| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 16, Number 9, September 2024, pages 423-435
Age-Specific Approach to Arterial Stiffness Prediction in Apparently Healthy Patients
Anna Braginaa, b , Yulia Rodionovaa, b
, Natalia Druzhininaa, b
, Timur Gamilovb, c, d
, Ekaterina Udalovad
, Artem Rogovb, c
, Lubov Vasilevaa, g
, Rustam Shikhmagomedova
, Oksana Avdeenkoe
, Anna Kazadaevaf
, Kirill Novikova
, Valeriy Podzolkova
aDepartment of Internal Medicine No. 2, Institute of Clinical Medicine, Sechenov First Moscow State Medical University, 19991 Moscow, Russia
bWorld-Class Research Center “Digital Biodesign and Personalized Healthcare”, Sechenov First Moscow State Medical University, 19991 Moscow, Russia
cMoscow Institute of Physics and Technology, 141701 Dolgoprudny, Russia
dDepartment of Mathematical Modelling of Processes and Materials, Sirius University of Science and Technology, 354340 Sochi, Russia
eDepartment of Therapeutic Dentistry, Institute of Dentistry Named After E.V. Borovsky, Sechenov First Moscow State Medical University, 19991 Moscow, Russia
fMedical Center Doctor Aleksandrovsky, Medgroup Fantasy Children’s Clinic, Ltd. 121059 Moscow, Russia
gCorresponding Author: Lubov Vasileva, Department of Internal Medicine No. 2, Institute of Clinical Medicine, Sechenov First Moscow State Medical University, Moscow, Russian
Manuscript submitted July 22, 2024, accepted September 2, 2024, published online September 12, 2024
Short title: Age-Specific Prediction of Arterial Stiffness
doi: https://doi.org/10.14740/jocmr5271
| Abstract | ▴Top |
Background: The high prevalence of traditional cardiovascular risk factors among the patients without cardiovascular disease (CVD) allows us to predict an increase in cardiovascular morbidity rate in the future. Arterial stiffness is one of the most important predictors and pathogenetic mechanisms of CVD development. The aim of our study was to evaluate the predictive differences of age-related and age-independent (universal) cardio-ankle vascular index (CAVI) reference values for detecting increased arterial stiffness in individuals without CVD.
Methods: The study included 600 patients (43% men and 57% women, mean age 36.0 ± 18.3 years). All the patients underwent anthropometric measurements with obesity markers evaluation, assessment of arterial stiffness by sphygmomanometry. To create predictive models, we used universal and age-related CAVI thresholds: ≥ 9.0 (CAVI≥ 9) and CAVIAge according to the “Consensus of Russian experts on the evaluation of arterial stiffness in clinical practice".
Results: In the < 50 years group, both the CAVIAge and CAVI≥ 9 models were significant (CAVIAge: b = 4.8, standard error b (st.err.b) = 0.27, P < 0.001; CAVI≥ 9: b = 3.2, st.err.b = 1.6, P < 0.001). The CAVIAge model demonstrated high sensitivity and specificity (> 70%) compared to the CAVI≥ 9 model (sensitivity 62%, specificity 58%). In the receiver operating characteristic (ROC) curve analysis, the CAVIAge model had a significantly higher area under the ROC curve (AUC) = 0.802 than the CAVI≥ 9 model: AUC = 0.674. In the ≥ 50 years group, both models were significant: CAVIAge (b = 2.6, st.err.b = 1.13, P < 0.001) and CAVI≥ 9 (b = 5.3, st.err.b = 0.94, P < 0.001). Both models demonstrated high sensitivity and specificity (> 70%). When ROC curves were analyzed for the CAVIAge model, the AUC value of 0.675 was significantly lower when compared to the CAVI≥ 9 model (AUC = 0.787, P = 0.031).
Conclusions: In the < 50 years group, the model based on age-specific CAVI thresholds has the higher predictive value, sensitivity, and specificity for identifying individuals with increased arterial stiffness. In contrast, in the ≥ 50 years group, a predictive model using a universal threshold value of CAVI≥ 9 has advantages.
Keywords: Arterial stiffness; Cardio-ankle vascular index; Predictive model; Metabolic parameters
| Introduction | ▴Top |
According to the Non-Communicable Diseases (NCD) Risk Factor Collaboration, which included information from 104 million individuals from 200 countries, the prevalence of essential hypertension (EH) has doubled over the past 20 years among those aged 30 - 79 years [1]. According to the Russian multicenter epidemiological study ESSE-RF2 (epidemiology of cardiovascular diseases in the regions of the Russian Federation, the second study) (2017), the incidence of EH at the age of 25 - 34 years is 25.5% in men and 11.3% in women, dyslipidemia/obesity - 32.9/14.3% and 38.8/10.7%, respectively, and increases in the following decades of life [2-4].
The high prevalence of traditional cardiovascular risk factors among the patients without cardiovascular disease (CVD) allows us to predict an increase in cardiovascular morbidity rate in the future. The Global Burden of Disease, Injuries, and Risk Factors Study (GBD) confirmed this trend through demonstrating CVD prevalence in patients aged 15 - 39 years increased by 0.38% annually, 0.08% of which was due to hypertension and ischemic heart disease (IHD) (data analyzed from 1990 to 2019) [5].
Therefore, CVD prediction at early preclinical stages is essential. Risk assessment scales such as Systematic Coronary Risk Evaluation 2 (SCORE2) and Framingham Risk Score are widely used to predict 10-year fatal and nonfatal cardiovascular risk [6, 7]. But there are some limitations: Framingham Risk Score (2008) is validated only for the US population (European Americans and African Americans aged 30 - 79 years) [6]; SCORE2 scale enables us to assess cardiovascular risk only for people aged 40 - 69 years [7]. Thus, younger patients are outside the ranges of the prognostic scales.
Arterial stiffness is one of the most important predictors and pathogenetic mechanisms of CVD development [8]. ENIGMA (enhancing neuroimaging genetics through meta-analysis) study (which includes 1,028 healthy students aged 17 - 27 years) revealed that arterial stiffness is an essential hemodynamic disturbance underlying hypertension [9].
A gradual increase in arterial stiffness is noted with increasing age, which is known as healthy vascular aging (HVA) [10]. Other patterns include supernormal vascular aging (SUPERNOVA), when patients have low arterial stiffness even in old age, and early vascular aging (EVA), characterized by the early development of increased arterial stiffness [10, 11].
Measurement of cardio-ankle vascular index (CAVI) with sphygmomanometry is one of the basic and accurate methods to assess arterial stiffness [8, 12, 13]. Based on the study of Tanaka et al, the level of CAVI ≥ 9.0 is generally accepted as the criterion of increased arterial stiffness [12]. In 2016, the Russian researchers also proposed age-specific CAVI thresholds ranging from > 7.2 in patients aged 21 - 30 years to > 9.8 in patients over 70 years [14]. Applying the universal threshold value can be associated with underestimation of arterial stiffness level in young and middle-aged individuals. To date, there are many approaches using different CAVI thresholds at different age groups, but no comparison of these models has been performed [14].
Therefore, the aim of our study was to evaluate the predictive differences of age-related and age-independent (universal) CAVI reference values for detecting increased arterial stiffness in individuals without CVD.
| Materials and Methods | ▴Top |
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by Sechenov University Local Ethic Committee (protocol No. 25-22, dated December 8, 2022). All patients provided written informed consent prior to enrollment.
We included 600 patients (43% men and 57% women, mean age 36.0 ± 18.3 years) who passed annual clinical examination in the University Clinical Hospital No. 4 of Sechenov University.
The inclusion criteria were apparently healthy adults ≥ 18 years of age, with written informed consent prior to enrollment and mental and physical ability to participate in the study. Exclusion criteria were: stage 3 hypertension, atherosclerosis obliterans of lower limb arteries, IHD, cerebrovascular disease, impaired liver function, glomerular filtration rate (GFR) < 30 mL/min/1.73m2, proteinuria ≥ 300 mg/day, type 1 and 2 diabetes mellitus, any chronic inflammatory disease, pregnancy and conditions limiting arterial stiffness assessment by sphygmomanometry (atrial fibrillation, aortic valve disease, bronchial asthma and chronic obstructive pulmonary disease exacerbation).
All the patients underwent anthropometric measurements with obesity markers evaluation: waist circumference (WC) and body mass index (BMI) [15]. Lipid profile (total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglyceride (TG)), glucose and creatinine levels were assessed by CardioChek PA (USA, 2017) express analyzer. Then lipid accumulation product (LAP) [16], visceral adiposity index (VAI) [17], body fat percentage (BFP) [18] indices were calculated. Impaired glucose tolerance (IGT) was determined by history taking and medical records according to the American Association of Clinical Endocrinology Consensus Statement: Comprehensive Type 2 Diabetes Management Algorithm - 2023 Update [19].
Stage and grade of hypertension were defined in accordance with the European Society of Cardiology 2023 guidelines [20]. Presence of dyslipidemia and abdominal obesity were assessed according to the Russian Society of Cardiology 2020 guidelines [15].
Arterial stiffness was obtained by sphygmomanometry on VaSera VS-1000 Fukuda Denshi scanner (Japan, 2010). All participants were given instructions prior to the measurements: the procedure was conducted in a supine position, in the morning, participants should be abstained from alcohol, caffeine, and smoking for 8 h prior to the measurement.
To create predictive models, we used universal and age-related CAVI thresholds: ≥ 9.0 (CAVI≥ 9) [12] and CAVIAge according to the “Consensus of Russian experts on the evaluation of arterial stiffness in clinical practice” (2016) [14]. The median reference values of CAVI ≥ 7.2 at the age of 21 to 30 years; CAVI ≥ 7.4 at the age of 31 to 40 years; CAVI ≥ 7.55 at the age of 41 to 50 years and CAVI ≥ 8.0 at the age of 51 to 60 years, and CAVI ≥ 9.8 at the age of over 70 years were used as CAVIAge thresholds [14].
All statistical analysis was performed with Statistica 12.0. For each pair of variables, including binomial variables (age, weight, hypertension, etc.), the Spearman correlation coefficient was calculated. Random Forest machine learning method was used to identify the significance of risk factors. Factors that demonstrated significance (variable rank ≥ 70 and importance ≥ 0.7) were included in further statistical analysis. We used the patient database to develop a machine learning algorithm that estimates the likelihood of developing arterial stiffness. We used logistic regression because it is able to handle different types of input variables (continuous, categorical) and can be used to obtain a clear formula to estimate the probability. We calculated the specificity, sensitivity and mixing matrix of the developed algorithm. The area under the receiver operating characteristic (ROC) curve (AUC) was also estimated. The results were considered significant when P < 0.05.
| Results | ▴Top |
Clinical and demographic characteristics of the patients are shown in Table 1. The incidence of hypertension, obesity and dyslipidemia was consistent with population-based rates. All patients (n = 600) were divided into two groups according to the age: < 50 (n = 378) and ≥ 50 years (n = 222).
 Click to view | Table 1. Clinical and Demographic Characteristics of Participants |
In the ≥ 50 years group there was a significantly higher incidence of overweight individuals with elevated anthropometric and metabolic parameters (WC, TC, LDL, glucose, LAP, VAI, BFP), hypertension (and corresponding systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels), higher creatinine concentrations and lower GFR. In the ≥ 50 years group, individuals with high arterial stiffness, defined by both the universal CAVI≥ 9 and CAVIAgе thresholds, were significantly more common. However, individuals with high arterial stiffness identified by CAVIAgе were significantly more common in the < 50 years group than in the CAVI≥ 9 group (χ2= 11.054, P < 0.001). In the older group, differences in the frequencies of increased stiffness detected according to CAVIAgе and CAVI≥ 9 criteria were not significant (χ2 = 2.476, P = 0.116).
The frequency of patients with increased arterial stiffness according to the CAVIAge or CAVI≥ 9 in different age groups is shown in Figure 1. When CAVIAge was used, arterial stiffness was detected significantly more often in younger age groups (P < 0.05) compared to CAVI≥ 9. Conversely, CAVI≥ 9 identified more patients with increased arterial stiffness in > 70 years group (P < 0.05) (Fig. 1).
 Click for large image | Figure 1. Age distribution of patients with elevated CAVI. CAVI: cardio-ankle vascular index. |
Single-factor correlation analysis was performed in the < 50 years (Fig. 2) and ≥ 50 years (Fig. 3) groups to determine the correlation between risk factors and CAVI values. The results are presented in correlation matrices. In the < 50 years group, there were significant positive correlations with varying strength between variables of CAVI and variables of age, DBP, LDL, LAP, VAI, and negative correlations between CAVI and GFR (Fig. 2).
 Click for large image | Figure 2. Correlation matrix between vascular stiffness markers, risk factors, clinical and biochemical measurements in patients < 50 years of age. HTN: hypertension; IGT: impaired glucose tolerance; BMI: body mass index; WC: waist circumference; HC: hip circumference; SBP: systolic blood pressure; DBP: diastolic blood pressure; TC: total cholesterol; LDL: low-density lipoprotein; HDL: high-density lipoprotein; TG: triglycerides; GFR: glomerular filtration rate; LAP: lipid accumulation product; VAI: visceral adiposity index; BFP: body fat percentage. |
 Click for large image | Figure 3. Correlation matrix between vascular stiffness markers, risk factors, clinical and biochemical measurements in patients ≥ 50 years. HTN: hypertension; IGT: impaired glucose tolerance; BMI: body mass index; WC: waist circumference; HC: hip circumference; SBP: systolic blood pressure; DBP: diastolic blood pressure; TC: total cholesterol; LDL: low-density lipoprotein; HDL: high-density lipoprotein; TG: triglycerides; GFR: glomerular filtration rate; LAP: lipid accumulation product; VAI: visceral adiposity index; BFP: body fat percentage. |
In ≥ 50 years group (Fig. 3), we revealed significant positive correlation with varying strength between variables of CAVI and variables of age, presence of hypertension, SBP, dyslipidemia, IGT, TC and glucose level. Factors most associated with CAVI levels in correlation analysis of the < 50 and ≥ 50 years groups were analyzed by Random Forest machine learning using CAVIAge and CAVI≥ 9 thresholds.
In the < 50 years group, age (variable rank = 100, importance = 1.0) and LDL level (variable rank = 79.5, importance = 0.795) played a significant role in the CAVIAge model (Fig. 4).
 Click for large image | Figure 4. Significance of CAVIAge model components for detecting high arterial stiffness (Random Forest estimation) in the < 50 years group. DBP: diastolic blood pressure; LDL: low-density lipoprotein; GFR (EPI): glomerular filtration rate (calculated by Chronic Kidney Disease Epidemiology Collaboration equation); LAP: lipid accumulation product index; VAI: visceral adiposity index. |
For the CAVI≥ 9 model, age (variable rank = 100, importance = 1.0) was also the most significant factor, and LDL level (variable rank = 72, importance = 0.72) was highly significant (Fig. 5).
 Click for large image | Figure 5. Significance of the CAVI≥ 9 model components for detecting high arterial stiffness (Random Forest estimation) in the < 50 years group. DBP: diastolic blood pressure; LDL: low-density lipoprotein; GFR (EPI): glomerular filtration rate (calculated by Chronic Kidney Disease Epidemiology Collaboration equation); LAP: lipid accumulation product index; VAI: visceral adiposity index. |
In the ≥ 50 years group, SBP level (variable rank = 100, importance = 1.0) played a fundamental role in the CAVIAge model, glucose level (variable rank = 99.5, importance = 0.995), TC (variable rank = 91, importance = 0.91), age (variable rank = 79, importance = 0.79) and hypertension duration (variable rank = 72, importance = 0.72) were highly significant (Fig. 6).
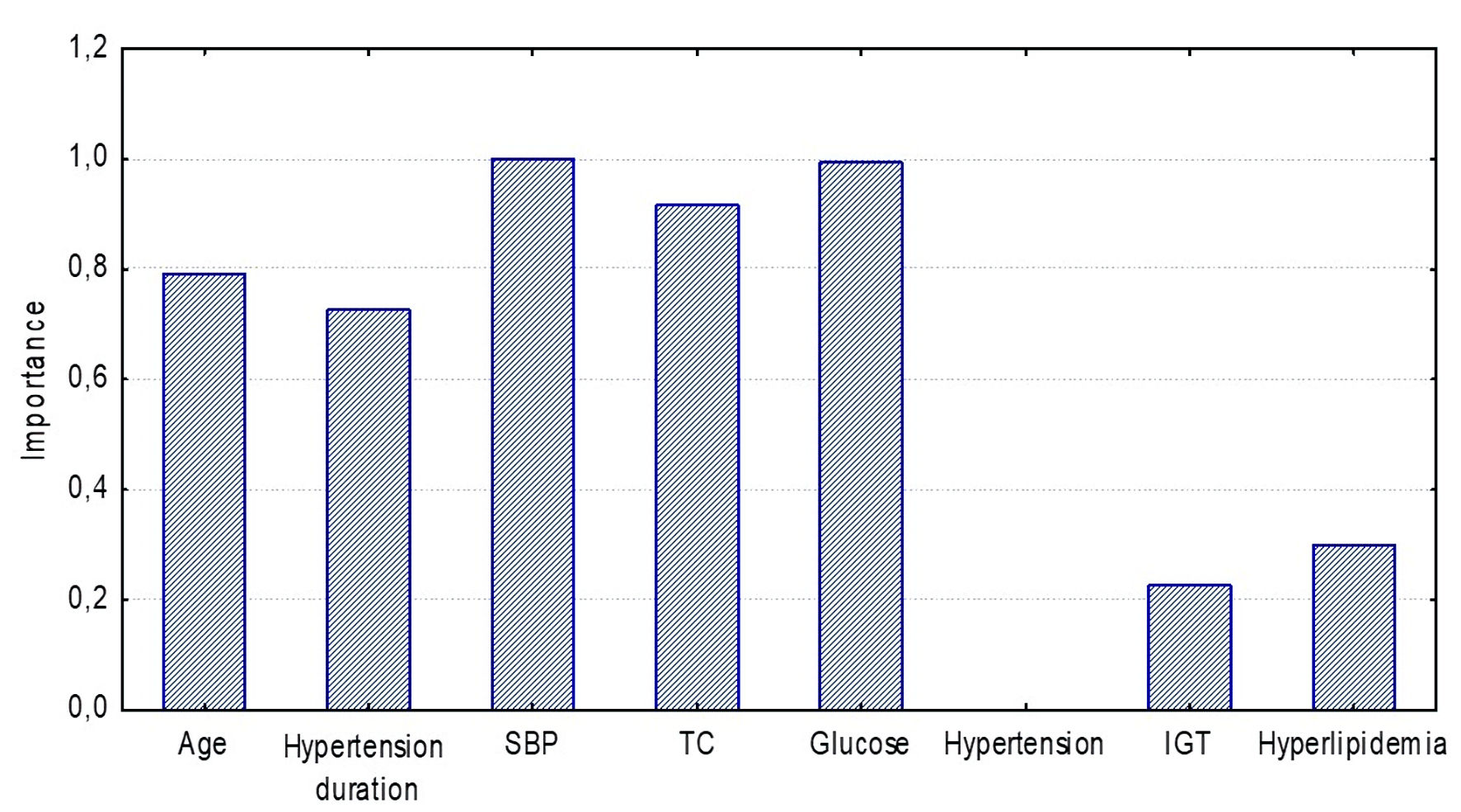 Click for large image | Figure 6. Significance of CAVIAge model components for detecting high arterial stiffness (Random Forest estimation) in the ≥ 50 years group. SBP: systolic blood pressure; TC: total cholesterol; IGT: impaired glucose tolerance. |
Age was the most significant factor (variable rank = 100, importance = 1.0) in the CAVI≥ 9 model, SBP (variable rank = 92, importance = 0.92), TC (variable rank = 85, importance = 0.85) and glucose level (variable rank = 83, importance = 0.83) were somewhat less important, hypertension duration (variable rank = 69, importance = 0.69) and other factors were less significant (Fig. 7).
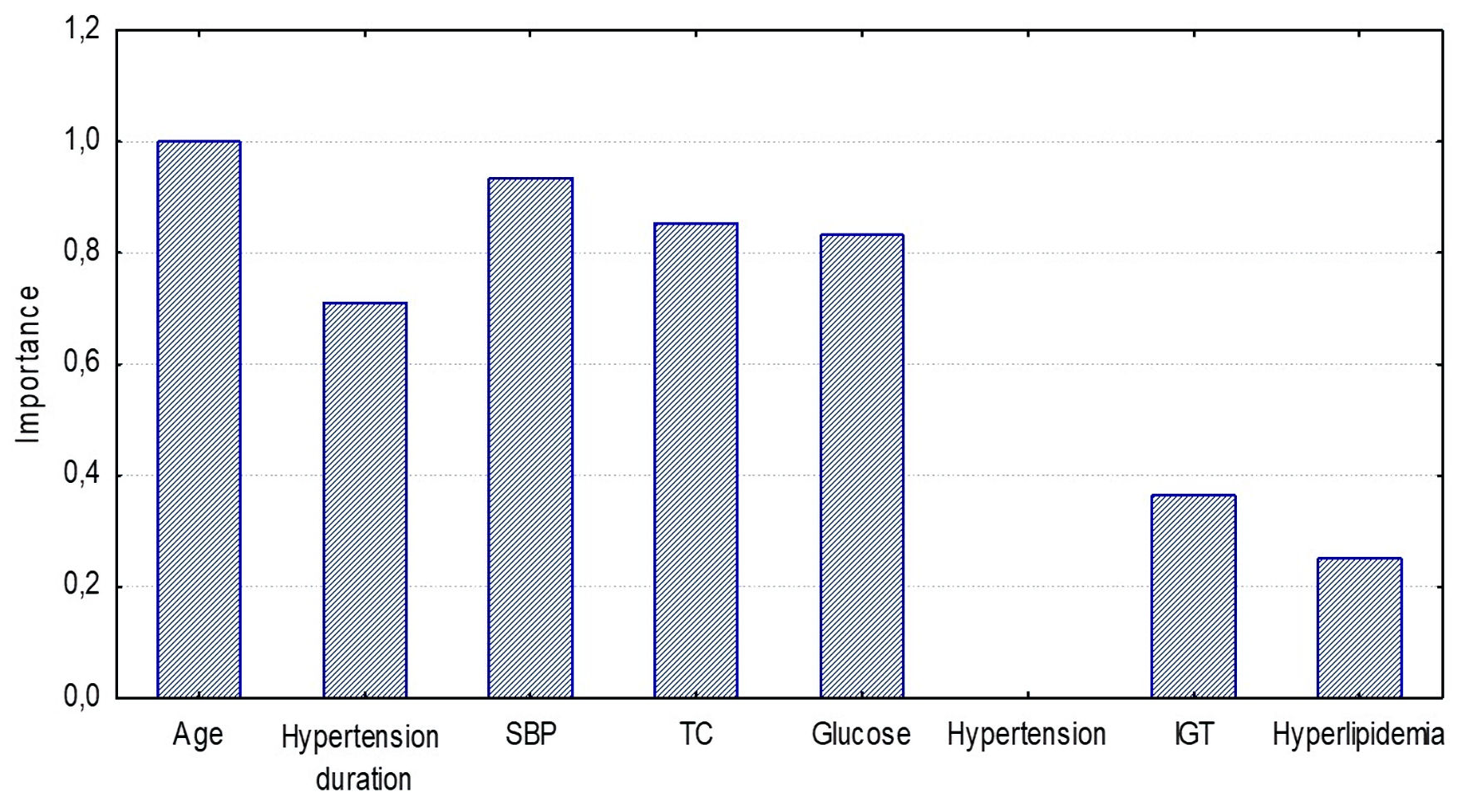 Click for large image | Figure 7. Significance of CAVI≥ 9 model components for detecting high arterial stiffness (Random Forest estimation) in the ≥ 50 years group. SBP: systolic blood pressure; TC: total cholesterol; IGT: impaired glucose tolerance. |
Factors with the highest significance in the Random Forest analysis were included in a multivariate analysis and predictive model formation to detect increased arterial stiffness using CAVIAge and CAVI≥ 9 in the < 50 and ≥ 50 years groups.
In the < 50 years group, age and LDL levels were included in the increased arterial stiffness predictive model (CAVIAge and CAVI≥ 9). Both the CAVIAge and CAVI≥ 9 models were significant (CAVIAge: b = 4.8, standard error b (st.err.b) = 0.27, P < 0.001; CAVI≥ 9: b = 3.2, st.err.b = 1.6, P < 0.001). Within these models, age had the highest independent association with CAVI value (CAVIAge: b = 2.1, st.err.b = 1.04, P < 0.001; CAVI ≥ 9: b = 0.9, st.err.b = 0.75, P = 0.008).
The CAVIAge model demonstrated high sensitivity and specificity (> 70%) compared to the CAVI≥ 9 model (sensitivity 62%, specificity 58%). In ROC curve analysis, the CAVIAge model had a significantly higher AUC = 0.802 (Fig. 8) than the CAVI≥ 9 model: AUC = 0.674 (P < 0.05) (Fig. 9).
 Click for large image | Figure 8. ROC curves for the logistic regression model determining arterial stiffness using CAVIAge in the < 50 years group. ROC: receiver operating characteristic. |
 Click for large image | Figure 9. ROC curves for the logistic regression model determining arterial stiffness using CAVI≥ 9 in the < 50 years group. ROC: receiver operating characteristic. |
In the ≥ 50 years group, age, SBP, TC and glucose level were included in the prediction models for high arterial stiffness (CAVIAge and CAVI≥ 9). Both models were significant: CAVIAge (b = 2.6, st.err.b = 1.13, P < 0.001) and CAVI≥ 9 (b = 5.3, st.err.b = 0.94, P < 0.001). For the CAVIAge model, SBP level was independently correlated with CAVI value (b = 2.1, st.err.b = 1.53, P < 0.001). For the CAVI≥ 9 model, age (b = 3.46, st.err.b = 2.18, P < 0.001), SBP (b = 2.07, st.err.b = 1.9, P < 0.001) and glucose (b = 1.43, st.err.b = 0.86, P < 0.001) levels were independently correlated with CAVI value.
Both models demonstrated high sensitivity and specificity (> 70%). When ROC curves were analyzed for the CAVIAge model, the AUC value of 0.675 (Fig. 10) was significantly lower when compared to the CAVI≥ 9 model (AUC = 0.787, P = 0.031) (Fig. 11).
 Click for large image | Figure 10. ROC curves for the logistic regression model determining arterial stiffness using CAVIAge in the ≥ 50 years group. ROC: receiver operating characteristic. |
 Click for large image | Figure 11. ROC curves for the logistic regression model determining arterial stiffness using CAVI≥ 9 in the ≥ 50 years group. ROC: receiver operating characteristic. |
| Discussion | ▴Top |
Arterial stiffness is a marker of vascular wall condition and an integral predictor of CVD risk. In our study, it was assessed by CAVI level, which is less dependent on blood pressure (BP) and heart rate (HR), in contrast to other markers of arterial stiffness [21, 22]. The study cohort (n = 600) was dominated by young and middle-aged individuals. The prevalence of traditional CVD risk factors such as arterial hypertension [23], obesity [24], and dyslipidemia [25] was similar to that in the general population [23-25]. To identify increased arterial stiffness, we compared two approaches: the universal, unified for all age categories CAVI≥ 9 [12] and the age-specific CAVIAge [14]. The CAVIAge method allowed us to identify significantly more individuals with increased arterial stiffness (1.81-fold) at the expense of younger patients. Only at the age of over 70 years CAVIAge identified significantly fewer individuals with increased arterial stiffness than CAVI≥ 9.
Patients were divided into the < 50 and ≥ 50 years of age groups to assess the contribution of the main risk factors in the development of increased arterial stiffness and for statistical analysis. The rationale for this division was the fact that a significant increase in arterial stiffness [8] with a shift of CAVI [14] into the gray zone (8 - 9) is observed in individuals over 50 years of age. In correlation analysis, CAVI was significantly associated with age in both < 50 and ≥ 50 years of age groups. The significance of age was confirmed in Random Forest and multiple regression analysis. Age is a major factor for increasing arterial wall stiffness in general [8] and CAVI marker in particular [26-28].
Otherwise, factors associated with arterial stiffness differed between the < 50 years and ≥ 50 years groups. In younger individuals, CAVI correlated with DBP, metabolic markers (LDL, LAP, VAI) and GFR. In the older group, CAVI was associated with hypertension, its stage and grade, SBP, and other metabolic markers (hyperlipidemia, IGT, TC and glucose level). The association of hypertension with increased arterial stiffness was demonstrated in the wide range of studies [8, 28-31]. High SBP and pulse pressure commonly described in elderly patients with isolated systolic hypertension are traditional markers of increased arterial stiffness [8]. Our data also showed the association of CAVI with the grade of SBP and characteristics of hypertension in the group ≥ 50 years of age. In the younger group, there was an association with DBP and no association with SBP. The phenomenon of isolated diastolic hypertension occurs in 2.5-7.8% of cases [20]. The highest prevalence of this disease is noted in individuals 30 - 39 years with its decrease in the fifth to sixth decade of life and almost complete absence in individuals older than 70 years [32].
Data on the relationship between CAVI and various metabolic markers (weight, BMI, HC, TC, LDL) are controversial. In the work of Safronova et al [28], a group of young healthy patients without hypertension and carbohydrate metabolism disorders (mean age 30.4 years) did not demonstrate significant correlations between CAVI and dyslipidemia markers. Apparently, it may be due to the peculiarities of the free from significant metabolic disorders sample. In our study, we found positive correlations of CAVI with integral metabolic indices (LAP and VAI) and LDL level in the < 50 years group, and with the presence of dyslipidemia, TC and glucose level in the ≥ 50 years group. Correlations of CAVI with integral metabolic indices (LAP and VAI) were previously described in apparently healthy young adults [33]. These correlations of CAVI with LAP and VAI indices reflect multifactorial relationships of arterial stiffness with anthropometric and laboratory metabolic indices [33]. Our data partially agree with the work of Kaveshnikov et al, which revealed direct correlations of CAVI with such marker of dyslipidemia as TG level [27]. In the study of Topouchian et al [34], older individuals with and without metabolic syndrome demonstrated positive correlation of CAVI with hypertension and hyperglycemia. A study by Lopes-Vicente et al found the association of pulse wave velocity, other markers of arterial stiffness, with age, SBP and such metabolic parameters as TG, HDL and glucose level [35]. However, the difference from our study was the older age of the participants (49 ± 8 years) and higher BMI (32 ± 4 kg/m2).
In our work, we attempted to predict increased arterial stiffness on the basis of screening parameters. Similar work for the prediction of EVA was performed by Antza et al. They used the more labor-intensive research method of daily BP monitoring as a predictor of arterial stiffness [36]. We also identified age-specific predictors of arterial stiffness: age and LDL level in individuals < 50 years and age, SBP, TC and glucose level in individuals ≥ 50 years. Two strategies for predicting increased stiffness showed different sensitivity and specificity with higher values for the model with age-specific thresholds (CAVIAge) in the < 50 years group and with universal thresholds (CAVI≥ 9) in the ≥ 50 years group.
An attempt to determine alternative age-specific CAVI references was also made in a study by Safronova et al [28]. The mean CAVI values calculated for patients < 50 years were also lower than the universal one [28]. However, this study did not compare sensitivity and specificity of universal and formula-derived CAVI references.
The inclusion of apparently healthy people in the main sample was the limitation of our study, making it difficult to extrapolate these results to CVD patients. In addition, this study did not divide patients into groups with and without obesity, metabolic syndrome and other significant risk factors. It might broaden our understanding of the efficacy of this diagnostic approach in individual patient groups.
Our study suggests the possibility of using the differentiated approach to screening patients of different age categories. The results obtained in our study require further validation and can only be used on practically healthy individuals. In the < 50 years group, the model based on age-specific CAVI thresholds has the higher predictive value, sensitivity, and specificity for identifying individuals with increased arterial stiffness. In contrast, in the ≥ 50 years group, a predictive model using a universal threshold value of CAVI≥ 9 has advantages. This approach (Fig. 12) can be used prospectively for screening stratification of apparently healthy people to identify groups for in-depth screening and inclusion of preventive measures.
 Click for large image | Figure 12. Algorithm for choosing CAVI reference values in apparently healthy adults. CAVIAge: age-specific cardio-ankle vascular index reference values; CAVI: cardio-ankle vascular index. |
Acknowledgments
None to declare.
Financial Disclosure
This work was financed by the Ministry of Science and Higher Education of the Russian Federation within the framework of state support for the creation and development of World-Class Research Centers “Digital biodesign and personalized healthcare” № 075-15-2022-304.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
All patients provided written informed consent.
Author Contributions
Conceptualization: AB, VP and ND; methodology: AB, ND and TG; software: TG; validation: AB and ND; formal analysis: ND, TG; data collection and analysis: ND, LV, EU, AR, RS, OA, AK and KN; interpretation of data: AB, YR, ND and LV; data curation: AB, YR, ND, LV and VP; writing - original draft preparation: YR, ND, KN and LV; writing - review and editing: AB, YR, ND, KN and VP; writing - literacy search: YR and ND; supervision: VP; project administration: VP. All authors approved the final version of the manuscript for submission.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
AUC: area under the ROC curve; BMI: body mass index; BFP: body fat percentage; BP: blood pressure; CAVI: cardio-ankle vascular index; CVD: cardiovascular disease; DBP: diastolic blood pressure; ENIGMA: enhancing neuroimaging genetics through meta-analysis; ESSE-RF2: epidemiology of cardiovascular diseases in the regions of the Russian Federation, the second study; EVA: early vascular aging; GBD: the Global Burden of Disease, Injuries, and Risk Factors Study; GFR: glomerular filtration rate; HC: hip circumference; HDL: high-density lipoprotein; HTN: hypertension; HVA: healthy vascular aging; IGT: impaired glucose tolerance; IHD: ischemic heart disease; LAP: lipid accumulation product; LDL: low-density lipoprotein; NCD: non-communicable diseases; ROC: receiver operating characteristic; SBP: systolic blood pressure; SCORE2: systematic coronary risk evaluation 2; st.err.b: standard error b; SUPERNOVA: supernormal vascular aging; TC: total cholesterol; TG: triglycerides; VAI: visceral adiposity index; WC: waist circumference
| References | ▴Top |
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398(10304):957-980.
doi pubmed pmc - Balanova YA, Shalnova SA, Imaeva AE, Kapustina АV, Muromtseva GA, Evstifeeva SV, Tarasov VI, et al. Prevalence, awareness, treatment and control of hypertension in Russian Federation (data of observational ESSERF-2 study). Rational Pharmacotherapy in Cardiology. 2019;15(4):450-466.
- Balanova YA, Shalnova SA, Deev AD, Imaeva AE, Kontsevaya AV, Muromtseva GA, Kapustina АV, et al. Obesity in Russian population - prevalence and association with the non-communicable diseases risk factors. Russian Journal of Cardiology. 2018;(6):123-130.
- Grinshtein YI, Shabalin VV, Ruf RR, Petrova MM, Shalnova SA. Prevalence of dyslipidemia among the population of a large region of Eastern Siberia and its association with sociodemographic and behavioral factors. Russian Journal of Preventive Medicine. 2018;21(5):63-69.
- Sun J, Qiao Y, Zhao M, Magnussen CG, Xi B. Global, regional, and national burden of cardiovascular diseases in youths and young adults aged 15-39 years in 204 countries/territories, 1990-2019: a systematic analysis of Global Burden of Disease Study 2019. BMC Med. 2023;21(1):222.
doi pubmed pmc - D'Agostino RB, Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753.
doi pubmed - Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Back M, Benetos A, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227-3337.
doi pubmed - Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res. 2021;128(7):864-886.
doi pubmed - McEniery CM, Yasmin, Wallace S, Maki-Petaja K, McDonnell B, Sharman JE, Retallick C, et al. Increased stroke volume and aortic stiffness contribute to isolated systolic hypertension in young adults. Hypertension. 2005;46(1):221-226.
doi pubmed - Nilsson PM. Early vascular aging in hypertension. Front Cardiovasc Med. 2020;7:6.
doi pubmed pmc - Laurent S, Boutouyrie P, Cunha PG, Lacolley P, Nilsson PM. Concept of extremes in vascular aging. Hypertension. 2019;74(2):218-228.
doi pubmed - Tanaka A, Tomiyama H, Maruhashi T, Matsuzawa Y, Miyoshi T, Kabutoya T, Kario K, et al. Physiological diagnostic criteria for vascular failure. Hypertension. 2018;72(5):1060-1071.
doi pubmed - Namba T, Masaki N, Matsuo Y, Sato A, Kimura T, Horii S, Yasuda R, et al. Arterial stiffness is significantly associated with left ventricular diastolic dysfunction in patients with cardiovascular disease. Int Heart J. 2016;57(6):729-735.
doi pubmed - Vasyuk YuA, Ivanova SV, Shkolnik EL, Kotovskaya YuV, Milyagin VA, Oleynikov VE, Orlova YaA, et al. Consensus of Russian experts on the evaluation of arterial stiffness in clinical practice. Cardiovascular Therapy and Prevention. 2016;15(2):4-19.
- Kobalava ZD, Konradi AO, Nedogoda SV, Shlyakhto EV, Arutyunov GP, Baranova EI, Barbarash OL, et al. Arterial hypertension in adults. Clinical guidelines 2020. Russian Journal of Cardiology. 2020;25(3):3786.
- Kahn HS. The lipid accumulation product is better than BMI for identifying diabetes: a population-based comparison. Diabetes Care. 2006;29(1):151-153.
doi pubmed - Mikael LR, Paiva AMG, Gomes MM, Sousa ALL, Jardim P, Vitorino PVO, Euzebio MB, et al. Vascular aging and arterial stiffness. Arq Bras Cardiol. 2017;109(3):253-258.
doi pubmed pmc - Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: age- and sex-specific prediction formulas. Br J Nutr. 1991;65(2):105-114.
doi pubmed - Samson SL, Vellanki P, Blonde L, Christofides EA, Galindo RJ, Hirsch IB, Isaacs SD, et al. American Association of Clinical Endocrinology Consensus Statement: comprehensive type 2 diabetes management algorithm - 2023 update. Endocr Pract. 2023;29(5):305-340.
doi pubmed - Mancia G, Kreutz R, Brunstrom M, Burnier M, Grassi G, Januszewicz A, Muiesan ML, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens. 2023;41(12):1874-2071.
doi pubmed - Miyoshi T, Ito H, Shirai K, Horinaka S, Higaki J, Yamamura S, Saiki A, et al. Predictive value of the cardio-ankle vascular index for cardiovascular events in patients at cardiovascular risk. J Am Heart Assoc. 2021;10(16):e020103.
doi pubmed pmc - Podzolkov V, Bragina A, Tarzimanova A, Vasilyeva L, Shvedov I, Druzhinina N, Rodionova Y, et al. Association of COVID-19 and Arterial Stiffness Assessed using Cardiovascular Index (CAVI). Curr Hypertens Rev. 2024;20(1):44-51.
doi pubmed pmc - Balanova YuA, Drapkina OM, Kutsenko VA, Imaeva AE, Kontsevaya AV, Maksimov SA, Muromtseva GA, et al. Hypertension in the Russian population during the COVID-19 pandemic: sex differences in prevalence, treatment and its effectiveness. Data from the ESSE-RF3 study. Cardiovascular Therapy and Prevention. 2023;22(8S):3785.
- Bizzoca ME, Campisi G, Muzio LL. COVID-19 pandemic: what changes for dentists and oral medicine experts? A narrative review and novel approaches to infection containment. Int J Environ Res Public Health. 2020;17(11):3793.
doi pubmed pmc - Messager S, Hazlerigg DG, Mercer JG, Morgan PJ. Photoperiod differentially regulates the expression of Per1 and ICER in the pars tuberalis and the suprachiasmatic nucleus of the Siberian hamster. Eur J Neurosci. 2000;12(8):2865-2870.
doi pubmed - Oliveira AC, Cunha P, Vitorino PVO, Souza ALL, Deus GD, Feitosa A, Barbosa ECD, et al. Vascular aging and arterial stiffness. Arq Bras Cardiol. 2022;119(4):604-615.
doi pubmed pmc - Kaveshnikov VS, Trubacheva IA, Serebryakova VN. Analysis of factors associated with arterial stiffness in the general working-age population. Russian Journal of Cardiology. 2022;27(5):5002.
- Safronova T, Kravtsova A, Vavilov S, Leon C, Bragina A, Milyagin V, Makiev R, et al. Model-based assessment of the reference values of CAVI in healthy Russian population and benchmarking with CAVI0. Am J Hypertens. 2024;37(1):77-84.
doi pubmed - Nagayama D, Imamura H, Sato Y, Yamaguchi T, Ban N, Kawana H, Ohira M, et al. Inverse relationship of cardioankle vascular index with BMI in healthy Japanese subjects: a cross-sectional study. Vasc Health Risk Manag. 2017;13:1-9.
doi pubmed pmc - Nagayama D, Sugiura T, Choi SY, Shirai K. Various obesity indices and arterial function evaluated with CAVI - is waist circumference adequate to define metabolic syndrome? Vasc Health Risk Manag. 2022;18:721-733.
doi pubmed pmc - Elosua-Bayes M, Marti-Lluch R, Garcia-Gil MDM, Camos L, Comas-Cufi M, Blanch J, Ponjoan A, et al. Association of classic cardiovascular risk factors and lifestyles with the Cardio-ankle Vascular Index in a general mediterranean population. Rev Esp Cardiol (Engl Ed). 2018;71(6):458-465.
doi pubmed - Romero CA, Tabares AH, Orias M. Is isolated diastolic hypertension an important phenotype? Curr Cardiol Rep. 2021;23(12):177.
doi pubmed pmc - Podzolkov VI, Bragina AE, Druzhinina NA, Rodionova YuN, Safronova TA, Shikhmagomedov RA, Novikov KK. Integral metabolic indices as markers of increased arterial stiffness in young and middle-aged individuals with hypertension and other cardiovascular risk factors. Cardiovascular Therapy and Prevention. 2024;23(4):3948.
- Topouchian J, Labat C, Gautier S, Back M, Achimastos A, Blacher J, Cwynar M, et al. Effects of metabolic syndrome on arterial function in different age groups: the Advanced Approach to Arterial Stiffness study. J Hypertens. 2018;36(4):824-833.
doi pubmed pmc - Lopes-Vicente WRP, Rodrigues S, Cepeda FX, Jordao CP, Costa-Hong V, Dutra-Marques ACB, Carvalho JC, et al. Arterial stiffness and its association with clustering of metabolic syndrome risk factors. Diabetol Metab Syndr. 2017;9:87.
doi pubmed pmc - Antza C, Doundoulakis I, Akrivos E, Stabouli S, Trakatelli C, Doumas M, Kotsis V. Early vascular aging risk assessment from ambulatory blood pressure monitoring: the early vascular aging ambulatory score. Am J Hypertens. 2018;31(11):1197-1204.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.