| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Case Report
Volume 16, Number 9, September 2024, pages 436-439
Physiological Stimulus for the Synthesis of Basement Membrane Proteins Leading to Its Reconstruction
Jose Maria Pereira de Godoya, b, g , Maria de Fatima Guerreiro Godoyc
, Ana Carolina Pereira de Godoyd, e
, Dalisio Santi Netof
aCardiology and Cardiovascular Surgery Department, Medicine School in Sao Jose do Rio Preto (FAMERP), Sao Jose do Rio Preto, Brazil
bCNPq (National Council for Research and Development), Brasilia, Brazil
cMedicine School in Sao Jose do Rio Preto (FAMERP), Member Research Group in the Clinica Godoy, Sao Jose do Rio Preto, Brazil
dIntensive Care, Pediatric Cardiology, Hospital da Crianca e Maternidade (HCM), Medicine School of Sao Jose do Rio Preto (FAMERP), Sao Jose do Rio Preto, Brazil
eBrazil and Member Research Group in the Clinica Godoy, Sao Jose do Rio Preto, Brazil
fHospital de Base, Medicine School in Sao Jose do Rio Preto (FAMERP), Sao Jose do Rio Preto, Brazil
gCorresponding Author: Jose Maria Pereira de Godoy, Cardiology and Cardiovascular Surgery Department, Medicine School in Sao Jose do Rio Preto (FAMERP), Sao Jose do Rio Preto, Brazil
Manuscript submitted July 19, 2024, accepted August 23, 2024, published online September 18, 2024
Short title: Physiological Stimulus of the Synthesis of Proteins
doi: https://doi.org/10.14740/jocmr5266
| Abstract | ▴Top |
The aim of the present study was to report the remodeling of the basement membrane through physiological stimulus during the treatment of fibrosis in a lower limb with lymphedema. A clinical trial was conducted involving the evaluation of the basement membrane in skin biopsies before and after treatment for clinical stage II lower limb lymphedema using the Godoy method for the reversal of lymphedema and skin fibrosis. The samples were stained with Gomori’s reticulin stain and evaluated using Weibel’s multipoint morphometric method at the Godoy Clinic. Prior to treatment for lymphedema, rupture and important discontinuity of the basement membrane was found. After treatment, structural continuity and thickness had returned to the regions of previous rupture. The difference was statistically significant (P < 0.05, paired t-test). The present study reports that physiological stimuli targeting the lymphatic system led to the clinical reversal of fibrosis, as well as stimulate the synthesis of extracellular matrix proteins and the reconstruction of the basal lamina of the skin.
Keywords: Physiological stimulus; Synthesis of proteins; Basement membrane
| Introduction | ▴Top |
Basement membranes (BMs) are thin lamina of extracellular matrix that provide support for epithelia, muscle fibers, blood vessels and peripheral nerves. According to the results of scanning electron microscopy (SEM), the standard BM has a thickness of < 100 nm [1, 2]. However, SEM micrographs of retinas of human primates and non-human adults show that the thickness gradually increases with age, reaching about 2 µm at around 80 years [3]. Under a light microscope, a BM is poorly revealed by hematoxylin and eosin but is more intensely revealed with periodic acid-Schiff reagent, measuring around 1.0 to 1.5 mm in width [4].
BMs are multifunctional and modulate cell behavior, regulate organogenesis, promote tissue repair, form a barrier to tumor filtration and metastases, link growth factors and mediate angiogenesis [4, 5]. Studies have shown that members of the laminin family, collagen IV, nidogen 1 and 2, proteoglycans, perlecan, agrin and collagen XVIII are the main constituents of BMs [6]. Collagen IV and laminin self-assemble into two supramolecular networks that link to nidogen and perlecan to form a morphologically discernable BM/basal lamina [5]. Laminin is the most abundant non-collagen glycoprotein in the membrane [5].
Fibrosis is a common form of target-organ insufficiency in a variety of organic systems, including the liver, lung, heart, pancreas and kidneys. In chronic lymphedema, collector vessels become progressively fibrotic and are eventually replaced with scar tissues, which obliterates the luminal area of the vessels [7]. Several studies have implicated the transforming growth factor (TGF)-β1 pathway as an important factor to the regulation of fibrosis in different organ systems [8]. Although the exact mechanism by which fibrosis induces lymphatic dysfunction is not yet fully clarified, it is likely the result of the progressive obliteration of the initial lymphatic system and collector vessels, along with impaired collateral regeneration. The aim of the present study was to report the remodeling of the BM through physiological stimulus during the treatment of fibrosis in a lower limb with lymphedema.
| Case Report | ▴Top |
A 67-year-old patient was assessed clinically for the diagnosis of lymphedema. Other causes of unilateral edema were investigated, such as chronic venous disease, heart disease, kidney disease and hypoproteinemia. The patient had a 12-year history of edema and had one episode of erysipelas in the period, which was treated with an antibiotic. The physical examination revealed intense fibrosis and the absence of Godet’s sign. Volumetry was performed using the water displacement method, which revealed that the limb had 1,200 additional grams in comparison to the contralateral limb. The diagnosis was late primary lymphedema aggravated by an episode of erysipelas. A biopsy was performed of the internal malleolar region of the right leg before and after clinical normalization of the fibrosis, which was characterized by the return of elasticity and the absence of edema. For the biopsies, the patient was submitted to asepsis and antisepsis; local anesthesia was performed using 2 mL of Xylocaine 2%, followed by a longitudinal wedge-shaped incision approximately 1 cm in length and 0.5 cm in width. The biopsied material was preserved in 10% formalin and embedded in paraffin. The histological slices were stained with Gomori’s reticulin stain and the evaluation was performed using Weibel’s multipoint morphometric method.
Treatment
The Godoy intensive treatment method [9] was performed, which consisted of cervical lymphatic therapy using the Godoy method (approximately 30 gentle movements on the skin in the supraclavicular region 15 - 20 min per day) [10], combined with 8 h of mechanical lymphatic therapy involving an electromechanical device that performs approximately 25 passive plantar flexion and extension movements per minute [11], 2 h per day of manual lymphatic therapy [12], and a compression mechanism. Hand-crafted stockings made with grosgrain fabric [13] were alternated with medium-stretch elastic bandages, which were maintained throughout the entire treatment. The duration of treatment was 2 months, when the clinical reversal of fibrosis was achieved, and the elasticity of the skin had improved. At this point, the post-intervention biopsy was performed.
Outcomes
Prior to treatment for lymphedema, rupture and important discontinuity of the BM was found. After treatment, structural continuity and thickness had returned to the regions of previous rupture. The difference was statistically significant (P < 0.05, paired t-test) (Table 1 and Fig. 1).
 Click to view | Table 1. Morphometry of Epidermis (µm) and Dermal Papillae (µm) |
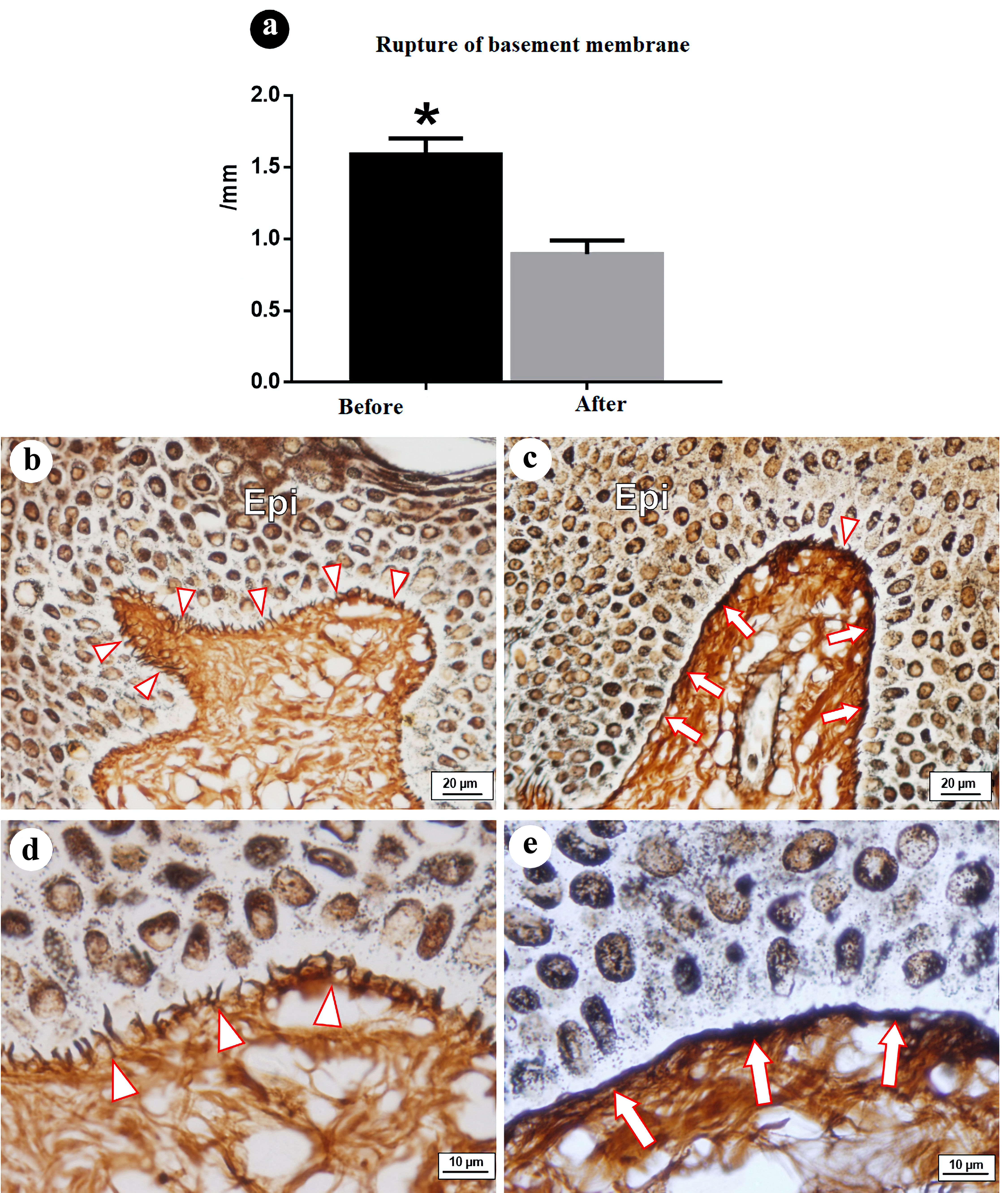 Click for large image | Figure 1. Rupture of basement membrane (ruptures/mm), before, and after treatment: epidermis-dermis interface, region of basement membrane - collagen III. (a) Graph of ruptures/mm of epidermis-dermis interface before and after treatment. Note significant difference between groups. (b, d) Region of basement membrane (papillary epidermis-dermis interface) prior to treatment, with reticular fibers (collagen IV) presenting ruptures and discontinuity. (c, e) Region of basement membrane showing return of structural continuity and thickness in regions of previous rupture. Epi: epidermis; arrowhead: rupture of collagen III in region of basement membrane; arrows: continuity and thickness of reticular fibers in region of basement membrane. Staining: Gomori’s reticulin. |
| Discussion | ▴Top |
The present study shows that it is possible to reconstruct the BM of fibrotic skin using specific stimuli on the lymphatic system. This is the first study in the literature to evaluate a specific form of lymphatic therapy for the treatment of stage II lymphedema that proposes the normalization or near normalization of lymphedema in all clinical stages, including elephantiasis, paving the way for a new line of research involving the reconstruction of the skin - in this case, specifically the basement basal lamina.
A previous study reports the loss of compliance of the soft tissues and lymphatic tissues, as seen in histological samples from patients with chronic lymphedema, which can considerably diminish lymphatic function, leading to the obliteration of the lymphatic vessels [14]. TGF-β1 is a well-known regulator in the synthesis of extracellular matrix, the function of which is to diminish fibrosis in practically all organ systems, such as the lung, liver, kidney, skin and cornea [14, 15]. Th2 cytokines interact and cooperate with TGF-β1 in the regulation of tissue fibrosis [15]. Besides its profibrotic effects, TGF-β1 inhibits the lymphatic endothelium, cell proliferation, migration and the formation of tubules [15]. These aspects have led to the hypothesis that the increased expression of TGF-β1 is the result of stasis of the lymphatic fluid.
There are several hypotheses and probable mechanisms in the involvement of skin in the fibrotic process. The initial changes in patients with lymphedema lead to the accumulation of proteins in the interstitial space, progressing to the development of lymphedema itself. Therefore, in terms of the treatment of fibrosis, the interference in its physiopathology, which is the main therapeutic basis, was a specific form of lymphatic therapy - the Godoy & Godoy method, which proposes the clinical reversal of fibrosis.
The present study evaluates the specific effect of treatment on the BM. However, the authors are currently performing a series of investigations in each region and the mechanisms involved. The most important aspect of the present study is that it paves the way for a line of therapy for fibrosis and the investigation of each phase of the process. The epidermis is significantly affected and is of fundamental importance in terms of medicinal therapies involving creams, pomades, etc. Therefore, new analyses and the use of SEM may identify and help analyze the dynamics of the changes that occur in the skin.
Conclusions
The present study reports that physiological stimuli targeting the lymphatic system led to the clinical reversal of fibrosis, as well as stimulate the synthesis of extracellular matrix proteins and the reconstruction of the basal lamina of the skin.
Acknowledgments
None to declare.
Financial Disclosure
No funding was used in this study.
Conflict of Interest
The authors declared no conflict of interest.
Informed Consent
This study received approval from the Institutional Review Board of the Sao Jose do Rio Preto School of Medicine (#4.398.518). The patient signed the consent form.
Author Contributions
Design and conduct of the study, collection data, management, analysis and interpretation of the data, preparation, review, approval of the manuscript, decision to submit the manuscript for publication: Godoy ACP, Godoy MFG, Godoy JMP, and Santi Neto D.
Data Availability
The data used to support the findings of this study are included within the article.
| References | ▴Top |
- Halfter W, Oertle P, Monnier CA, Camenzind L, Reyes-Lua M, Hu H, Candiello J, et al. New concepts in basement membrane biology. FEBS J. 2015;282(23):4466-4479.
doi pubmed - Halfter W, Candiello J, Hu H, Zhang P, Schreiber E, Balasubramani M. Protein composition and biomechanical properties of in vivo-derived basement membranes. Cell Adh Migr. 2013;7(1):64-71.
doi pubmed pmc - Cummings CF, Hudson BG. Lens capsule as a model to study type IV collagen. Connect Tissue Res. 2014;55(1):8-12.
doi pubmed pmc - Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17(12):1063-1072.
doi pubmed - Mak KM, Mei R. Basement membrane type IV collagen and laminin: an overview of their biology and value as fibrosis biomarkers of liver disease. Anat Rec (Hoboken). 2017;300(8):1371-1390.
doi pubmed - Uechi G, Sun Z, Schreiber EM, Halfter W, Balasubramani M. Proteomic view of basement membranes from human retinal blood vessels, inner limiting membranes, and lens capsules. J Proteome Res. 2014;13(8):3693-3705.
doi pubmed - Li CY, Kataru RP, Mehrara BJ. Histopathologic features of lymphedema: a molecular review. Int J Mol Sci. 2020;21(7):2546.
doi pubmed pmc - Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199-210.
doi pubmed pmc - Pereira de Godoy JM, Guerreiro Godoy MF, Barufi S, Pereira de Godoy HJ. Intensive treatment of lower-limb lymphedema and variations in volume before and after: a follow-up. Cureus. 2020;12(10):e10756.
doi pubmed pmc - Pereira de Godoy JM, Pereira de Godoy HJ, Pereira de Godoy AC, Guerreiro Godoy MF. Case Report: Godoy & Godoy method of cervical lymphatic therapy - indirect evaluation of the effect of the duration of stimulation on ocular edema. F1000Res. 2022;11:112.
doi pubmed pmc - Pereira de Godoy HJ, Pereira de Godoy AC, Lopes Pinto R, Baruffi S, Pereira de Godoy JM, Guerreiro Godoy MF. Mechanical lymphatic therapy to maintain the results of treatment for lymphedema. Acta Phlebol. 2021;22:51-54.
doi - de Godoy JMP, de Godoy ACP, Maria FGG. Evolution of Godoy & Godoy manual lymph drainage. Technique with linear Movements. Clin Pract. 2017;7(4):1006.
doi pubmed pmc - Guerreiro Godoy MdF, Barufi S, Pereira de Godoy Capeletto P, Pereira de Godoy HJ, Pereira de Godoy JM. Grosgrain stockings in the treatment of primary congenital lymphedema. Electron J Gen Med. 2022;19(2):354.
doi - Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, Rockson SG, Mehrara BJ. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am J Pathol. 2010;177(6):3202-3214.
doi pubmed pmc - Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med. 2001;194(6):809-821.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.