| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 16, Number 12, December 2024, pages 625-634
Higher Processed Blood Volume of Granulocyte and Monocyte Adsorption Apheresis Ameliorates Long-Term Disease Activity in Ulcerative Colitis Patients
Yoshifumi Hamasakia, f, Ryo Matsuurab, Takahide Shinagawac, Soichiro Ishiharac, Sozaburo Iharad, Mitsuhiro Fujishirod, Kent Doie, Masaomi Nangakua, b
aDepartment of Hemodialysis and Apheresis, The University of Tokyo Hospital, Tokyo, Japan
bDepartment of Nephrology and Endocrinology, The University of Tokyo Hospital, Tokyo, Japan
cDepartment of Surgical Oncology, The University of Tokyo, Tokyo, Japan
dDepartment of Gastroenterology, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
eDepartment of Emergency and Critical Care Medicine, The University of Tokyo Hospital, Tokyo, Japan
fCorresponding Author: Yoshifumi Hamasaki, Department of Hemodialysis and Apheresis, The University of Tokyo Hospital, Tokyo 113-8655, Japan
Manuscript submitted September 14, 2024, accepted December 9, 2024, published online December 20, 2024
Short title: Higher GMA Dose Suppresses UC Exacerbation
doi: https://doi.org/10.14740/jocmr6071
| Abstract | ▴Top |
Background: Granulocyte and monocyte adsorption apheresis (GMA) is a therapeutic option for remission induction in the active ulcerative colitis (UC) patients. Effects of high processed blood volume of GMA as remission induction therapy on the long-term prognosis of UC patients have remained unclear. For this study, we investigated the relation between re-exacerbation of UC and the processed blood volume of GMA performed as induction therapy.
Methods: Data from UC patients treated using a total of 10 GMA sessions as remission induction therapy during 2012 - 2022 were retrospectively collected and analyzed. The relation between the GMA dose, processed blood volume of GMA divided by body weight, and UC re-exacerbation requiring inpatient treatment within 1 year was evaluated.
Results: This study examined data of 72 active UC patients, with median age of 44.4 years (65% male) and median GMA dose of 34.2 mL/kg/session. Kaplan-Meier analysis showed the 1-year exacerbation-free rate was significantly higher in the higher GMA dose group than in the lower GMA dose group (P = 0.008). Cox proportional hazards regression analyses revealed a higher GMA dose as inversely associated with the re-exacerbation of UC within 1 year (hazard ratio: 0.36, 95% confidence interval: 0.17 - 0.78). Extended treatment time of GMA session beyond 60 min contributed to achieving the higher GMA dose and did not increase unexpected treatment termination because of clotting.
Conclusion: Greater processed blood volume of GMA per patient body weight may be associated with a lower 1-year exacerbation rate in UC patients.
Keywords: Ulcerative colitis; Granulocyte and monocyte adsorption apheresis; Remission induction therapy
| Introduction | ▴Top |
Granulocyte and monocyte adsorption apheresis (GMA) is applied as a non-pharmacological treatment strategy for induction and/or maintenance of remission in active ulcerative colitis (UC) patients. GMA treatment, performed using the adsorption column fulfilled with carrier beads that interact with fragment crystallizable-gamma receptor (FcγR) and complement receptor expressing on the surface of granulocyte and monocytes/macrophages, selectively adsorbs and removes these cells from the circulating blood. In fact, GMA is based on the hypothesis that removal of proinflammatory cells from a patient’s blood is beneficial for modulating disease activity [1-5]. Pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β are reduced as myeloid leukocytes producing these humoral factors are directly adsorbed and removed from circulation by GMA. Apoptotic cells resulting from contact of leukocytes with GMA columns are known to trigger the differentiation of CD19-positive B cells into regulatory B cells producing a major anti-inflammatory cytokine IL-10 [2, 4, 5]. Clinical efficacies of GMA for UC have been reported from randomized controlled trials [6-9]. A recent meta-analysis has shown that GMA, as an adjunctive therapy, is more effective for both inducing and maintaining remission in UC patients than conventional pharmacological therapy alone [3]. It has been suggested that treatment strategies including GMA for remission induction affect the maintenance of long-term remission in UC patients [10-12]. Some studies have demonstrated a relation between the therapeutic dose of GMA at remission induction and relapse-free rate until 6 months [13, 14]. However, it remains unclear whether the therapeutic dose of GMA at remission induction is associated with long-term patient prognosis.
The aim of this study was investigation of whether the GMA dose, the processed blood volume per session divided by patient body weight at remission induction therapy, is associated with UC exacerbation requiring hospitalization for 1 year. We also evaluated the efficacy and safety of treatment time extension, which is a simple procedure to increase the GMA dose.
| Materials and Methods | ▴Top |
Study design and patients
We conducted a retrospective cohort study. The inclusion criteria were: 1) adult (age 18 years and older) active UC patients treated using GMA between April 1, 2012 and April 30, 2022 at a blood purification unit in a tertiary care hospital, and 2) UC patients who started GMA twice weekly and completed a total of 10 sessions. Twice weekly GMA treatment has been reported as associated with a higher remission induction rate than that of the weekly method and which is the standard GMA treatment protocol at our hospital [6]. The decision of GMA induction was made by the attending doctors of each UC patient, independently of the physicians responsible for blood purification. The exclusion criteria were the following: 1) patients who had undergone fewer than 10 GMA sessions, 2) patients for whom all GMA sessions were performed once a week at the request of the patient or attending doctors, 3) patients who had undergone a series of GMA treatment in the past 6 months, 4) patients who did not show clinical improvement by GMA and could not be evaluated for re-exacerbation, and 5) patients who indicated refusal to participate in this study.
Institutional Review Board approval and ethical compliance
All procedures performed in this retrospective study were approved by the Research Ethics Committee of the Faculty of Medicine of the University of Tokyo (approval number, 2269). All procedures performed in this study were in accordance with the principles outlined in the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
GMA treatment and definition of GMA dose
All GMA sessions were performed as remission induction therapy for UC using an adsorptive column (Adacolumn®; Jimro Co., Ltd, Gunma, Japan) in the extracorporeal circuit. Vascular access was obtained by puncturing two upper extremity veins with 17 G needles at each GMA session. During the treatment session, blood was drained from the patient’s body and was circulated through the circuit at a rate of 30 mL/min according to recommended manufacturing protocols. Nafamostat mesylate, chosen as an anticoagulant considering the risk of bleeding from the colon, was administered continuously into the circuit during the treatment session. If nafamostat mesylate was considered unsuitable for the patient, sodium heparin was used. Acid citrate dextrose (ACD)-A solution was not available at our center as an anticoagulant for extracorporeal circulation. The standard treatment time of a GMA session was 60 min. When the patient agreed with the physician who explained the expected advantages and disadvantages of the extended treatment time before starting a series of sessions, GMA treatment time was scheduled for 90 min, with due consideration for patient safety. After May 2019, all patients were briefed on the extension of treatment time for GMA. The GMA dose was defined as the processed blood volume per treatment session divided by the body weight.
Data collection
Basic information of the participants was obtained from their electronic medical records: age, gender, duration of UC, extent of UC lesion, body weight, comorbidities (diabetes, hypertension, dyslipidemia, and chronic kidney disease with less than 60 mL/min/1.73 m2 of estimated glomerular filtration rate), smoking, alcohol drinking (more than three times per week), processed blood volume and treatment time of GMA, medications for UC, and use of biological agents. Laboratory data were also obtained from electronic medical records: white blood cell count (WBC), hemoglobin (Hb), serum albumin (Alb), serum C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR).
The clinical severity of UC before starting GMA was assessed according to the criteria proposed based on the Truelove-Witts index, as in previous reports [15-17]. Briefly, the severity of UC in each participant was assessed based on six variables (number of diarrhea instances, presence of bloody stool, fever, tachycardia, anemia, and ESR) and was classified into one of three categories: mild, moderate, or severe. We also evaluated the Seo index to quantify the disease activity of UC before GMA [18]. The Seo index score was calculated based on data of bloody stool, number of defecations, ESR, Hb, and serum Alb. Based on the score, we classified UC severity into three levels: mild (less than 150), moderate (150 - 220), and severe (more than 220). Clinical improvement and remission after completion of GMA treatment were defined as a decrease in Seo index score of 30 or more from the pre-GMA value and a Seo index score of less than 120 after GMA, respectively [19].
Outcomes
Patient prognostic data were obtained through electronic medical records. The primary outcome was UC exacerbation, defined as clinical worsening of UC requiring hospitalization within 1 year after GMA. Although intensified outpatient treatment might be given for worsening symptoms and laboratory findings by individual physicians, we defined UC exacerbations as described above to determine it more objectively. The occurrence and date of the first observed outcome for each patient were investigated.
Statistical analysis
All statistical analyses were conducted using software (BellCurve for Excel, version 4.05; Social Survey Research Information Co., Ltd, Tokyo, Japan). Continuous data are expressed as the median (interquartile range). Mann-Whitney U-tests were used to compare continuous variables between two groups. Chi-square test and Fisher’s exact test were used to compare categorical variables. The Kaplan-Meier method and the log-rank test were used to compare differences in primary outcomes between the groups divided according to the GMA dose and/or treatment time of GMA. Univariate and multivariate Cox proportional hazards regression analyses were conducted to examine significant factors associated with UC exacerbation within 1 year after GMA. Results for which a P value less than 0.05 was obtained were inferred as representing significant difference.
| Results | ▴Top |
Study participant characteristics
Of the 104 UC patients treated by GMA during the study period, 72 patients were included in the analysis (Fig. 1). Patient characteristics are presented in Table 1. The median age was 44.4 years; 47 (65%) were male. The median duration from UC diagnosis to GMA was 5.3 years. Clinical severity of UC assessed by criteria proposed in our country was mild in six, moderate in 57, and severe in nine patients. Severity of UC classified according to the Seo index was mild in 20 patients, moderate in 41, and severe in 11. The median GMA dose was 34.2 mL/kg/session. Outcome occurrence, hospitalization because of worsening of UC within 1 year after GMA, occurred in 34 (47%) patients.
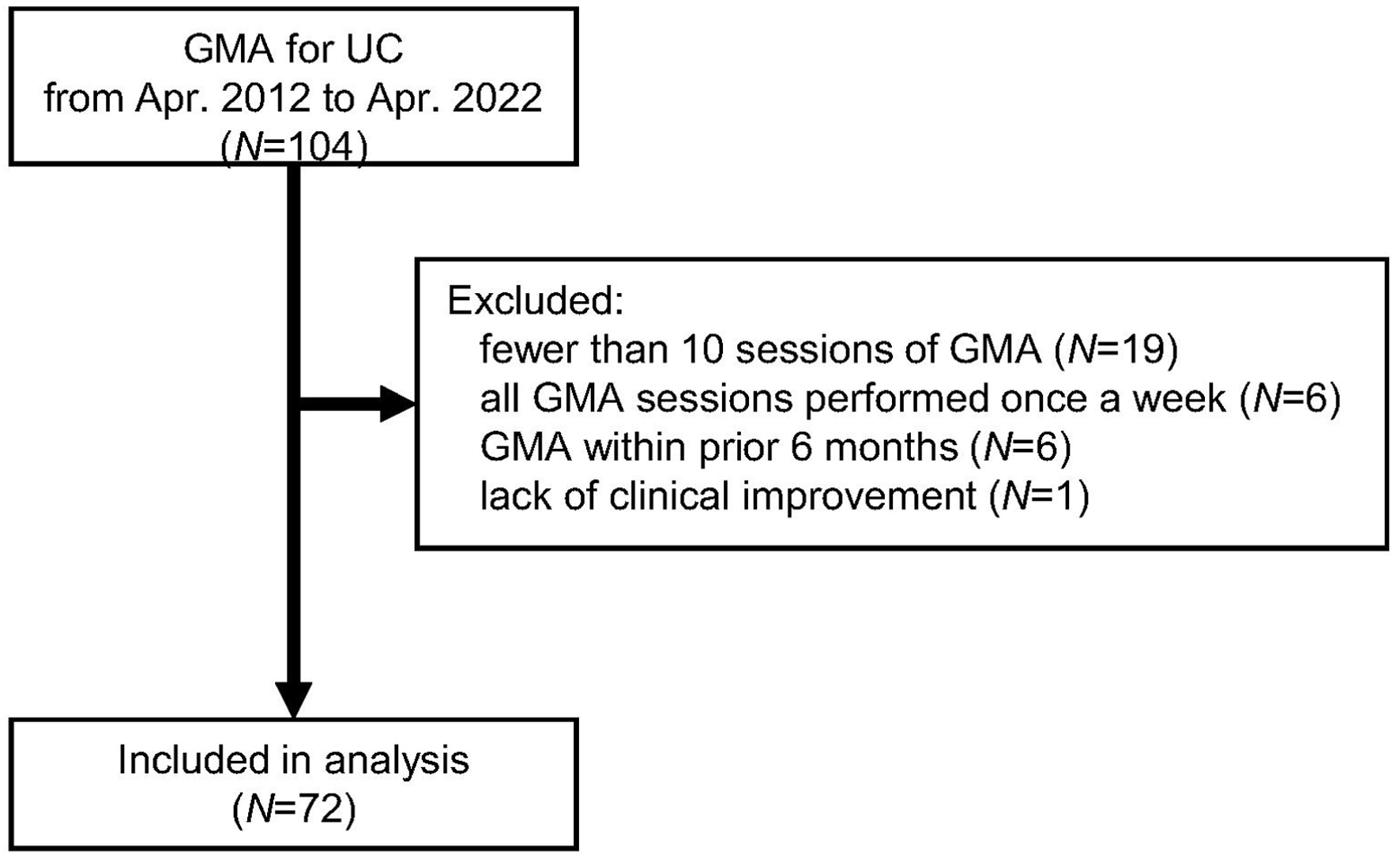 Click for large image | Figure 1. Flow diagram of the present study. GMA: granulocyte and monocyte adsorption apheresis; UC: ulcerative colitis. |
 Click to view | Table 1. Patient Characteristics and Comparisons Between Groups Divided by GMA Dose |
When all patients were divided into two groups based on the median value of the GMA dose (34.2 mL/kg/session), the lower GMA dose (LGD) group had a significantly higher proportion of males (81% vs. 50%, P = 0.01) and greater body weight (63.0 (57.8 - 68.7) vs. 51.0 (47.8 - 54.0), P < 0.01) than the higher GMA dose (HGD) group. Duration, extent, and severity of UC before starting GMA treatment were not significantly different between the two groups. The HGD group had significantly lower Hb levels (11.2 (10.6 - 12.1) vs. 12.7 (11.8 - 13.5) g/dL, P < 0.01). Medications at initiation of GMA and use of biological agents during follow-up period were not significantly different between the two groups. The exacerbation rate of UC within 1 year after GMA was significantly higher in the LGD group than in the HGD group (64% vs. 31%, P < 0.01).
Regarding laboratory data after a series of GMA treatment, the HGD group had significantly lower CRP (0.02 (0.02 - 0.10) vs. 0.06 (0.03 - 0.23) mg/dL, P = 0.03) and a trend toward lower WBC (8.8 (6.6 - 11.2) vs. 10.5 (9.0 - 11.7) × 103/µL, P = 0.09) than the LGD group (Table 2). The rate of clinical improvement and/or remission after a series of GMA treatment was not significantly different between the LGD and HGD groups (78% vs. 89%, P = 0.34). No significant differences were found in medication at the beginning and end of GMA treatment between the LGD and HGD groups (Tables 1 and 2). Sixty-five of the 72 patients were using 5-aminosalicylic acid (5-ASA) at the start of GMA. Of the seven patients who were not on 5-ASA at the initiation of GMA, three were started on 5-ASA medication after GMA initiation. The remaining four patients did not use 5-ASA because of allergies (N = 3) or followed a physician’s decision considering stable clinical course after GMA initiation (N = 1). Four patients were not using prednisolone (PSL) before the initiation of GMA but started PSL during a series of GMA treatments. Two of these patients started PSL soon after the first GMA session. The remaining two patients did not initiate PSL before the first session of GMA because of patient preference or physician concern about hyperglycemia, but were subsequently started on PSL. The rate of oral steroid use was significantly lower in the HGD group than in the LGD group for patients in the follow-up period at 6 months post-GMA (Supplementary Material 1, jocmr.elmerjournals.com).
 Click to view | Table 2. Disease Severity, Laboratory Data, and Medications After a Series of GMA Treatments |
Ten patients (28%) in the LGD group and 11 (31%) in the HGD group had received another series of GMA treatments more than 6 months before the GMA in this study, with no significant difference found between the two groups (P = 1.0).
Relation between outcome and GMA dose
The UC exacerbation rate within 1 year after GMA was compared between groups with LGD and HGD groups using Kaplan-Meier analysis and log-rank testing (Fig. 2). Results showed that the 1-year UC exacerbation-free rate in the HGD group was significantly higher than in the LGD group (P = 0.008). All-cause death, which could be a competing risk, was not found during the follow-up period.
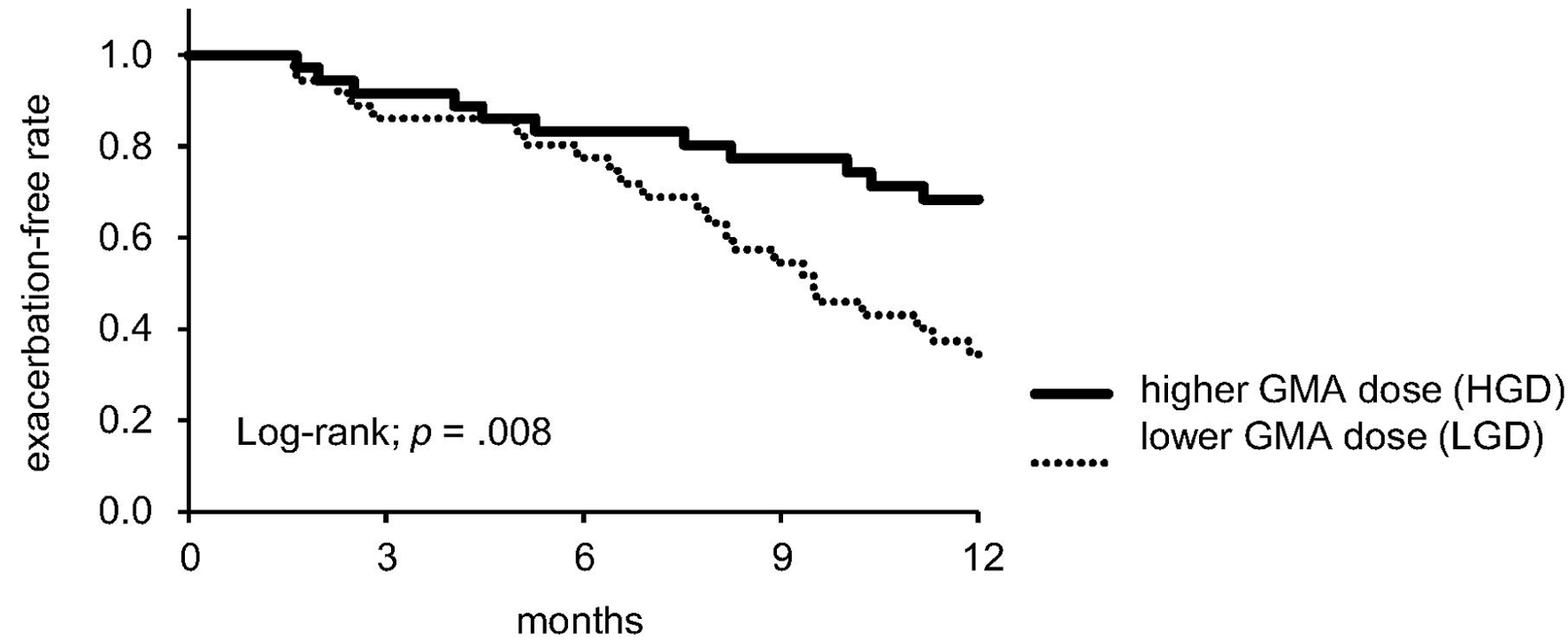 Click for large image | Figure 2. Kaplan-Meier analysis for UC exacerbation within 1 year after GMA. GMA: granulocyte and monocyte adsorption apheresis; UC: ulcerative colitis. |
Analyses using univariate and multivariate Cox proportional hazard models were conducted to evaluate the relation between outcome and variables including GMA dose (Tables 3 and 4). Results from univariate Cox regression analysis showed significant relations between reduced risk of UC exacerbation within 1 year and HGD (hazard ratio (HR): 0.39; 95% confidence interval (CI): 0.19 - 0.80; P = 0.01, Table 3). Multivariate analyses were performed with adjustment for factors which have been reported as associated with UC exacerbation and factors with P values < 0.1 in univariate analysis [20-22]. HGD was associated significantly with a reduced risk of 1-year UC exacerbation in all models (Table 4).
 Click to view | Table 3. Results of Univariate Cox Regression Analysis for 1-Year UC Exacerbation After GMA |
 Click to view | Table 4. Results From Multivariate Cox Proportional Hazards Model for UC Exacerbation Within 1 Year After GMA |
Comparison between groups with standard and extended treatment times of GMA
A total of 180 GMA sessions for 18 patients extended beyond 60 - 90 min of treatment time. A total of 540 sessions for 54 patients were scheduled for 60 min (Table 5). When groups with standard and extended treatment times were compared, the frequency of sessions in which the actual processed blood volume did not reach the standard treatment volume of 1,800 mL was not significantly different between the two groups. Session termination because of circuit coagulation was not different between the two groups, but termination because of defecation was significantly more common in the extended treatment time group (2.8% vs. 0.4%, P = 0.01).
 Click to view | Table 5. Safety of GMA Sessions With Standard and Extended Treatment Time |
Kaplan-Meier analysis was performed to compare outcomes between patient groups with standard and extended treatment time of GMA. No significant difference in the 1-year UC exacerbation rate was found between standard (N = 54) and extended (N = 18) treatment time groups (Fig. 3a). However, when patients with a standard 60 min treatment time were divided into two subgroups, one with a higher GMA dose (≥ 34.2 mL/kg, N = 19) and one with a lower GMA dose (< 34.2 mL/kg, N = 35), and compared among the three groups, the exacerbation-free rate was significantly lower in the group with standard treatment time and lower GMA dose than in the other two groups (log-rank; P = 0.016, Fig. 3b). Seventeen of 18 patients in the group with extended treatment time (94%) achieved GMA dose of 34.2 mL/kg or higher.
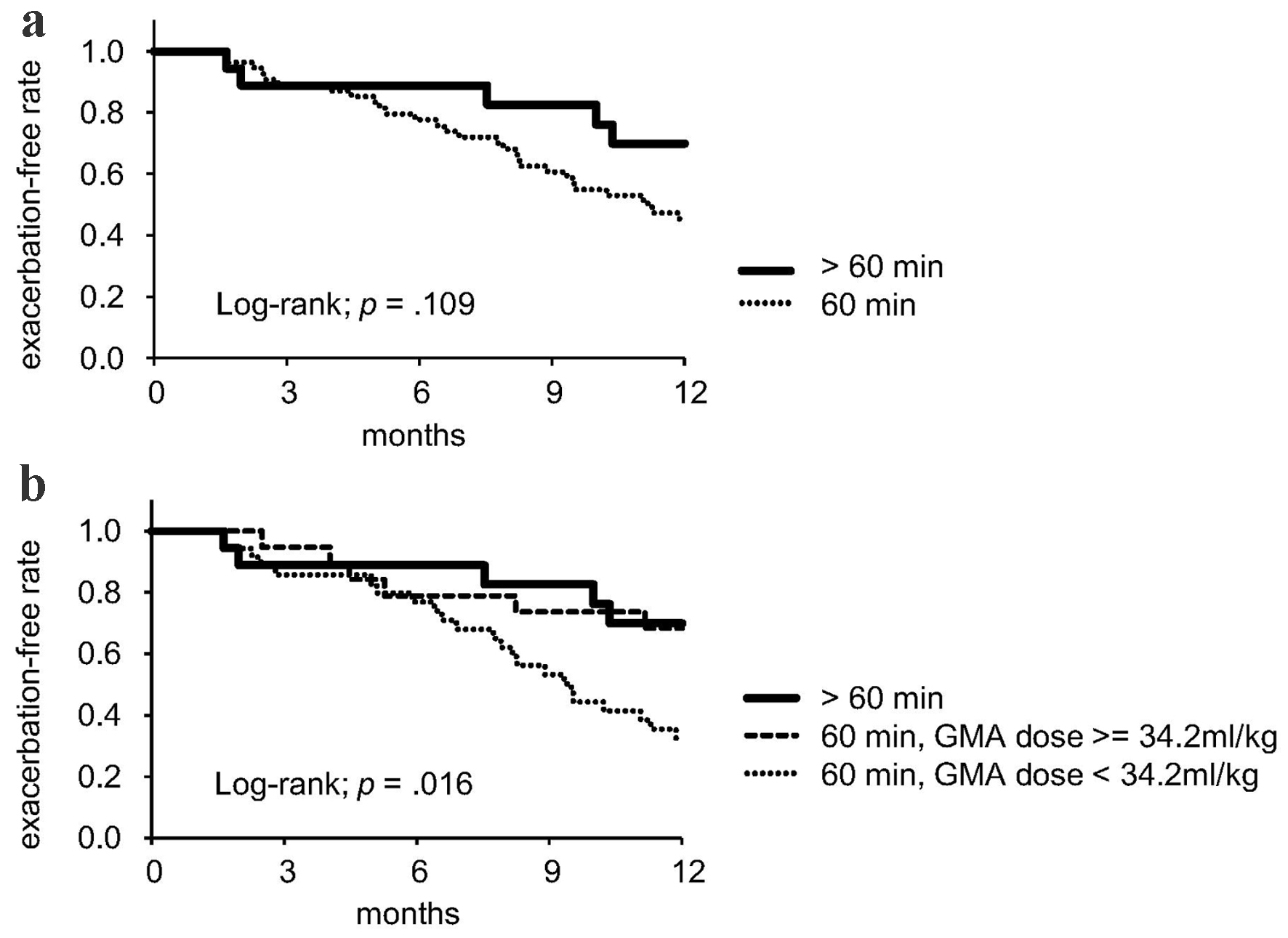 Click for large image | Figure 3. Comparison of groups divided by scheduled treatment time (a) and GMA dose (b). GMA: granulocyte and monocyte adsorption apheresis. |
| Discussion | ▴Top |
Our study demonstrated that a higher GMA dose, as calculated by dividing the processed blood volume by patient body weight, might be associated significantly with reduced risk of re-exacerbation of UC within 1 year. The UC exacerbation rate was significantly lower in the group of patients with extended treatment time, a simple method of increasing GMA dose, compared to the group of patients who had lower GMA dose by standard treatment time. Unexpected termination of GMA sessions because of any cause, such as circuit coagulation, was not increased significantly by the extended treatment time, suggesting that the treatment time extension was safely conducted.
The association between processed blood volume per session and clinical efficacy in GMA treatment as remission induction therapy for UC has been demonstrated in earlier studies [13, 14]. Yoshimura et al compared a group with a standard processed blood volume of 1,800 mL and a group with adjusted processed blood volume of 60 mL/kg according to the patient’s body weight. They found that the latter group had significantly reduced disease activity after a series of GMA than the former, as well as higher response maintenance rate at 6 months after GMA [14]. Kikuyama et al compared three groups of patients who received standard therapeutic dose of GMA, divided according to their weight. Their results revealed the remission rate at week 6 as significantly higher in the lowest body weight group, indicating that the ideal treatment dose was 38.7 mL/kg or higher [13]. Our results from analyses including multivariate Cox regression hazards model showed that a GMA therapeutic dose of 34.2 mL/kg or higher was significantly associated with a lower 1-year UC exacerbation rate, which apparently does not conflict with results presented in earlier reports.
Results of our study show that, although the 1-year outcome was similar between the extended treatment time group and the standard treatment time group with GMA dose of 34.2 mL/kg or higher, the 60 min treatment time group only achieving GMA dose of less than 34.2 mL/kg had a worse outcome compared with the other two groups. Clinical efficacies of GMA have been reported from around the world. The reported response or remission rates vary widely from above 80% to non-significant between publications [3, 7, 23]. In most earlier studies, the processed blood volume of each GMA session was fixed to 1,800 mL (at 30 mL/min blood flow for 60 min), although the body weight of participants might have varied from study to study. Therefore, in addition to heterogeneity of gender, disease activity, and concomitant medications, differences in GMA dose caused by differences in body weight might have affected the clinical efficacy for UC. Establishing the appropriate processed blood volume in a GMA session based on body weight might contribute to effective treatment for UC [13, 14]. Extending the treatment time, as was done for our study subjects, would be a simple method to achieve adequate GMA dose. It might be considered aggressively for patients with greater body weight, whose GMA dose is inadequate for 60 min treatment.
The mechanism of the therapeutic effect obtained from increased GMA dose by extended treatment time remains a subject of debate. Although results suggest that the surface of the GMA carrier saturates after 60 min of treatment, one report has described that leukocytes in the outflow remain reduced after prolonged (average 109 min) treatment, suggesting that the adsorption capacity might be maintained [2, 14, 24]. Our study showed that post-treatment white blood cell counts tended to be lower in the HGD group, in which nearly half of patients were treated for extended time, than in the LGD group. It remains unclear whether the effect of GMA on increasing anti-inflammatory humoral factors in the circulating blood is enhanced with prolonged treatment time. That subject remains open to study.
In the study described herein, no severe adverse effect related to the use of adsorption column for GMA was observed, although allergic reactions to the anticoagulant were observed in three patients. Flushing and headache, adverse effects commonly described in reports of earlier studies, were not observed in our study, at least not to a degree requiring treatment termination [3, 14, 23, 25]. Extending the treatment time did not increase session termination because of circuit coagulation, but it increased the frequency of treatment termination because of defecation compared to the standard treatment time. In active UC patients for whom the defecation interval became shorter, it would be desirable to set the adequate treatment time for GMA sessions considering not only the target GMA dose but also patient comfort and safety. For the present participants, GMA sessions were terminated when they complained of defecation even when the treatment time had not reached the planned time, suggesting that GMA was conducted considering the patient’s burden.
Crohn’s disease, which is classified as an inflammatory bowel disease similarly to UC, is sometimes treated using GMA to control disease activity. However, data demonstrating its efficacy have been limited. In a randomized controlled study, GMA did not improve the remission induction rate in patients with moderate to severe Crohn’s disease [26, 27]. Even in the same inflammatory bowel disease, UC and Crohn’s disease seem to respond differently to GMA treatment.
Our study had several limitations. First, because this study included a small number of patients at a single center, it remains unclear whether the results are applicable to patients at other hospitals. Second, because this retrospective study was based on data from medical records, it was not possible to assess the clinical activity index by Lichtiger or Rachmilewitz or to collect data of the endoscopic findings including Rachmilewitz’s index. We attempted to assess disease severity using indices such as the Seo index, which can be scored from the medical record data. Considering an earlier report describing that the Seo index correlates with disease activity indices including endoscopic items, and demonstrating that endoscopic items provide little additional information to indices of disease activity of UC, the evaluation of disease activity of UC in this study might be sufficient [28]. Third, although medication of GMA treatment was evaluated, cumulative doses of steroids during the follow-up period were not evaluated. Fourth, the patients with severe disease activity in UC were few, but this fact might indicate that our clinical practice is able to treat patients early before they become severely ill. A large multicenter prospective cohort study is warranted to address these limitations.
Conclusions
This study demonstrated that high GMA dose at remission induction therapy was associated with a low 1-year re-exacerbation rate in UC patients. Extension of the treatment time of GMA session might be useful to earn processed blood volume for an adequate GMA dose without increasing severe adverse events. Additional studies must be conducted to clarify the significance of GMA as remission induction therapy for UC on long-term prognosis.
| Supplementary Material | ▴Top |
Suppl 1. Medications 3 and 6 months after GMA treatments.
Acknowledgments
We are grateful to Fastek, Ltd for assistance with English proofreading.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Conflict of Interest
The authors report there are no competing interests to declare.
Informed Consent
Written informed consent was waived for the nature of retrospective study. All participants were guaranteed the opportunity to opt-out. Opt-out informed consent protocol was used for use of participant data for research purposes and this consent procedure was reviewed and approved by the Research Ethics Committee of the Faculty of Medicine of the University of Tokyo (approval number, 2269).
Author Contributions
All authors contributed to the study conception and design. This study was designed by YH. Material preparation, data collection, and analyses were performed by YH and RM. The first draft of the manuscript was written by YH, and was revised by RM, TS, SI, SI, MF, KD, and MN. All authors have read and approved the final manuscript.
Data Availability
The data which support the findings of this study are available from the corresponding author, YH, upon reasonable request.
Abbreviations
ACD: acid citrate dextrose; Alb: albumin; AZA: azathioprine; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; FcγR: fragment crystallizable-gamma receptor; GMA: granulocyte and monocyte adsorption apheresis; Hb: hemoglobin; HGD: higher GMA dose; IL: interleukin; LGD: lower GMA dose; PSL: prednisolone; TNF: tumor necrosis factor; UC: ulcerative colitis; WBC: white blood cell count; 5-ASA: 5-aminosalicylic acid; 6-MP: 6-mercaptopurine
| References | ▴Top |
- Sawada K. Cytapheresis (CAP) with leukocyte removal filter/bead column as one therapeutic option for inflammatory bowel disease. Transfus Apher Sci. 2017;56(5):689-697.
doi pubmed - Hanai H, Takeda Y, Eberhardson M, Gruber R, Saniabadi AR, Winqvist O, Lofberg R. The mode of actions of the Adacolumn therapeutic leucocytapheresis in patients with inflammatory bowel disease: a concise review. Clin Exp Immunol. 2011;163(1):50-58.
doi pubmed - Kiss S, Nemeth D, Hegyi P, Foldi M, Szakacs Z, Eross B, Tinusz B, et al. Granulocyte and monocyte apheresis as an adjunctive therapy to induce and maintain clinical remission in ulcerative colitis: a systematic review and meta-analysis. BMJ Open. 2021;11(5):e042374.
doi pubmed - Saez-Gonzalez E, Moret I, Alvarez-Sotomayor D, Diaz-Jaime FC, Cerrillo E, Iborra M, Nos P, et al. Immunological mechanisms of adsorptive cytapheresis in inflammatory bowel disease. Dig Dis Sci. 2017;62(6):1417-1425.
doi pubmed - Saniabadi AR, Tanaka T, Yamamoto T, Kruis W, Sacco R. Granulomonocytapheresis as a cell-dependent treatment option for patients with inflammatory bowel disease: Concepts and clinical features for better therapeutic outcomes. J Clin Apher. 2019;34(1):51-60.
doi pubmed - Sakuraba A, Motoya S, Watanabe K, Nishishita M, Kanke K, Matsui T, Suzuki Y, et al. An open-label prospective randomized multicenter study shows very rapid remission of ulcerative colitis by intensive granulocyte and monocyte adsorptive apheresis as compared with routine weekly treatment. Am J Gastroenterol. 2009;104(12):2990-2995.
doi pubmed - Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Kageoka M, Ikeya K, et al. Intensive granulocyte and monocyte adsorption versus intravenous prednisolone in patients with severe ulcerative colitis: an unblinded randomised multi-centre controlled study. Dig Liver Dis. 2008;40(6):433-440.
doi pubmed - Sakuraba A, Sato T, Naganuma M, Morohoshi Y, Matsuoka K, Inoue N, Takaishi H, et al. A pilot open-labeled prospective randomized study between weekly and intensive treatment of granulocyte and monocyte adsorption apheresis for active ulcerative colitis. J Gastroenterol. 2008;43(1):51-56.
doi pubmed - Domenech E, Panes J, Hinojosa J, Annese V, Magro F, Sturniolo GC, Bossa F, et al. Addition of granulocyte/monocyte apheresis to oral prednisone for steroid-dependent ulcerative colitis: a randomized multicentre clinical trial. J Crohns Colitis. 2018;12(6):687-694.
doi pubmed - Tanida S, Mizoshita T, Nishie H, Ozeki K, Katano T, Kubota E, Kataoka H, et al. Combination therapy with adalimumab plus intensive granulocyte and monocyte adsorptive apheresis in patients with refractory ulcerative colitis. J Clin Med Res. 2015;7(11):884-889.
doi pubmed - Fukuchi T, Kawashima K, Koga H, Utsunomiya R, Sugiyama K, Shimazu K, Eguchi T, et al. Induction of mucosal healing by intensive granulocyte/monocyte adsorptive apheresis (GMA) without use of corticosteroids in patients with ulcerative colitis: long-term remission maintenance after induction by GMA and efficacy of GMA re-treatment upon relapse. J Clin Biochem Nutr. 2022;70(2):197-204.
doi pubmed - Ueno N, Sugiyama Y, Kobayashi Y, Murakami Y, Iwama T, Sasaki T, Kunogi T, et al. Concomitant pharmacologic medications influence the clinical outcomes of granulocyte and monocyte adsorptive apheresis in patients with ulcerative colitis: A multicenter retrospective cohort study. J Clin Apher. 2023;38(4):406-421.
doi pubmed - Kikuyama R, Fukunaga K, Kawai M, Yokoyama Y, Kamikozuru K, Hida N, Ohda Y, et al. Relevance of the processed blood volume per granulocyte and monocyte apheresis session to its clinical efficacy in patients with ulcerative colitis. Ther Apher Dial. 2011;15(4):360-366.
doi pubmed - Yoshimura N, Tadami T, Kawaguchi T, Sako M, Yoshimoto H, Yamaka T, Takazoe M. Processed blood volume impacts clinical efficacy in patients with ulcerative colitis undergoing adsorptive depletion of myeloid lineage leucocytes. J Gastroenterol. 2012;47(1):49-55.
doi pubmed - Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2(4947):1041-1048.
doi pubmed - Hibi T, Sameshima Y, Sekiguchi Y, Hisatome Y, Maruyama F, Moriwaki K, Shima C, et al. Treating ulcerative colitis by Adacolumn therapeutic leucocytapheresis: clinical efficacy and safety based on surveillance of 656 patients in 53 centres in Japan. Dig Liver Dis. 2009;41(8):570-577.
doi pubmed - Sakamoto J, Munakata A. [The concept, criteria for diagnosis, and disease classification of ulcerative colitis]. Nihon Rinsho. 2005;63(5):744-749.
pubmed - Seo M, Okada M, Yao T, Ueki M, Arima S, Okumura M. An index of disease activity in patients with ulcerative colitis. Am J Gastroenterol. 1992;87(8):971-976.
pubmed - Higgins PD, Schwartz M, Mapili J, Krokos I, Leung J, Zimmermann EM. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut. 2005;54(6):782-788.
doi pubmed - Nakano R, Iwakiri R, Ikeda Y, Kishi T, Tsuruoka N, Shimoda R, Sakata Y, et al. Factors affecting short- and long-term effects of leukocyte removal therapy in active ulcerative colitis. J Gastroenterol Hepatol. 2013;28(2):303-308.
doi pubmed - Yokoyama Y, Watanabe K, Ito H, Nishishita M, Sawada K, Okuyama Y, Okazaki K, et al. Factors associated with treatment outcome, and long-term prognosis of patients with ulcerative colitis undergoing selective depletion of myeloid lineage leucocytes: a prospective multicenter study. Cytotherapy. 2015;17(5):680-688.
doi pubmed - Yamasaki S, Sakata Y, Yoshida H, Shirai S, Tanaka Y, Nakano R, Yukimoto T, et al. Shorter Relapse-Free Period after Leukocyte Removal Therapy in Younger than Older Patients with Ulcerative Colitis. Digestion. 2019;100(4):247-253.
doi pubmed - Sands BE, Sandborn WJ, Feagan B, Lofberg R, Hibi T, Wang T, Gustofson LM, et al. A randomized, double-blind, sham-controlled study of granulocyte/monocyte apheresis for active ulcerative colitis. Gastroenterology. 2008;135(2):400-409.
doi pubmed - Kashiwagi N, Sugimura K, Koiwai H, Yamamoto H, Yoshikawa T, Saniabadi AR, Adachi M, et al. Immunomodulatory effects of granulocyte and monocyte adsorption apheresis as a treatment for patients with ulcerative colitis. Dig Dis Sci. 2002;47(6):1334-1341.
doi pubmed - Hanai H, Watanabe F, Yamada M, Sato Y, Takeuchi K, Iida T, Tozawa K, et al. Adsorptive granulocyte and monocyte apheresis versus prednisolone in patients with corticosteroid-dependent moderately severe ulcerative colitis. Digestion. 2004;70(1):36-44.
doi pubmed - Connelly-Smith L, Alquist CR, Aqui NA, Hofmann JC, Klingel R, Onwuemene OA, Patriquin CJ, et al. Guidelines on the use of therapeutic apheresis in clinical practice - evidence-based approach from the writing committee of the american society for apheresis: the ninth special issue. J Clin Apher. 2023;38(2):77-278.
doi pubmed - Sands BE, Katz S, Wolf DC, Feagan BG, Wang T, Gustofson LM, Wong C, et al. A randomised, double-blind, sham-controlled study of granulocyte/monocyte apheresis for moderate to severe Crohn's disease. Gut. 2013;62(9):1288-1294.
doi pubmed - Higgins PD, Schwartz M, Mapili J, Zimmermann EM. Is endoscopy necessary for the measurement of disease activity in ulcerative colitis? Am J Gastroenterol. 2005;100(2):355-361.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.