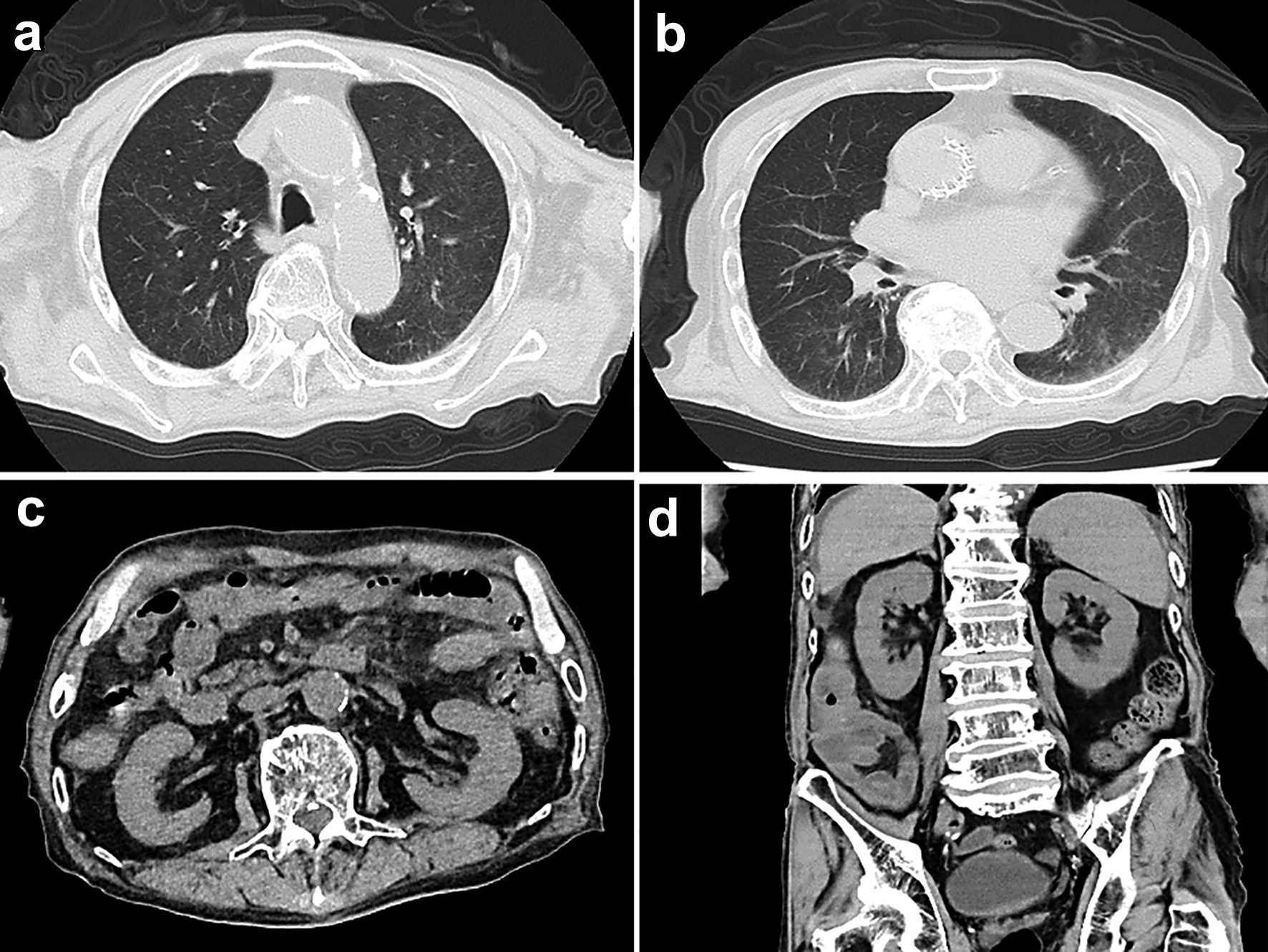 Click for large image | Figure 2. Computed tomography (CT) scan images of the thorax and abdomen on admission. (a, b) Chest CT scan shows no signs of lesion in both lungs, but an enlarged left atrium and congestion in the heart are observed. (c, d) CT scan of the abdomen shows no obvious abnormal findings such as mass lesions or ascites. |
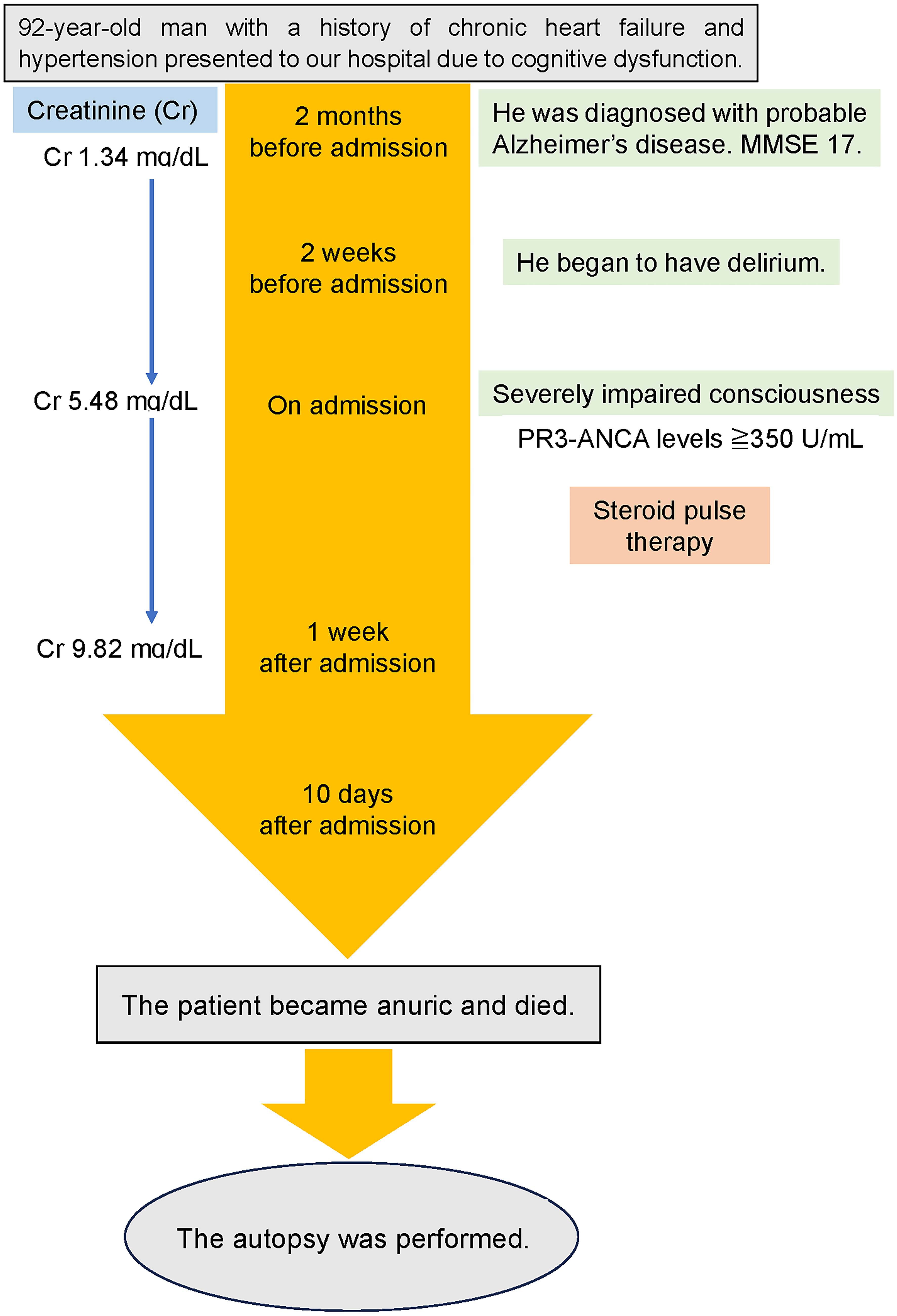 Click for large image | Figure 3. Timeline of this case report. MMSE: mini-mental state examination. |
Autopsy findings
The questionable points in the clinical course of this case are as follows. 1) Was the patient’s cognitive impairment caused by AD? 2) Did the CNS involvement by GPA affect consciousness disturbance and decline in cognitive function? 3) Was GPA the cause of the acute renal failure with strong PR3-ANCA positivity? 4) Assuming that it is GPA, are there histological findings in other organs aside from the kidneys? Considering the aforementioned points, an autopsy was performed with the consent of the family.
Brain
Upon gross autopsy examination, the fresh brain weighed 1,015 g. Mild atrophy was observed in the temporal lobe. Histologically, senile plaques were found in the parietal lobe and hippocampus; neurofibrillary tangles in the putamen, parietal lobe, and hippocampus; and neuropil threads in the frontal lobe (Fig. 4). These findings are consistent with the histopathological features of AD. There was no direct expansion of granulomatous processes, vasculitis of cerebral small vessels, and direct formation of granulomas within the brain parenchyma, suggesting GPA.
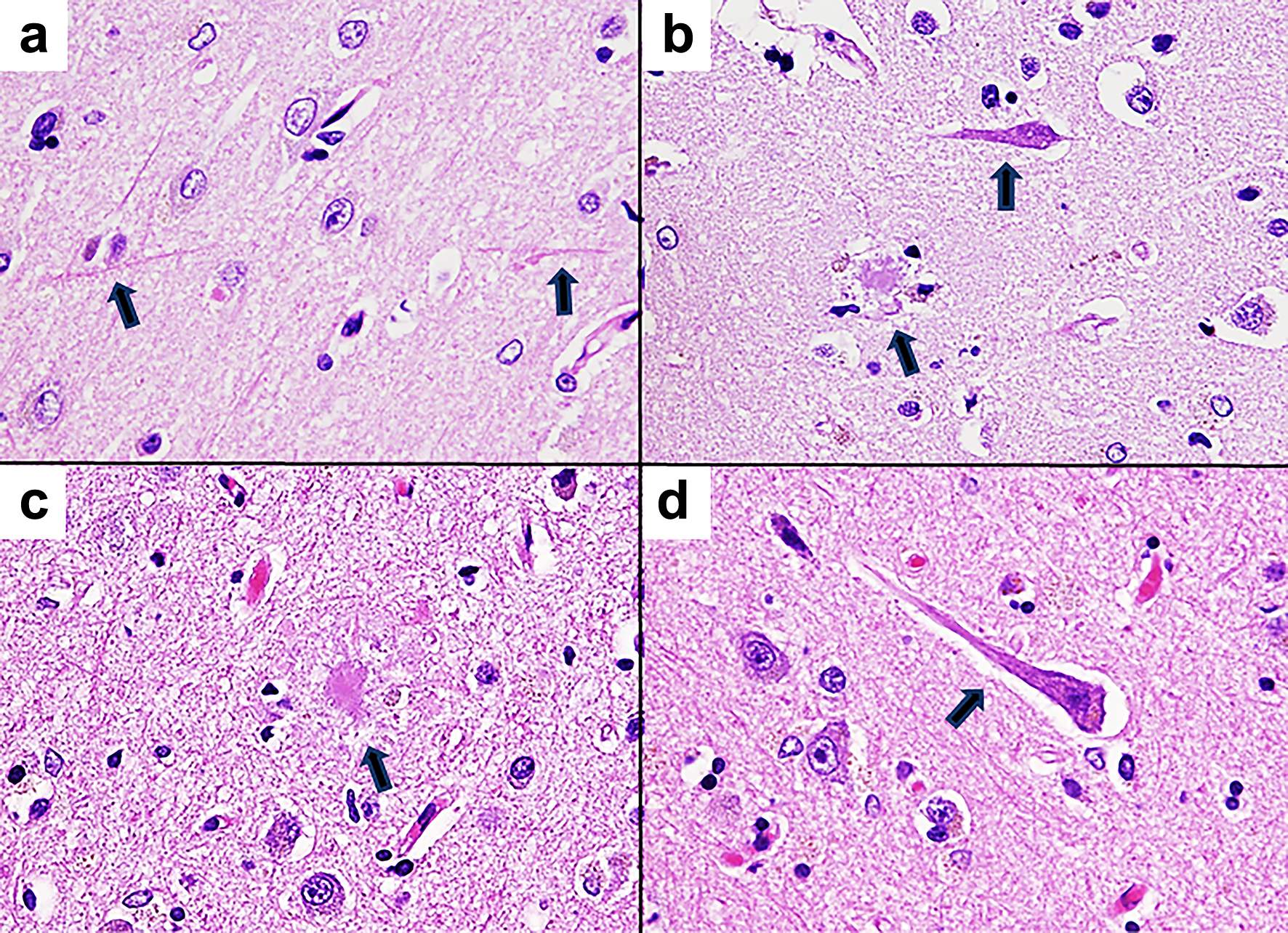 Click for large image | Figure 4. Pathological microscopic images of the brain tissue (hematoxylin and eosin staining, × 400). (a) Neuropil threads are observed in the cortical tissue of the frontal lobe. The arrows indicate neuropil threads. (b) In the cortical tissue of the hippocampus, senile plaques and neurofibrillary tangles are observed. The senile plaque appears to be a neuritic plaque with surrounding neurites. The arrows indicate senile plaque and neurofibrillary tangle. (c) Senile plaque of the cortical tissue of the parietal lobe. The arrow indicates senile plaque. (d) Neurofibrillary tangle of the cortical tissue in the parietal lobe. The arrow indicates neurofibrillary tangle. |
Kidney
Both kidneys were turbid and swollen. Approximately 15% of the glomeruli had crescents (cellular and fibrous) (Fig. 5). A moderate inflammatory cell infiltrate, mainly lymphocytes, was found in the submucosa of the renal pelvis and in the renal interstitium. These histopathological findings supported GPA.
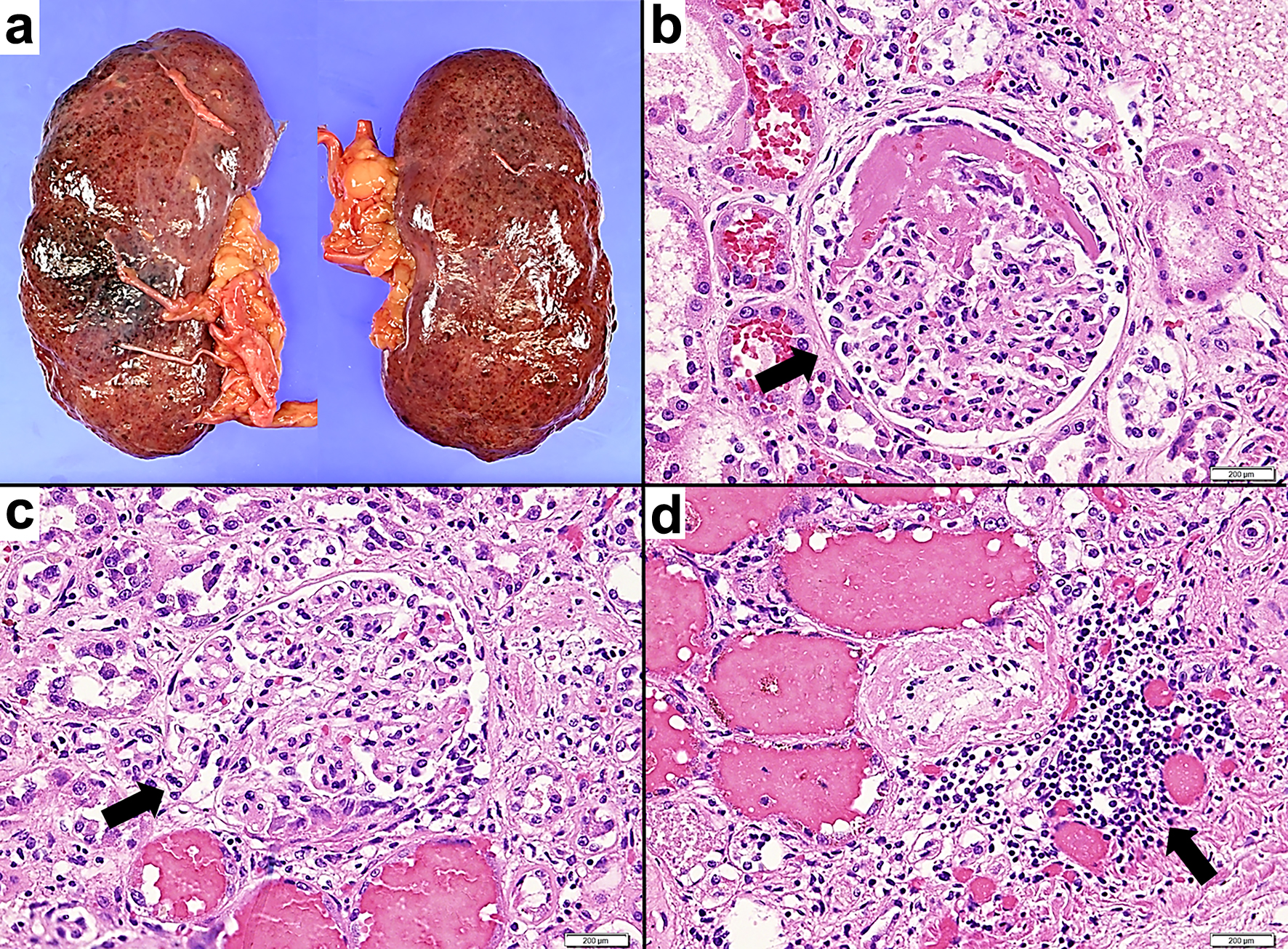 Click for large image | Figure 5. Pathological macroscopic and microscopic images of the kidney tissue. (a) The fresh both kidneys exhibit turbid coloration and swelling. (b, c) Hematoxylin and eosin staining, × 400. (b) Glomerular lesions exhibiting fibrous crescent formation are observed. The arrow indicates fibrous crescentic glomerulonephritis. (c) Glomerular lesions exhibiting cellular crescent formation are observed. Inflammatory cells including neutrophils accumulate in areas demonstrating fibrin precipitation and loop rupture. The arrow indicates cellular crescentic glomerulonephritis. (d) Hematoxylin and eosin staining, × 200. Inflammatory cells such as lymphocytes and neutrophils accumulate in the tubulointerstitium. The arrow indicates peritubular inflammatory cell infiltration. |
Lung
Both lungs were fibrinously adherent to the chest wall. Grossly, predominant lower lobe congestion was observed, and a hemosiderin-laden macrophage was prominent in the left lung. There were no findings indicating GPA, such as granulomatous inflammation, vasculitis, or mixed cellular infiltration of neutrophils, lymphocytes, or plasma cells.
Heart
The patient had a mild image of acute-stage infarction showing fresh myocardial necrosis in the left ventricular anterior wall and septum.
Others
There were no histological findings indicating granulomatous lesions or vasculitis of small-sized vessels in other organs, including the upper respiratory tract.
| Discussion | ▴Top |
In this case, we confirmed the absence of systemic disease or other brain disease that could cause progressive cognitive impairment. According to the diagnostic criteria of the National Institute of Neurological and Communicative Disorders and Stroke AD and Related Disorders Association (NINCDS-ADRDA), a clinical diagnosis of probable AD was made [7]. Based on the NINCDS-ADRD, the definitive diagnosis of AD was based on neuropathological evidence from a biopsy or autopsy. Thus, in actual clinical practice, extremely few patients are definitively diagnosed with AD. The autopsy revealed neuropathological findings that suggest AD (Fig. 4). Thus, the patient was diagnosed with definite AD. The coexistence of moderate AD may have influenced the early detection and diagnosis of acute renal failure. He exhibited subacute deterioration in cognitive function such as the appearance of BPSD or delirium. The autopsy revealed that the aforementioned neurological findings were not related to CNS involvement by GPA. Therefore, the deterioration of the patient’s general condition due to dehydration and uremia due to acute renal failure was considered to have caused delirium and eventually severe consciousness disturbance. CNS involvement in GPA is more common in the severe form and reportedly occurs in about 10% of patients [6, 8, 9]. However, in the present case, although PR3-ANCA was abnormally high and GPA was severe, the patient’s neurological symptoms were not associated with GPA.
The patient had a strongly positive PR3-ANCA and acute progressive renal failure, but histopathological findings were necessary to make a definitive diagnosis of GPA [10]. At autopsy, necrotizing crescentic glomerulonephritis and peritubular inflammatory cell infiltration were observed in the kidney, which led to a definitive diagnosis of GPA (Fig. 5). This case also met the criteria for GPA according to the 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology, which requires a total score ≥ 5 [11]. GPA begins in the upper respiratory tract and often progresses to the lower respiratory tract (lung) and kidney. The presence of all three lesions indicates a systemic type, whereas the presence of only one or two lesions indicates a limited type. More than 90% of patients with GPA have been reported to have upper airway involvement [12]. Lung lesions are reported to be present in almost 50% of patients with GPA initially, and lung disease eventually develops in 85-90% of patients in the course of the disease [13]. However, in the autopsy of this case, there were no pathological findings indicating GPA in other parts of the body (e.g., skin, eye, ear, or nervous system), including these major lesions, aside from the kidney. To the best of our knowledge, there are a few studies on PR3-ANCA-positive GPA presenting as a solitary renal mass accompanying glomerulonephritis like this case, although there was no renal mass in our patient [14-16]. Thus, the case of PR3-ANCA-positive GPA with renal-limited vasculitis and acute renal failure as the initial manifestation is rare, as in the present case. Furthermore, renal involvement is clinically evident in only 11% to 20% of cases at onset, but 77% to 85% of patients with GPA eventually develop glomerulonephritis within 2 years of GPA onset [10]. Additionally, necrotizing glomerulonephritis is present in approximately 70% to 80% of patients with generalized GPA, together with pulmonary involvement [17]. GPA supposedly begins with limited organ involvement and may change to a more systemic form in which the nose, lungs, and kidneys are involved [10]. In the present case, if the patient had not died of acute renal failure, other lesions would have appeared in the upper airway, lungs, etc., and the GPA would have progressed to a systemic form. In the diagnosis of renal-limited GPA, microscopic polyangiitis (MPA) is the most difficult disease to differentiate when the observation period is short, as in this case. Because the development of necrotizing crescentic glomerulonephritis is much more common in patients with MPA than in those with GPA, a certain period of observation will be necessary to improve the diagnostic accuracy of renal-limited GPA and MPA [2]. Moreover, PR3-ANCA is detected not only in about 65% to 90% of patients with GPA but also in approximately 10-20% of patients with MPA [2, 17, 18]. Conversely, in a small number of patients with GPA, MPO-ANCA may be detected or ANCA may not be detected, and most patients with MPA (65-90%) have MPO-ANCA [2, 17, 18]. Therefore, PR3-ANCA positivity alone should not be used to diagnose GPA or determine the treatment. However, the frequency of ANCA being detected as a nonpathogenic autoantibody in patients aged 70 to 90 years is extremely low, less than 1%, and PR3-ANCA is supposed to be correlated with GPA severity and relapse potential, making it highly disease-specific (specificity > 90%) [2, 3]. Based on these backgrounds, although there are certain exceptions, the patient was diagnosed with renal-limited GPA based on the combination of the strong positivity of PR3-ANCA (≥ 350 U/mL), negative MPO-ANCA, onset of acute kidney failure, and consistent renal pathology.
Conclusion
The patient clinically diagnosed with probable AD was confirmed to have definite AD by autopsy. The subacute cognitive deterioration and impaired consciousness observed in the patient on admission were found to be caused by delirium resulting from severe dehydration or uremia, not by CNS involvement due to GPA. Necrotizing crescentic glomerulonephritis and peritubular inflammatory cell infiltration were also found. Therefore, the cause of the acute progressive renal failure in this case was determined to be PR3-ANCA-positive GPA, and the autopsy revealed no GPA-related lesions in other parts of the body aside from the kidney. It is rare to encounter cases of PR3-ANCA-positive GPA with renal-limited vasculitis and acute renal failure as the initial manifestation, as in the present case. Making an accurate clinical diagnosis of older patients suffering from various diseases in multiple organs is extremely challenging. Although an autopsy has the limitation of a terminal image, it is extremely useful in elucidating the pathophysiology of older patients as it enables observation over a wide area, unlike biopsy. We report this case to utilize the results of this autopsy for the development of clinical medicine.
Acknowledgments
None to declare.
Financial Disclosure
No funding was received.
Conflict of Interest
The authors declare that there is no conflict of interest relevant to this article.
Informed Consent
Written informed consent was obtained from the patient’s family for publication of this case report.
Author Contributions
S. Tetsuka drafted the original manuscript. Y. Dobashi performed the autopsy and described the histopathological findings. All authors reviewed and revised the manuscript draft and approved the final version for submission.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
GPA: granulomatosis with polyangiitis; ANCA: antineutrophil cytoplasmic antibody; PR3-ANCA: ANCA directed against proteinase 3; CNS: central nervous system; AD: Alzheimer’s disease; FLAIR: fluid-attenuated inversion recovery; MRI: magnetic resonance imaging; Cr: creatinine; BUN: blood urea nitrogen; BPSD: behavioral and psychological symptoms of dementia; CT: computed tomography; MPA: microscopic polyangiitis; MPO: myeloperoxidase; NINCDS-ADRDA: National Institute of Neurological and Communicative Disorders and Stroke AD and Related Disorders Association
| References | ▴Top |
- Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1-11.
doi pubmed - Kitching AR, Anders HJ, Basu N, Brouwer E, Gordon J, Jayne DR, Kullman J, et al. ANCA-associated vasculitis. Nat Rev Dis Primers. 2020;6(1):71.
doi pubmed - Granel J, Korkmaz B, Nouar D, Weiss SAI, Jenne DE, Lemoine R, Hoarau C. Pathogenicity of proteinase 3-anti-neutrophil cytoplasmic antibody in granulomatosis with polyangiitis: implications as biomarker and future therapies. Front Immunol. 2021;12:571933.
doi pubmed pmc - Mattioli F, Capra R, Rovaris M, Chiari S, Codella M, Miozzo A, Gregorini G, et al. Frequency and patterns of subclinical cognitive impairment in patients with ANCA-associated small vessel vasculitides. J Neurol Sci. 2002;195(2):161-166.
doi pubmed - Zheng Y, Zhang Y, Cai M, Lai N, Chen Z, Ding M. Central nervous system involvement in ANCA-associated vasculitis: what neurologists need to know. Front Neurol. 2018;9:1166.
doi pubmed pmc - Kang A, Antonelou M, Wong NL, Tanna A, Arulkumaran N, Tam FWK, Pusey CD. High incidence of arterial and venous thrombosis in antineutrophil cytoplasmic antibody-associated vasculitis. J Rheumatol. 2019;46(3):285-293.
doi pubmed - Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, Delacourte A, et al. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010;9(11):1118-1127.
doi pubmed - Jazayeri SB, Rahimian A, Ahadi MS, Tavakolpour S, Alesaeidi S. Neurologic involvement in granulomatosis with polyangiitis: a comparative study. Biology and Life Sciences Forum. 2022;19(1):19.
doi - Sherri A, Mortada MM, Makowska J, Lewandowska-Polak A. Primary angiitis of the CNS and ANCA-associated vasculitis: from pathology to treatment. Rheumatol Int. 2024;44(2):211-222.
doi pubmed pmc - Kubaisi B, Abu Samra K, Foster CS. Granulomatosis with polyangiitis (Wegener's disease): An updated review of ocular disease manifestations. Intractable Rare Dis Res. 2016;5(2):61-69.
doi pubmed pmc - Robson JC, Grayson PC, Ponte C, Suppiah R, Craven A, Judge A, Khalid S, et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for granulomatosis with polyangiitis. Ann Rheum Dis. 2022;81(3):315-320.
doi pubmed - Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, Rottem M, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116(6):488-498.
doi pubmed - Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener's granulomatosis. A clinical and imaging study of 77 cases. Chest. 1990;97(4):906-912.
doi pubmed - Kapoor A, Balfour-Dorsey RA, George DL. Wegener's granulomatosis presenting as multiple kidney masses. Am J Med. 2002;112(1):82-83.
doi pubmed - Ruiz Carazo E, Medina Benitez A, Lopez Milena G, Rabaza Espigares J, Leon L, Marquez B. Multiple renal masses as initial manifestation of Wegener's granulomatosis. AJR Am J Roentgenol. 2001;176(1):116-118.
doi pubmed - Xu H, Zhang J, Wang Y, Yu S, Zhou R, Zhang J. Clinicopathological analysis of renal inflammatory pseudotumors presenting as the unilateral solitary masses. Int J Clin Exp Pathol. 2017;10(7):7734-7742.
pubmed pmc - Binda V, Moroni G, Messa P. ANCA-associated vasculitis with renal involvement. J Nephrol. 2018;31(2):197-208.
doi pubmed - Salvador F. ANCA associated vasculitis. Eur J Intern Med. 2020;74:18-28.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.