| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 16, Number 12, December 2024, pages 608-624
A Retrospective Chart Analysis Comparing Breast Cancer Detection Rates Between Annual Versus Biennial Mammograms
Pavan Patela, Hifza Sakhia, d, Devaki Kalvapudia, Angelo Changasa, Mukhamed Sulaimanova, Brian Criollo Gutierrezb, Idopise Umanac, Jake A. Slatonb, Hardeep Singhb
aTransitional Year, Northeast Georgia Medical Center, Gainesville, GA, USA
bGME Research, Northeast Georgia Medical Center, Gainesville, GA, USA
cInternal Medicine, Northeast Georgia Medical Center, Gainesville, GA, USA
dCorresponding Author: Hifza Sakhi, Transitional Year, Northeast Georgia Medical Center, Gainesville, GA 30501, USA
Manuscript submitted September 21, 2024, accepted November 14, 2024, published online December 20, 2024
Short title: Annual vs. Biennial Breast Cancer Screening
doi: https://doi.org/10.14740/jocmr6081
| Abstract | ▴Top |
Background: Per American Cancer Society, breast cancer is one of the most prevalent causes of cancer-related mortality in women in the United States. Different organizations vary in their recommendations regarding frequency of mammograms, with the United State Preventive Service Taskforce recommending biennial screening and other organizations like American College of Radiology promoting annual screening. The purpose of this study was to analyze institutional data to compare breast cancer detection rates among women undergoing annual vs. biennial mammograms.
Methods: In this retrospective chart review, we analyzed deidentified records of women aged 25 to 74 at Northeast Georgia Health System, who had undergone at least two screening mammograms and were diagnosed with primary breast cancer. We analyzed several variables including Breast Imaging Reporting and Data System (BI-RADS) categorization, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) status, age, race, ethnicity, nodal involvement, smoking status, insurance status, grade, tumor size, number of screening mammograms, personal history of breast cancer, family history of breast cancer, and their correlation to screening frequency (annual vs. biennial vs. less than biennial).
Results: Among the total 2,219 records that satisfied the inclusion criteria, we observed that BI-RADS categorization (P < 0.001), ER status (P = 0.003), and PR status (P = 0.001) were associated with mammogram screening frequency while the other variables were not statistically significant. Post-hoc analysis revealed that biennially screened patients exhibited less N2 node involvement than expected (P = 0.022). Additionally, Hispanic/Latino(a) patients had a greater frequency of biennial screenings than expected (P = 0.050). Lastly, post-hoc analysis revealed that current smokers had a greater incidence of less-frequent-than-biennial screenings (P = 0.023).
Conclusions: Annual mammograms were associated with a lower BI-RADS stage and lower stage of breast cancer diagnosis.
Keywords: Breast cancer; Mammogram; Annual; Biennial; ACR; USPSTF
| Introduction | ▴Top |
According to the American Cancer Society, breast cancer is the most common cancer among females in the United States with an annual incidence of about 287,850 (approximately one in three women) reported in 2022 [1-3]. While breast cancer is generally considered to be better managed compared to other cancers, it still presents significant challenges in terms of treatment and outcomes. While American College of Radiology (ACR), American College of Obstetricians and Gynecologists (ACOG), and American Cancer Society (ACS) National Comprehensive Cancer Network recommend annual screening for breast cancer, United State Preventive Service Taskforce (USPSTF) guidelines recommend biennial screening for breast cancer starting at the age of 40 [4-7]. Discordant guidelines for breast cancer screening have created confusion and challenges in determining the most effective approach for early detection.
The current guidelines defined by the USPSTF recommend screening for breast cancer every 2 years (biennially) in women aged 40 years or older [4]. However, other medical organizations including ACR recommend screening annually starting at the age of 40 for women at average risk, and earlier and more intensive screening (starting at age 25 - 30) for high-risk patients [5, 8], as higher risk women tend to develop more aggressive lesions and have more node-positive tumors [9]. Those with a personal history of breast cancer, family history of breast cancer, high-risk mutations (BRCA1, BRCA2, PTEN, SK1, and Li-Fraumeni syndrome), and minority ethnicity groups such as African American and Hispanic women are all at higher risk [5, 8]. Additional factors that increase the risk of breast cancer include genetic mutations, family history, chest radiation of ≥ 10 Gy at a young age, breast density, and race/ethnicity [9]. African American women have a 39% higher chance of mortality from breast cancer as compared to non-Hispanic White women [10]. Furthermore, non-Hispanic African American women were almost twice as likely (9% vs. 5-6%) to be diagnosed with a distant stage breast cancer than other ethnic groups [10]. The median age of breast cancer diagnosis in black women is 59 years as compared to 63 years in white women [11].
Both ACR and USPSTF recommend mammography as the preferred imaging modality for screening. Screening mammograms are effective tools for identifying abnormal lesions that could be the first signs of breast cancer, including a mass changing in size or presenting with characteristics such as being taller than wider, having spiculations, or displaying microcalcifications [12].
In agreement with ACR and other organizations that support annual breast cancer screening, most insurers pay for annual screening with no cost to the patients [13, 14]. In fact, ACR guidelines suggest using a risk assessment tool to stratify all women by age 25 based on risk factors to provide screening recommendations [4]. Women with calculated lifetime risk of 20% or more are recommended to undergo magnetic resonance imaging (MRI) and mammography starting at ages 25 to 30 [4]. The detection rate with annual mammograms is 2.32 per thousand screens, whereas with biennial mammograms it is 3.32 per 1,000 screens [15]. This increase in detection rate is associated with a 1.2% increase in 10-year survival among women aged 50 - 74 years who are diagnosed with breast cancer [15]. However, annual breast cancer screening leads to detection of cancers at earlier stages and results in fewer interval cancers diagnosed between mammogram screenings as compared to biennial or less frequent screenings [16]. Current barriers to annual screening from the patients’ perspective include differing guidelines, physician perspectives on guidelines, concerns about job security when taking time off for attending screening appointments, lack of access to transportation or childcare, cultural perceptions, language barriers, inaccessibility to primary care physicians, and underinsured status, which could result in patients having to self-pay up to $735 per mammogram [17-20].
Additionally, women who are apprehensive about healthcare and fearful of the mammography procedure have the longest screening times and are less likely to pursue further evaluation of abnormal screening results [18]. The other barrier to screening mammograms is the inability to access digital breast tomography at certain institutions [20]. Some of the proposed interventions to mitigate barriers to screening include building mammography centers closer to the target population, adjusting hours of operation to meet population needs, offering non-clinical options such as mobile mammography units, and removing administrative obstacles that can delay mammogram scheduling and delivery [21]. Regularly screening patients at higher risk for breast cancer is essential for decreasing the stage of diagnosis, which will result in better management and overall patient well-being [16]. Variations in screening frequency as recommended by different organizations can significantly influence tumor size, treatment options, and patient mortality [16]. Further investigation is needed to determine whether annual screening is more effective at detecting breast cancer at earlier stages, potentially providing patients with better treatment options and improving survival. While ACR, ACOG, and ACS National Comprehensive Cancer Network recommend annual screening for breast cancer, USPSTF guidelines recommend biennial screening for breast cancer starting at the age of 40 [4-7]. Due to differences in mammography screening guidelines published by the USPSTF (biennial) and ACR (annual), performing a retrospective study on our institutional data will provide more insight into which guidelines offer the best outcomes for patients [4, 6, 7].
While ACR does recommend an annual screening mammogram and further endorses the enforcement of a risk assessment tool at the age of 25 to determine duration and frequency of screening, it can lead to increased patient burden. On the other hand, the USPSTF recently updated its breast cancer screening guidelines, recommending that women at average risk begin regular mammograms at age 40, a shift from the previous recommendation of starting at age 50. Evidence showed that earlier screening led to earlier detection and therefore better outcomes, particularly since younger women have denser breasts and a higher propensity for developing more rapidly growing cancers [22]. The American Academy of Family Physicians (AAFP) supports these guideline changes, stating that this expansion in age range could save 19% more lives than before [23]. USPSTF also emphasizes the importance of personalized decision-making between health care providers and patients regarding screening intervals. Some women may opt for annual or biennial screenings based on individual risk factors and preferences. The guidelines further stress the need for additional research into optimal screening strategies for diverse populations [22].
When faced with different guidelines and recommendations, health care providers may have a hard time coming up with the best option for their patients. The aim of conducting this retrospective study in our institute was to compare the incidence of breast cancer diagnosis in those who were screened annually, biannually, or less frequently than biannually. With that information, we would be able to determine which organization’s screening guidelines are more effective for early diagnosis and therefore, early treatment.
Out of the seven prominent organizations which publish screening guidelines, most of them, including ACR, recommend annual screening, whereas USPSTF recommends biannual screening. Annual screening leads to early diagnosis but can lead to higher false positivity rates requiring more invasive investigations. Biannual screening reduces the incidence of false positive cases but does not catch early-stage breast cancer in those who are particularly susceptible. Both have their merits and demerits. Our study was conducted to compare which is more beneficial for patient outcome.
| Materials and Methods | ▴Top |
In this study, we performed a retrospective, non-interventional chart review of Northeast Georgia Health System (NGHS) records of women diagnosed with breast cancer, who had screening mammograms between January 2018 and 2023 with a goal to compare groups based on screening frequency and to elucidate a potential link to stage of breast cancer diagnosis. As data were planned to be pulled from the EPIC (EPIC Systems Corporation - electronic medical records) database retrospectively, hence study was determined to be exempted from our Institutional Review Board, and data were collected in compliance with all the applicable institutional ethical guidelines and health insurance portability and accountability act. Data were collected from the NGHS Clinical Research Data Platform by the blinded Graduate Medical Education Data Administrator, and 10% of the dataset was validated by the Data Administrator and investigating co-resident.
Inclusion and exclusion criteria were developed to minimize the effect of confounding variables on the results of screening frequency and the severity of breast cancer at detection. Inclusion criteria included females between the ages of 25 and 74 years at Northeast Georgia Health System (NGHS), who had at least two screening mammograms and were diagnosed with primary breast cancer. This resulted in 2,219 records that satisfied the inclusion criteria. Exclusion criteria included males, detection of cancer through imaging modalities other than a mammogram, greater than a 4-year screening interval, and non-primary breast cancer.
The independent variables in this study include screening frequency (annual/biennial/ more than 2 years), race (Asian, White, Hispanic, African American, and others), age (number), ethnicity (Hispanic vs. non-Hispanic), and lifetime risk (percentage, high-risk vs. low-risk). Dependent variables included primary breast cancer, Breast Imaging Reporting and Data System (BI-RADS) rating (0 - 6, as described in Table 1), TNM (tumor, node, metastasis) stage (Tables 2, 3) [24], tumor grade (1 = low, 2 = intermediate, 3 = high), estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) status, personal history of malignant neoplasm of the breast, family history of malignant neoplasm of the breast (yes/no; if yes, which family member), screening mammogram (yes/no, number), screening interval time (number of months: annual: 9 - 15 months (0.75 - 1.25 years), biennial: 21 - 27 months (1.75 - 2.25 years), > 3 years: 30 months or more (> 2.5 years), and mutations.
 Click to view | Table 1. Breast Imaging Reporting and Data System (BI-RADS) Categorization |
 Click to view | Table 2. TNM Staging |
 Click to view | Table 3. Elaboration of Tumor Spread Based on TNM Staging |
Chi-squared tests of independence were used to examine the association between screening frequency and other nominal variables. If the omnibus Chi-squared test rejected the null hypothesis of independence, it was deemed statistically significant, and post-hoc analysis was performed. Post-hoc analysis entailed the examination of the adjusted standardized residuals to determine which frequency table cells contribute the most to the bivariate association [25]. To account for the influence that frequency table size may have when examining multiple residuals within a Chi-squared test [26], a Benjamini-Hochberg correction was applied to control for false discovery rate [27]. Cramer’s V effect size statistic was calculated for any Chi-squared test that was statistically significant. Patient age is the sole continuous variable; thus, a multinomial logistic regression model was fit to screening frequency with age as a single predictor. This model was then compared to an intercept-only model using a likelihood ratio test to delineate if age as a predictor improves the model fit. The alpha criterion for null hypothesis rejection was set to P < 0.05 for all statistical tests. Statistical analyses were performed with the programming language R (version 4.3.3) in the RStudio IDE (Posit Software, PBC, Boston, MA, US) [28]. The risk stratification tool recommended by the ACR is available through the provided link [9].
| Results | ▴Top |
A total of 2,129 women aged 25 - 74 had mammograms between 2018 and 2023 and were diagnosed with primary breast cancer. The severity of breast cancer at the time of diagnosis in high-risk women between those who were screened at different intervals is analyzed Table 4. Totally, 1,728 women had annual screening mammograms, 384 women had biennial screenings, and 107 women had mammograms less frequently than every 2 years.
 Click to view | Table 4. Demographic Characteristics of 2,129 Women Diagnosed With Breast Carcinoma |
Demographic characteristics
Demographic characteristics of the women enrolled in the study is presented in Table 4.
Age
The differences in screening frequency with respect to age were not statistically significant. Age does not appear to be associated with whether women had biennial or less-frequent-than-biennial screenings compared to annual ones. The multinomial logistic regression model with age as a predictor did not perform significantly better than the intercept-only model (χ2 = 1.4, P = 0.503). This is evident by the model coefficients (Fig. 1), with biennial (odds ratio (OR) = 0.99, 95% confidence interval (CI): 0.97 - 1.02, P = 0.465) or less frequently than biennial (OR = 0.99, 95% CI: 0.98 - 1.01, P = 0.320) not displaying significantly differing ages compared to patients with annual screening frequency (Figs. 1, 2).
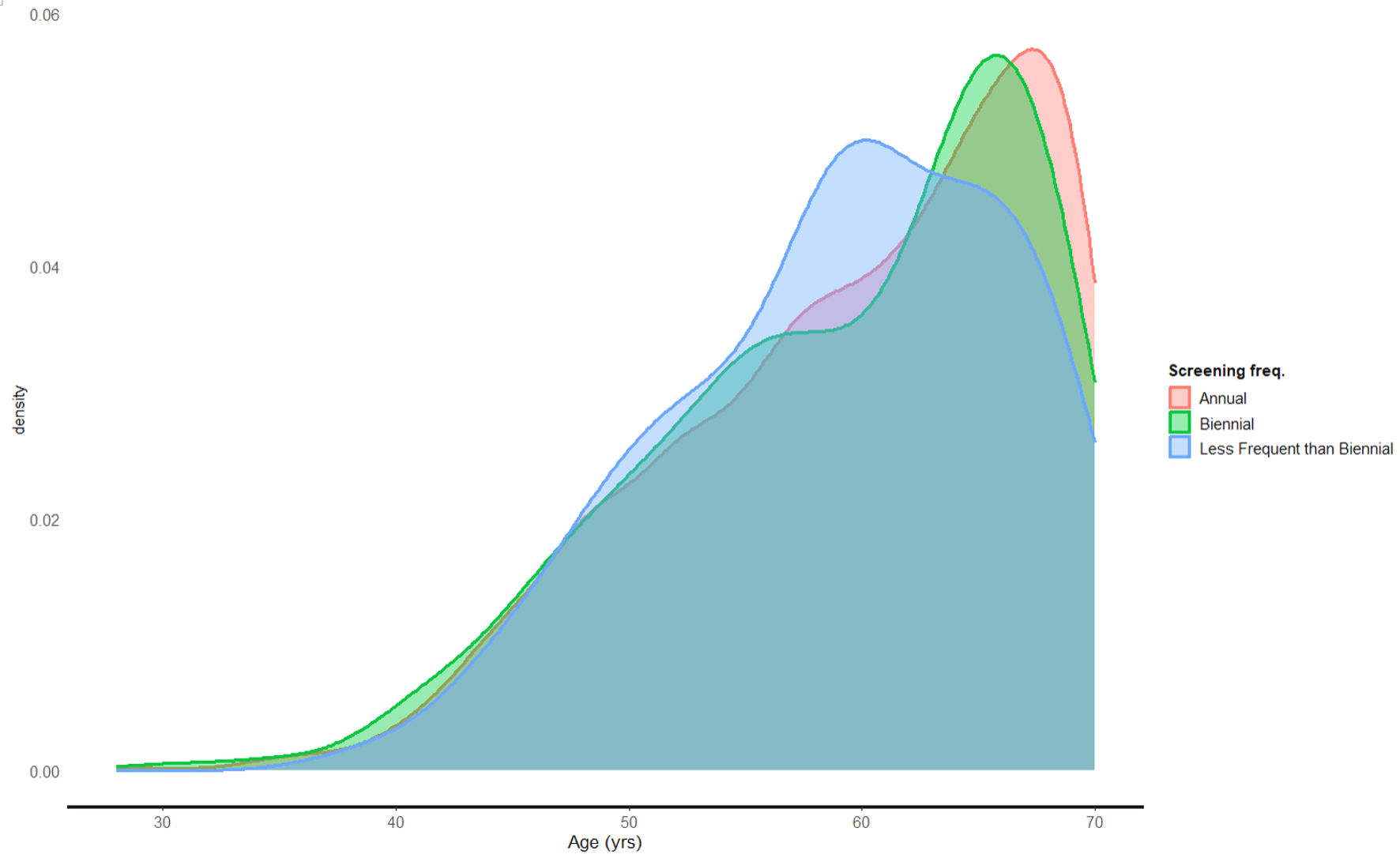 Click for large image | Figure 1. Screening frequency according to age. |
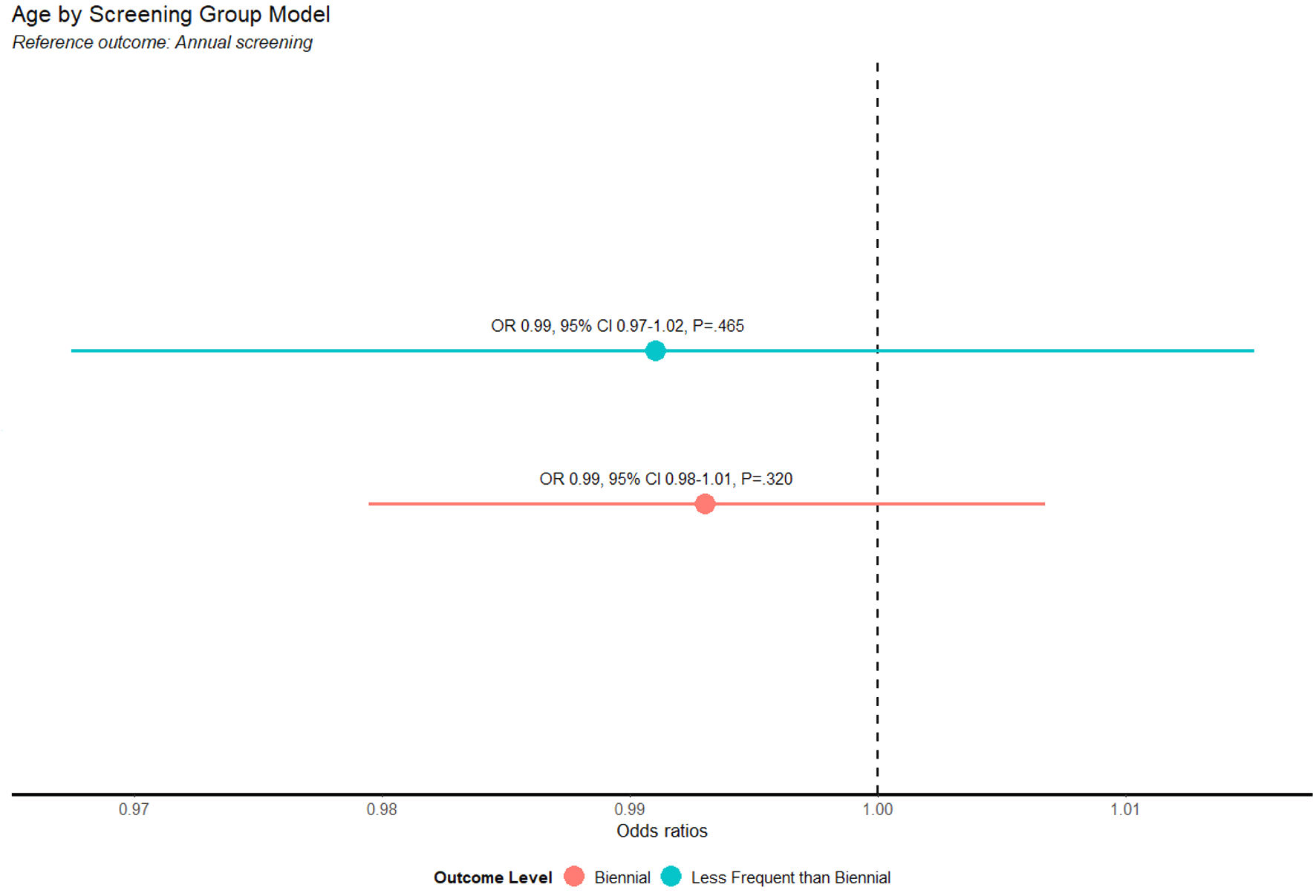 Click for large image | Figure 2. Age by biennial or less-frequent-than-biennial screening. OR: odds ratio; CI: confidence interval. |
Tumor characteristics: BI-RADS, ER status, PR status
BI-RADS classification, ER status, and PR status were significantly associated with patient screening frequency.
BI-RADS status and screening frequency
BI-RADS classification was statistically significant when compared to association with patient screening frequency, as depicted in Table 5 (χ2 = 72.2, P value < 0.001, effect size V = 0.13). Annually screened patients were more likely than expected to have “no malignancy” (z = 8.19, P < 0.001), whereas biennially screened patients (z = -7.36, P < 0.001) and less frequent than biennially screened patients (z = -2.85, P = 0.013) were less likely than expected to have “no malignancy” on BI-RADS. Annually screened patients were less likely to have a BI-RADS of “probably benign” than expected (z = -2.50, P = 0.031). Additionally, annually screened patients were less likely to have a BI-RADS of “suspicion of malignancy” (z = -4.82, P < 0.001) than expected, whereas biennially screened patients were more likely than expected to possess this BI-RADS staging (z = 4.77, P < 0.001) (Fig. 3).
 Click to view | Table 5. Screening Frequency and Resultant BI-RADS Classification of Carcinoma Breast Diagnosis in Women Undergoing Annual, Biennial, and Less-Frequent-Than-Biennial Screenings |
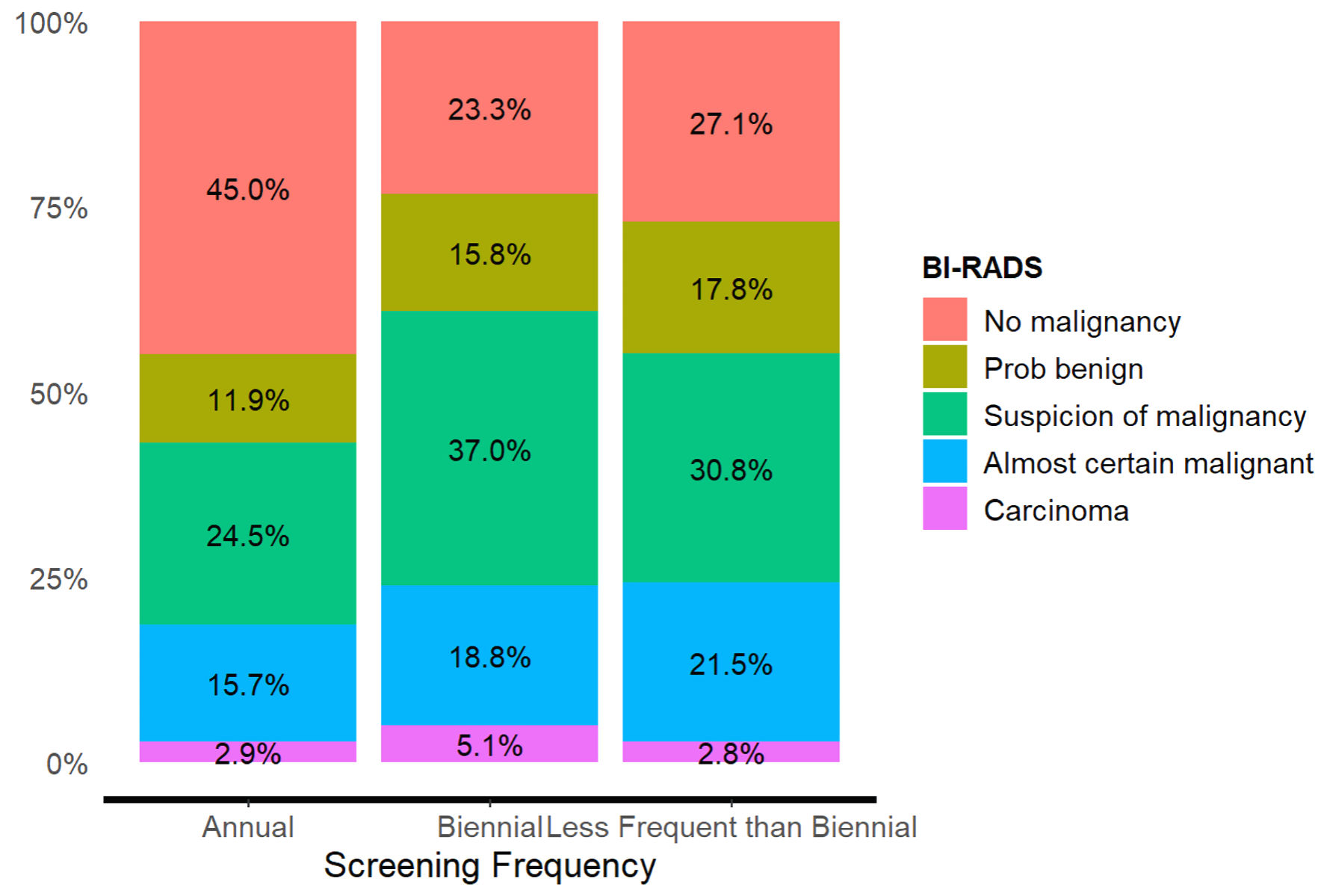 Click for large image | Figure 3. BI-RADS and screening frequency adjusted residuals (P < 0.050). BI-RADS: Breast Imaging Reporting and Data System. |
As the screening frequency decreased, women were diagnosed with carcinoma breast or more severe BI-RADS categories (Fig. 3).
ER status and screening frequency
ER status is statistically significantly associated with patient screening frequency (χ2 = 16.2, P = 0.003, effect size V = 0.08) (Table 6).
 Click to view | Table 6. ER Status in Women Who Were Diagnosed With Breast Carcinoma Undergoing Annual, Biennial, and Less-Frequent-Than-Biennial Screening |
Patients screened annually had a higher rate of undetermined ER status than expected (z = 3.78, P = 0.001). In contrast, patients screened biennially (z = -2.60, P = 0.021) and those screened less frequently than biennially (z = -2.78, P = 0.016) had lower rates of undetermined ER status than expected. Additionally, patients screened annually had a lower rate of positive ER status than expected (z = -2.86, P = 0.016), while those screened biennially (z = 2.00, P = 0.068) and less frequently than biennially (z = 2.03, P = 0.068) had higher rates of positive ER status than expected.
Annually screened patients had more undetermined ER status and fewer positive ER status cases than expected (Fig. 4). Conversely, biennially and less frequently than biennially screened patients had fewer undetermined ER status cases and more positive ER status cases than expected.
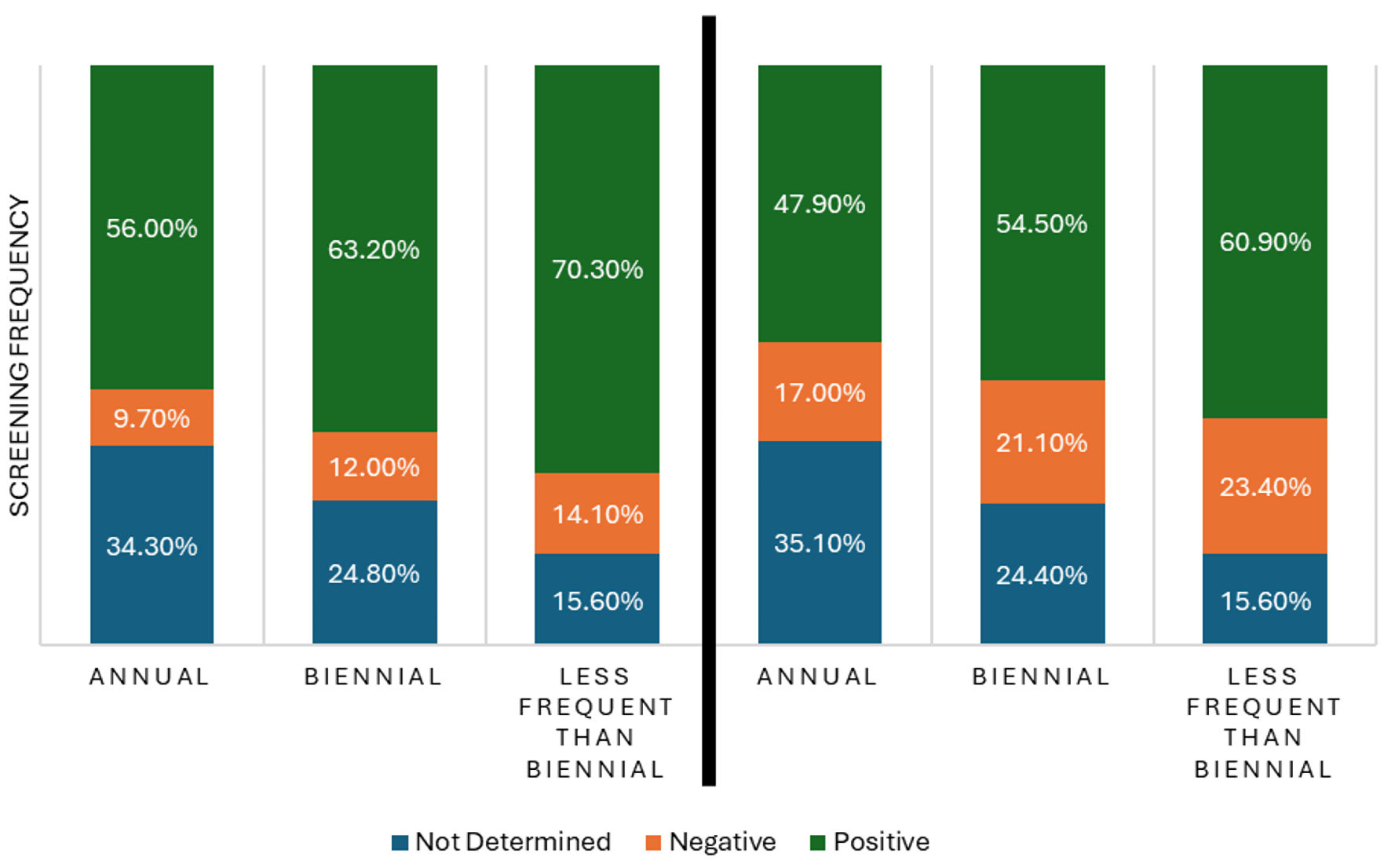 Click for large image | Figure 4. ER status, PR status and screening frequency adjusted residuals (P < 0.050). ER: estrogen receptor; PR: progesterone receptor. |
PR status and screening frequency
PR status is significantly linked to screening frequency (χ2 = 18.9, P = 0.001, effect size V = 0.08) (Table 7).
 Click to view | Table 7. PR Status in Women With Undergoing Annual, Biennial, and Less-Frequent-Than-Biennial Breast Cancer Screening |
Post-hoc analysis revealed that annually screened patients possessed a greater rate of undetermined PR status than expected (z = 4.15, P < 0.001). Conversely, patients who were screened biennially (z = -2.95, P = 0.013) and those screened less frequently than biennially (z = -2.86, P = 0.013) exhibited a lesser rate of undetermined PR status than expected. Annually screened patients exhibited a lesser rate than expected of positive PR status (z = -2.67, P = 0.017).
Annually screened patients having higher rates of undetermined PR status and lower rates of positive PR status than expected, whereas patients screened biennially or less frequently than biennially show lower undetermined PR status and higher positive PR status (Fig. 4).
Smoking, ethnicity, nodal involvement - clinically relevant factors
Though smoking status, ethnicity and nodal involvement were not statistically significant possibly due to trivial effect size (V = 0.05), we found them to be clinically relevant on post-hoc analysis.
Ethnicity
There is evidence of an association between ethnicity and screening frequency (χ2 = 5.9, P = 0.051). Subsequent analysis revealed this association was driven by Hispanic/Latina patients possessing a greater frequency of biennial screenings than expected (z = -2.40, P = 0.050). Non-Hispanic/Latina patients, therefore, exhibited a lesser frequency of biennial screenings than expected (z = 2.40, P = 0.050).
Smoking status
Our data show an association between smoking status and screening frequency (χ2 = 9.3, P = 0.053). This is likely due to current smokers having less-frequent-than-biennial screenings than expected (z = 3.02, P = 0.023).
Nodal involvement
Our data indicate that there is still evidence of a clinically significant association between node involvement and screening frequency despite the P value (χ2 = 14.8, P = 0.062), with a small effect size (V = 0.07), as shown in Table 8 and Figure 5. Biennially screened patients exhibited less N2 node involvement than expected (z = -3.19, P = 0.022).
 Click to view | Table 8. Nodal Involvement in Breast Cancer Screening Frequencies Occurring Annually, Biennially, and Less Frequent Than Biennially |
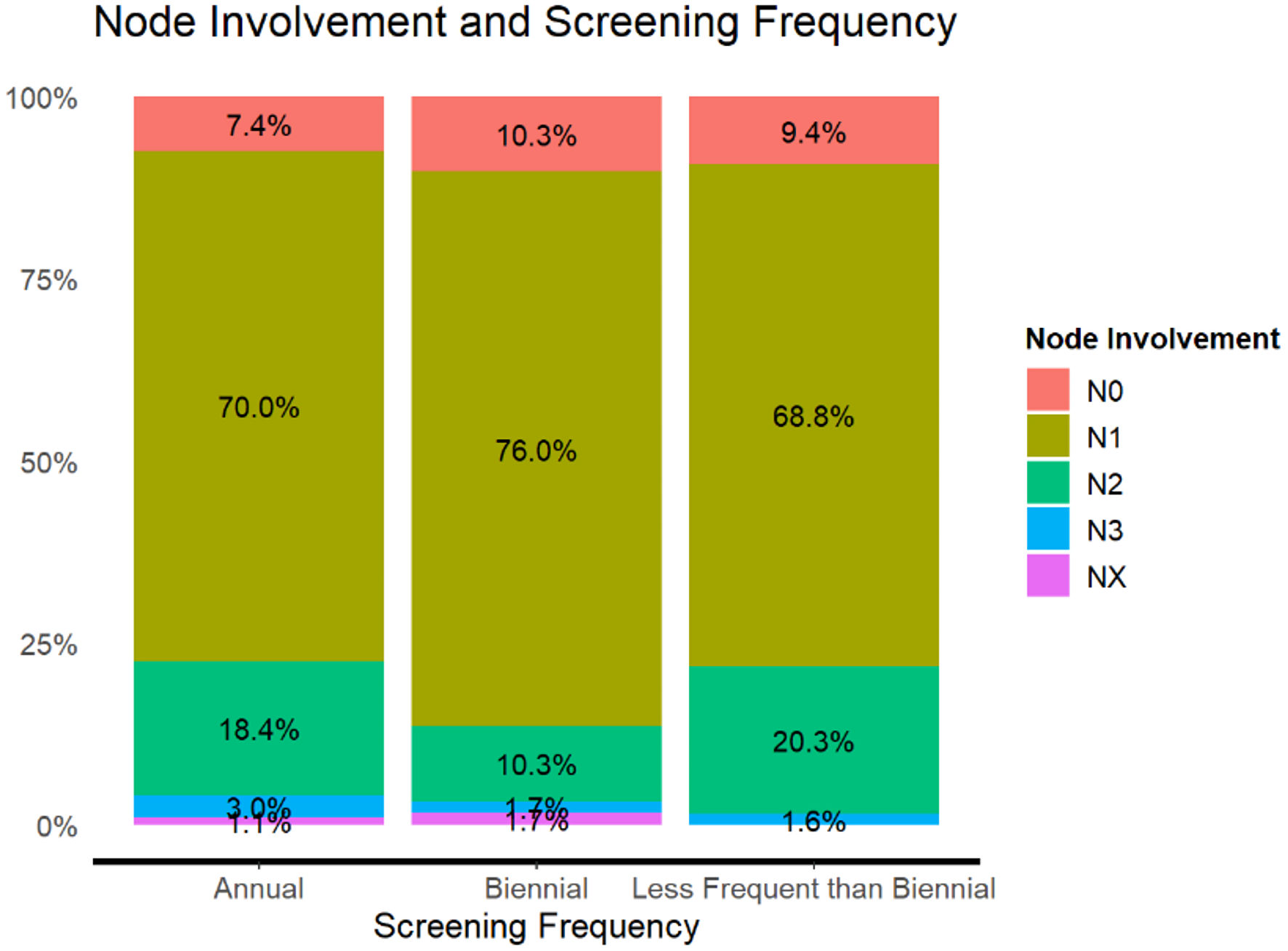 Click for large image | Figure 5. Node involvement and screening frequency adjusted residuals (P < 0.050). |
Race (χ2 = 5.9, P = 0.430), personal history (χ2 = 2.6, P = 0.278), family history (χ2 = 1.8, P = 0.404), insurance status (χ2 = 8.0, P = 0.239), tumor grade at the time of diagnosis (χ2 = 9.4, P = 0.155), tumor size at the time of diagnosis (χ2 = 20.2, P = 0.064), HER2 status (χ2 = 8.5, P = 0.076), or presence of metastasis (χ2 = 4.2, P = 0.377) were not statistically significantly associated with patient screening frequency.
| Discussion | ▴Top |
The study highlights the significance of annual mammograms over biennial screenings in detecting breast cancer at an earlier stage. Patients are stratified to receiving either screening or diagnostic mammograms depending on whether the patient has any presenting symptoms or concerning findings on physical exams or previous mammograms such as breast pain, breast discharge, or a palpable lump, at which point the mammogram is designated to be diagnostic [29]. Diagnostic mammograms are performed in the presence of a radiologist to ensure all relevant imaging to adequately assess the suspicious lesions can be obtained [29]. BI-RADS is a standardized framework created by ACR for reporting breast density and classification of breast lesions on imaging with their corresponding risk of malignancy and subsequent recommendations [29]. Breast density is a quantification of the fibroglandular tissue in the breast and is classified with grades A - D: A (fatty), B (scattered fibroglandular density), C (heterogeneously dense), and D (extremely dense) [30]. Breast density is an important parameter, as higher density decreases the sensitivity of mammograms [29-31]. Any masses that are present are described by their shape (round, oval, or irregular), margin (circumscribed, obscured, microlobulated, indistinct, and spiculated), and density (high density, equal density, low density, and fat-containing) [29]. Any associated findings such as skin or nipple retraction, skin thickening, and axillary lymphadenopathy are also reported [29]. All these findings are succinctly reported via a BI-RADS score that ranges from 0 to 6 (Table 1). Higher BI-RADS scores correlate with a higher likelihood of breast cancer, with a score of 6 representing biopsy-proven malignancy. Patients who receive a score of 4 to 5 are recommended to undergo a biopsy to perform a histopathological assessment of breast tissue to determine the presence of cancer [32]. A study from 2019 found that 34.8% of the biopsies conducted for BI-RADS category 4 were tumorous [32]. Another study from 2022 found that up to 85% of biopsies for BI-RADS 4 rating may be benign, highlighting substantial intra- and inter-radiologist variability when reporting BI-RADS scores [33]. Another reason accounting for substantial rates of biopsy-proven false-positive results is the wide-range in the BI-RADS categorization itself, since a category of 4 indicates a 2-95% likelihood of malignancy [33]. Nonetheless, BI-RADS scores provide a standardized method of communicating recommendations when any suspicious lesions may be present.
In our study, we found that BI-RADS categorization was statistically significant with respect to frequency of breast cancer screening (P < 0.001), indicating that annual screening results in lower BI-RADS categorization and earlier stage of breast cancer diagnosis. However, previous studies also show that annual mammography is associated with a higher rate of false-positives (higher BI-RADS categorization), with a cumulative 10-year probability of 61.3% in annual screens and 41.6% in biennial screens [34, 35]. Other negatives associated with false-positive results include delaying future mammograms and heightened anxiety associated with pursuing invasive workup [34, 35]. Cost-wise, these unnecessary interventions amount to healthcare expenditures totaling $4 billion a year. These contentious findings highlight the need for a balanced approach to screening intervals, weighing the benefits of early cancer detection (evidenced through lower BI-RADS categorization) against the risks of overdiagnosis, heightened anxiety, and expenditures incurred with unnecessary interventions.
Earlier studies also show that mammograms conducted annually result in fewer interval tumors as compared to those conducted at longer intervals [30]. Interval tumors occur in the interval period between successive mammograms. Notably, in women with dense breast tissue, there is a 13 - 31 times greater incidence of interval cancers [30]. Studies also show that interval tumors tend to be larger and more aggressive, resulting in more node-positive tumors [30]. A quandary to the screening recommendation is the higher incidence of false-positive results (higher BI-RAD categorization) with annual mammograms in women with dense breast tissue which necessitate biopsy recommendations that occur at a rate of 49.5% in annual screens vs. 30.7% in biennial screens [36]. False-positive results occur because dense breast tissue makes it difficult to distinguish tumorous lesions from normal tissue and thus reduces the sensitivity of mammograms [36]. On the other hand, studies show that women with non-dense breast tissue achieve a 41% mortality benefit from the conduction of mammograms whereas women with dense breast tissue only receive a 13% mortality benefit [37].
Although annual screenings incur higher monetary costs, require patients to take more time off work to complete procedures, cause greater radiation exposure, and may result in higher incidence of anxiety-provoking procedures such as biopsies for benign lesions, the downstream benefit lower morbidity and mortality as well as lower costs of treatment (up to $250,000 for metastatic tumors) outweigh the aforementioned negatives for most women [37]. Regarding the effect of radiation exposure through mammography, studies estimate that annual screening results in 236 additional lives saved vs. theoretically four lives lost from radiation-induced breast cancer [37].
Studies show that African American women tend to have a greater incidence of invasive breast cancers which are node-positive and metastatic but negative for estrogen, progesterone, and HER-2-neu receptors [37]. In this population, it is strongly advised to follow the annual screening protocol as opposed to biennial [37]. Additionally, studies demonstrate that Hispanic women have larger and later-stage tumors when undergoing biennial as compared to annual screens [37]. Furthermore, Asian women undergoing biennial screens also have a higher incidence of node-positive tumors [37]. In our data, although race was not significantly associated with frequency of breast cancer screening (P = 0.43), further delving into the ethnicity portion of our data revealed that Hispanic patients had a greater degree of biennial screens. This is aligned with studies which show that although Hispanic women tend to have lower incidence of breast cancer, when they are diagnosed, it is at a later stage, due to lower rate of mammography utilization [38]. Furthermore, studies show that foreign-born Hispanic/Latina women as compared to US-born Hispanic/Latina women had a higher likelihood of never having received breast cancer screening in their lifetime (even after accounting for sociodemographic confounders), ultimately placing them at a higher risk for late-stage diagnosis [38]. This is likely due to disparity in access to screening which is further exacerbated by lower educational attainment and income status of Hispanic/s/Latino populations in the USA, which ultimately results in underutilization of preventive services [38]. This is further exacerbated by the cost of obtaining a mammogram ($735 per scan), not accounting for any further workup in the case of a positive result [39].
In this study, we found that family history, personal history, and HER2-neu receptor status were not correlated with screening frequency. With regards to age and family history, it is an interesting finding as previous studies do show that family history and age correlate with the density of breast tissue [40, 41]. In fact, prior research shows that a family history of breast cancer results in higher stages of diagnosis, including N2 and TNM stage III [41]. We postulate that this was a consequence of class imbalance in our data as we had 1,728 annual screens, 384 biennial, and 107 less-than-biennial results, which makes it challenging to elucidate relationships.
Younger patients have greater density breast tissue whereas older women have lower density tissue and hence, we would have expected decreased cancer rates in both biennial and annual screens [42]. Our study found that there is a greater incidence of breast cancer with the ER status, and the findings were reversed for the PR, between the annual vs. biennial screens. Additionally, while smoking status (P = 0.053) and screening frequency were not statistically significant per the criterion of P = 0.05, but post-hoc analysis highlights that current smokers underutilize preventive screenings and more likely to undergo less-frequent-than-biennial screenings (P = 0.023).
Breast cancer is the most common type of cancer found in women with Li-Fraumeni syndrome, an autosomal dominant disorder associated with aberrant expression of tumor suppressor P53 [43]. Studies show that numerous genes are implicated in breast cancer development, including high penetrance genes such as BRCA1, BRCA2, and TP53 (resulting in triple negative breast cancers) and low penetrance genes such as CHEK2, CDH1, NBS1, RAD50, BRIP1 and PALB2 [42]. Interestingly, the genes of lower penetrance are more often implicated in breast cancer development as they are more likely to be mutated in the general population [42].
Regarding the node involvement, annual screens were associated with a greater propensity for N2 nodal involvement than biennial screens. We postulate this is attributed to patients with a greater node involvement pursuing further diagnostic workups and closely attending follow-up appointments as compared to those with lower node tumors. Studies delving into nodal involvement and screening frequency have found that women in their 40s tend to obtain the most benefit from annual screens, as biennial screenings in this age group are associated with higher incidence of late-stage disease [44].
Our retrospective observational study results support the recommendation for annual mammograms, aligning with ACR guidelines. This has implications for healthcare providers and policymakers in reinforcing the importance of guideline adherence to optimize breast cancer detection and management. Several organizations have developed a consensus in guidelines to determine the appropriate ages for screening mammography in women at average risk for breast cancer. Some organizations’ guidelines are listed in Table 9 [45-49].
 Click to view | Table 9. A Comparison Between Screening Guidelines From Various Societies in the USA |
The ages at which women should start, and end mammography examinations and their frequency have been a matter of debate for the past three decades. ACR and ACS suggest annual screenings have a better benefit and outcome for women who undergo annual screening mammographic studies as opposed to biennial screenings recommended by the USPSTF. This was also the outcome of the study conducted in our institution. Women undergoing annual screenings had better BIRADS outcomes and therefore, better prognosis. Additionally, there seems to be a broad agreement among all societies that high-risk women need to undergo additional or earlier screening [50, 51]. While more frequent screenings have their own clinical benefits and advantages, they introduce a variety of additional complications. Earlier research has shown that annual mammograms had higher sensitivity but lower specificity than biennial mammograms, which resulted in more false positives leading to unnecessary interventions [52]. Additionally, a study reported that the increased sensitivity of annual mammograms did not result in a proportional reduction in advanced stage disease [53]. Concerns for overdiagnosis, false-positive results, radiation harm, and unnecessary interventions cause undue burden on the women undergoing them.
Effective implementation of clinical guidelines optimizes patient care and minimizes the potential for unforeseen complications [54]. As Ricci-Cabello et al observed, for every 1,000 women screened according to clinical guidelines compared to those who were not, there were 138 more survivors and 336 more free of recurrence of breast cancer over a 5-year follow-up period [55]. The standardized approach of USPSTF screening guidelines makes it popular among insurance companies as it has a broader healthcare perspective [56]. However, priority should be given to individual preferences and values, as more emphasis is placed on shared decision-making between patients and clinicians, especially in high-risk demographic patients [56]. Therefore, to effectively promote adherence to breast cancer detection and management guidelines, a collaborative effort between healthcare providers and policymakers is essential [24].
Variations in screening rates among different ethnic minorities are influenced by factors such as access to healthcare, socio-economic status, and cultural barriers [57]. We observed the same in our study. Though evidence-based recommendations based on screening guidelines can serve as a rule, they often overlook the more vulnerable population that is at higher risk. African American, Hispanic/Latina women and Ashkenazi Jewish women have a higher genetic predisposition and are also less often screened due to barriers in accessing healthcare [58, 59]. Mobilization of appropriate resources that improve their access to mammography and break down of health care system barriers to such women can greatly improve their outcome [60]. Hispanic/Latina women had less frequent screening mammograms than those of other ethnicities. According to a study done by Moore et al (2018), geographic areas of high breast cancer mortality or “hot spots” were prevalent throughout the southeastern USA for African American women and Hispanic women within the southwest region of the USA [61, 62]. African American and Hispanic women are approximately 30% more likely to be diagnosed with late-stage breast cancer compared to Caucasian women [11], therefore they are more likely to receive chemotherapy. An interesting analysis by Babatunde et al (2022) noted that African American women were more likely to receive surgery 8 days later, chemotherapy 7 days later, radiation therapy 3 days later, and adjuvant hormone therapy 28 days later than their Caucasian counterparts because of late diagnoses [63].
A study by Aleshire et al noted barriers to mammography were identified for each of the five dimensions of access to care: accessibility, affordability, availability, accommodation, and acceptability [64]. Improving transportation options, ensuring insurance coverage, and providing health care literacy can address these barriers. Noting these discrepancies, some policies have been put in place that have helped to a marginal degree. The Patient Protection and Affordable Care Act (ACA) of 2010 joined forces with state-level bodies to increase breast screening program support [65]. The ACA policy resulted in an immediate increase in breast cancer screenings per 1,000 encounters, as Steenland et al noted in the state of Massachusetts in 2019 [66]. Medicaid expansion also made breast cancer screening services more affordable [65].
A personalized schedule, based on each patient’s risk as determined by the patient and their health care provider, is one possible solution to optimize breast cancer screening. However, the lack of a single, all-encompassing consensus among insurance policies can lead to difficulty in obtaining appropriate coverage, further hindering efforts to come up with an effective, cost-affordable screening practice. As of now, USPSTF recommendation is widely followed, because it is tied to payment and thus improves screening access [46].
Implementing a risk-calculator or developing a universal tool to identify high-risk women and ensure they have adequate insurance coverage to undergo mammograms as often as they need will greatly improve access and provide optimized, patient-focused treatment options. Breast cancer risk assessment is important for guiding personalized screening strategies and risk-reducing interventions [67]. The most comprehensively used tool is the Tyrer-Cuzick model, which assesses the probability of carrying a BRCA mutation and the risk for developing in situ or invasive breast cancer [67, 68]. Other available models used for risk assessment include BRCAPRO (BRCA (gene) PRO (probability) - breast cancer genetic risk assessment tool), BOADICIEA (Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm - breast cancer risk prediction model), Breast Cancer Surveillance Consortium (BCSC), and Gail, which are all comparable [69-75]. The consensus is that a calculated lifetime risk of more than 20% warrants annual breast cancer screening [5]. No matter which tool is used, ACR has emphasized the importance of risk assessment at the age of 25, early enough to allow supplemental screening to be used [5].
Advancing health equity should be central to breast cancer control policy and practice in the United States. Significant progress in treatment has reduced breast cancer mortality. However, these mortality benefits are not experienced equally throughout all populations [76]. The 2024 USPSTF recommendations focus on health equity, aligning with the task force’s commitment to addressing race, antiracism, and health disparities [77-79]. These recommendations are still to be implemented among a population that has a higher incidence and a worse prognosis. African American women, for example, experience a higher incidence of breast cancer in their 30s and 40s compared to White women and are more likely to develop aggressive subtypes like triple-negative cancer, potentially due to environmental factors and systemic racism [80]. However, randomized controlled trials (RCTs) and large observational studies of mammography screening have often lacked sufficient representation of women of color. We are limited in understanding of breast cancer screening merits and demerits across various racial and ethnic groups. Many models suggest that African American women would benefit more from earlier screening, even starting in their 40s [80]. Additionally, socioeconomic factors such as race, income, and insurance status play a role in screening adherence. Women without a regular source of care, those living below the federal poverty level, or those who are uninsured or publicly insured are less likely to report recent mammograms [81, 82]. Previous studies have also shown that low-income patients, even with insurance including Medicare, tend to utilize fewer preventive services compared to higher-income patients [82, 83]. Addressing these disparities through targeted health education and support for vulnerable populations could help improve screening rates and outcomes [82, 84]. There is potential for future research and more comprehensive studies in certain demographic groups, vulnerable populations such as African Americans, Hispanic/Latina women, Ashkenazi Jews, and those with genetic risk factors. The importance of early diagnosis in prognosis of breast cancer can be further established by studying these populations’ screening frequency.
A limitation of our study was that we did not examine parity or breastfeeding status of women in our study, as nulliparity increases breast density due to greater estrogen exposure, which in turn is associated with a higher breast cancer risk [50]. Conversely, breastfeeding shuts down the hypothalamus-pituitary-gonadal axis and results in less estrogen production, which could correlate with lower incidence of breast cancer [85]. Likewise, our study did not examine the incidence of breast cancer in the male cohort. Future research and more comprehensive studies in certain demographic groups, vulnerable populations, and those with genetic risk factors would help create a new set of guidelines for those who are at high risk. Our retrospective observational study was restricted to a single center, and therefore, restricted to the population demographic of the surrounding area. It was not large enough to represent an entire population subset, nor all minorities. We also did not assess financial status directly as we were limited by the hospital database, however we did check patient’s insurance status to obtain some information to infer financial status. While selection bias is a risk in observational studies, we avoided that by deidentification of data by the data developer for all stake holders including the investigating team. Additionally, confounding cannot be entirely eliminated with observational research, but we tried to exclude any patients who did not meet both the inclusion and exclusion criteria variables. As with any observational study, only correlation and association can be drawn, but no causal relation can be established. We have de-selected anyone who does not fit all the criteria/variables we have chosen for our study and has incomplete data as per our requirements.
Conclusions
Based on our population demographic, annual screening for breast cancer is recommended, aligning with ACR guidelines. BI-RADS status at time of diagnosis, ER status and PR status of the women who received annual mammographic studies, were shown to have less aggressive tumors, and therefore had a better prognosis and decreased breast cancer related mortality. Women screened less frequently had severe breast cancer diagnoses and higher BI-RADS categories. Annually screened women had more undetermined ER/PR status and fewer positive cases, whereas biennially or less frequently screened women had fewer undetermined ER/PR cases and more positive cases. Hispanic/Latino(a) patients were more likely to have biennial screenings compared to non-Hispanic/Latino(a) patients. Current smokers were less likely to have annual screenings. Biennially screened patients had less N2 node involvement as compared to those screened annually. Our study’s data demonstrate that more frequent screenings lead to earlier detection and less aggressive tumor characteristics, ultimately contributing to improved prognoses and reduced mortality rates, in line with ACR recommendations. To enhance outcomes, more frequent and earlier screenings are necessary to ensure that all women receive timely and effective care.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable. Hospital database was used to pull data retrospectively.
Author Contributions
Pavan Patel: project proposal and development, data validation. Hifza Sakhi: literature search, manuscript drafting, writing, and review, submission. Devaki Kalvapudi: literature search, manuscript drafting, writing, and review, submission. Angelo Changas: project proposal and development, data validation. Mukhamed Sulaimanov: project proposal and development, data validation. Brian Criollo Gutierrez: project proposal, development, data validation. Idopise Umana: project proposal, faculty mentor and sponsor, reviewing and approving final manuscript. Jake A Slaton: validating results and data analysis. Hardeep Singh: project proposal, development, study design, data validation, manuscript review, and submission. All authors met all the ICMJE criteria.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor receptor 2; BI-RADS: Breast Imaging Reporting and Data System; ACR: American College of Radiology, USPSTF, United States Preventative Service Task Force; ACOG: American College of Obstetricians and Gynecologists; ACS: American Cancer Society; MRI: magnetic resonance imaging; BRCA: breast cancer gene; PTEN: phosphatase and TENsin homolog gene; SK11: sphingosine kinase 1; AAFP: American Academy of Family Physicians; NGHS: Northeast Georgia Health System; EPIC: EPIC Systems Corporation - electronic medical records; TNM stage: tumor, node, metastasis stage; DCIS: ductal carcinoma in situ; OR: odds ratio; CI: confidence interval; ACA: Affordable Care Act; BRCAPRO: BRCA (gene) PRO (probability) - breast cancer genetic risk assessment tool; BOADICIEA: Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm - breast cancer risk prediction model; BCSC: Breast Cancer Surveillance Consortium; RCTs: randomized control trials
| References | ▴Top |
- U.S. Department of Health and Human Services CfDCaPaNCI. U.S. Cancer Statistics Working Group. U.S. Cancer statistics data visualizations tool, based on 2022 submission data. CDC1999-2020.
- American Cancer Society. Cancer facts and figures 2023. American Cancer Society; 2023.
- Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, Jemal A, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72(6):524-541.
doi pubmed - Siu AL, Force USPST. Screening for breast cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2016;164(4):279-296.
doi pubmed - Monticciolo DL, Newell MS, Moy L, Lee CS, Destounis SV. Breast cancer screening for women at higher-than-average risk: updated recommendations from the ACR. J Am Coll Radiol. 2023;20(9):902-914.
doi pubmed - Network NCCN. National Comprehensive Cancer Network Breast Cancer. NCCN Clinical Practice Guidelines in Oncology version. 1.
- Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YC, Walter LC, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American cancer society. JAMA. 2015;314(15):1599-1614.
doi pubmed - Kleibl Z, Kristensen VN. Women at high risk of breast cancer: molecular characteristics, clinical presentation and management. Breast. 2016;28:136-144.
doi pubmed - Breast Cancer Risk Assessment Tool, National Cancer Institute, bcrisktool.cancer.gov/calculator.html. Accessed 6 Nov. 2024.
- DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67(6):439-448.
doi pubmed - Yedjou CG, Sims JN, Miele L, Noubissi F, Lowe L, Fonseca DD, Alo RA, et al. Health and racial disparity in breast cancer. Adv Exp Med Biol. 2019;1152:31-49.
doi pubmed - Franquet T, De Miguel C, Cozcolluela R, Donoso L. Spiculated lesions of the breast: mammographic-pathologic correlation. Radiographics. 1993;13(4):841-852.
doi pubmed - Richman IB, Long JB, Kyanko KA, Xu X, Gross CP, Busch SH. Insurance Coverage Mandates and the Adoption of Digital Breast Tomosynthesis. JAMA Netw Open. 2022;5(3):e224208.
doi pubmed - Brem Foundation. Know your screening options. Available at: https://www.bremfoundation.org/screening-options. Accessed: Nov 06, 2024.
- Wai E, D'yachkova Y, Olivotto IA, Tyldesley S, Phillips N, Warren LJ, Coldman AJ, et al. Comparison of 1- and 2-year screening intervals for women undergoing screening mammography. Br J Cancer. 2005;92(6):961-966.
doi pubmed - Moorman SEH, Pujara AC, Sakala MD, Neal CH, Maturen KE, Swartz L, Egloff H, et al. Annual screening mammography associated with lower stage breast cancer compared with biennial screening. AJR Am J Roentgenol. 2021;217(1):40-47.
doi pubmed - Tsapatsaris A, Babagbemi K, Reichman MB. Barriers to breast cancer screening are worsened amidst COVID-19 pandemic: A review. Clin Imaging. 2022;82:224-227.
doi pubmed - Castaldi M, Smiley A, Kechejian K, Butler J, Latifi R. Disparate access to breast cancer screening and treatment. BMC Womens Health. 2022;22(1):249.
doi pubmed - Sarma EA. Barriers to screening mammography. Health Psychol Rev. 2015;9(1):42-62.
doi pubmed - Henderson M. Access, cost and awareness among the barriers to screening mammography. RSNA. Radiological Society of North America; 2022. October 17, 2022.
- Reducing structural barriers for clients - breast cancer. The guide to community preventive services. Accessed August 7, 2023.
- BCRF. USPSTF breast cancer screening guidelines 2024: BCRF, Breast Cancer Research Foundation. Available at: https://www.bcrf.org/blog/uspstf-new-breast-cancer-screening-guidelines-2023/ (Accessed: November 06, 2024).
- Staff N. AAFP backs updated USPSTF breast cancer screening guidance. AAFP Backs Updated USPSTF Breast Cancer Screening Guidance | Patient Care. Available at: https://www.aafp.org/news/health-of-the-public/2024-breast-cancer-screening-update.html (Accessed: November 06, 2024).
- Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67(2):93-99.
doi pubmed - Agresti A. Categorical data analysis. 3rd ed. Hoboken (NJ): Wiley; 2013.
- MacDonald PL, Gardner RC. Type I error rate comparisons of post hoc procedures for I × J chi-square tables. Educ Psychol Meas. 2000;60(5):735-754.
doi - Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57(1):289-300.
- Posit team. RStudio: integrated development environment for R. Boston (MA): Posit Software, PBC; 2024. Available from: http://www.posit.co/.
- Magny SJ, Shikhman R, Keppke AL. Breast imaging reporting and data system. In: StatPearls. Treasure Island (FL). 2024.
pubmed - Seely JM, Peddle SE, Yang H, Chiarelli AM, McCallum M, Narasimhan G, Zakaria D, et al. Breast density and risk of interval cancers: the effect of annual versus biennial screening mammography policies in Canada. Can Assoc Radiol J. 2022;73(1):90-100.
doi pubmed - Breast disease. In: Hoffman BL, Schorge JO, Halvorson LM, Hamid CA, Corton MM, Schaffer JI, editors. Williams gynecology, 4th ed. McGraw-Hill Education; 2020. Available from: https://accessmedicine.mhmedical.com/content.aspx?bookid=2658§ionid=218608871. Accessed: Nov 06, 2024.
- Kozielek K, Stranz-Walczak N, Gajdzis P, Karmelita-Katulska K. Evaluation of the positive predictive value (PPV3) of ACR BI-RADS category 4 and 5 based on the outcomes of Invasive Diagnostic Office in an outpatient clinic. Pol J Radiol. 2019;84:e185-e189.
doi pubmed - Ezeana CF, Puppala M, Wang L, Chang JC, Wong STC. A comparative efficacy study of diagnostic digital breast tomosynthesis and digital mammography in BI-RADS 4 breast cancer diagnosis. Eur J Radiol. 2022;153:110361.
doi pubmed - Ro V, Jones T, Silverman T, McGuinness JE, Guzman A, Amenta J, Kukafka R, et al. Patient, primary care provider, and stakeholder perspectives on mammography screening frequency: lessons learned from a qualitative study. BMC Cancer. 2022;22(1):819.
doi pubmed - Sprague BL, Chen S, Miglioretti DL, Gard CC, Tice JA, Hubbard RA, Aiello Bowles EJ, et al. Cumulative 6-year risk of screen-detected ductal carcinoma in situ by screening frequency. JAMA Netw Open. 2023;6(2):e230166.
doi pubmed - Kerlikowske K, Zhu W, Hubbard RA, Geller B, Dittus K, Braithwaite D, Wernli KJ, et al. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807-816.
doi pubmed - Eby PR. Evidence to support screening women annually. Radiol Clin North Am. 2017;55(3):441-456.
doi pubmed - Talham CJ, Montiel Ishino FA, O'Brien KM, Sandler DP, Williams F. Breast cancer screening among Hispanic and non-Hispanic White women by birthplace in the Sister Study. Cancer Med. 2022;11(8):1913-1922.
doi pubmed - Meissner HI, Klabunde CN, Breen N, Zapka JM. Breast and colorectal cancer screening: U.S. primary care physicians' reports of barriers. Am J Prev Med. 2012;43(6):584-589.
doi pubmed - Bodewes FTH, van Asselt AA, Dorrius MD, Greuter MJW, de Bock GH. Mammographic breast density and the risk of breast cancer: A systematic review and meta-analysis. Breast. 2022;66:62-68.
doi pubmed - Liu L, Hao X, Song Z, Zhi X, Zhang S, Zhang J. Correlation between family history and characteristics of breast cancer. Sci Rep. 2021;11(1):6360.
doi pubmed - Aedma SK, Kasi A. Li-Fraumeni syndrome. In: StatPearls. Treasure Island (FL). 2024.
pubmed - Sheikh A, Hussain SA, Ghori Q, Naeem N, Fazil A, Giri S, Sathian B, et al. The spectrum of genetic mutations in breast cancer. Asian Pac J Cancer Prev. 2015;16(6):2177-2185.
doi pubmed - White E, Miglioretti DL, Yankaskas BC, Geller BM, Rosenberg RD, Kerlikowske K, Saba L, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004;96(24):1832-1839.
doi pubmed - American Cancer Society. American Cancer Society recommendations for the early detection of breast cancer. Published 2021. https://www.cancer.org/cancer/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html.
- US Preventive Services Task Force. Final recommendation statement: breast cancer screening. US Preventive Services Task Force; 2016.
- Hoover LE. Breast cancer screening: ACP releases guidance statements. Am Fam Physician. 2020;101(3):184-185.
pubmed - Monticciolo DL, Malak SF, Friedewald SM, Eby PR, Newell MS, Moy L, Destounis S, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and society of breast imaging. J Am Coll Radiol. 2021;18(9):1280-1288.
doi pubmed - National Comprehensive Cancer Network. Breast Cancer (NCCN Guidelines for Patients). Accessed September 18, 2024. Available from: https://www.nccn.org/patientresources/patient-resources/guidelines-for-patients/guidelines-for-patients-details?patientGuidelineId=66.
- Reynolds H. The big squeeze: a social and political history of the controversial mammogram. ILR Press/Cornell University Press. 2012.
- Abu Abeelh E, AbuAbeileh Z. Impact of mammography screening frequency on breast cancer mortality rates. Cureus. 2023;15(11):e49066.
doi pubmed - Mandelblatt JS, Cronin KA, Bailey S, Berry DA, de Koning HJ, Draisma G, Huang H, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151(10):738-747.
doi pubmed - Kerlikowske K, Hubbard RA, Miglioretti DL, Geller BM, Yankaskas BC, Lehman CD, Taplin SH, et al. Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: a cohort study. Ann Intern Med. 2011;155(8):493-502.
doi pubmed - Davis DA, Taylor-Vaisey A. Translating guidelines into practice. A systematic review of theoretic concepts, practical experience and research evidence in the adoption of clinical practice guidelines. CMAJ. 1997;157(4):408-416.
pubmed - Ricci-Cabello I, Vasquez-Mejia A, Canelo-Aybar C, Nino de Guzman E, Perez-Bracchiglione J, Rabassa M, Rigau D, et al. Adherence to breast cancer guidelines is associated with better survival outcomes: a systematic review and meta-analysis of observational studies in EU countries. BMC Health Serv Res. 2020;20(1):920.
doi pubmed - Brawley OW. On assessing the effect of breast cancer screening schemes. Cancer. 2017;123(19):3656-3659.
doi pubmed - Jensen B, Khan H, Layeequr Rahman R. Sociodemographic determinants in breast cancer screening among uninsured women of West Texas. Medicina (Kaunas). 2022;58(8):1010.
doi pubmed - Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D, Dolan NC, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97(6):439-448.
doi pubmed - John EM, Miron A, Gong G, Phipps AI, Felberg A, Li FP, West DW, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA. 2007;298(24):2869-2876.
doi pubmed - Bourgois P, Holmes SM, Sue K, Quesada J. Structural vulnerability: operationalizing the concept to address health disparities in clinical care. Acad Med. 2017;92(3):299-307.
doi pubmed - Moore JX, Andrzejak SE, Jones S, Han Y. Exploring the intersectionality of race/ethnicity with rurality on breast cancer outcomes: SEER analysis, 2000-2016. Breast Cancer Res Treat. 2023;197(3):633-645.
doi pubmed - Moore JX, Royston KJ, Langston ME, Griffin R, Hidalgo B, Wang HE, Colditz G, et al. Mapping hot spots of breast cancer mortality in the United States: place matters for Blacks and Hispanics. Cancer Causes Control. 2018;29(8):737-750.
doi pubmed - Babatunde OA, Eberth JM, Felder TM, Moran R, Hughes-Halbert C, Truman S, Hebert JR, et al. Racial disparities and diagnosis-to-treatment time among patients diagnosed with breast cancer in South Carolina. J Racial Ethn Health Disparities. 2022;9(1):124-134.
doi pubmed - Aleshire ME, Adegboyega A, Escontrias OA, Edward J, Hatcher J. Access to care as a barrier to mammography for black women. Policy Polit Nurs Pract. 2021;22(1):28-40.
doi pubmed - Le Blanc JM, Heller DR, Friedrich A, Lannin DR, Park TS. Association of medicaid expansion under the affordable care act with breast cancer stage at diagnosis. JAMA Surg. 2020;155(8):752-758.
doi pubmed - Steenland M, Sinaiko A, Glynn A, Fitzgerald T, Cohen J. The effect of the Affordable Care Act on patient out-of-pocket cost and use of preventive cancer screenings in Massachusetts. Prev Med Rep. 2019;15:100924.
doi pubmed - Amir E, Freedman OC, Seruga B, Evans DG. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102(10):680-691.
doi pubmed - Quante AS, Whittemore AS, Shriver T, Strauch K, Terry MB. Breast cancer risk assessment across the risk continuum: genetic and nongenetic risk factors contributing to differential model performance. Breast Cancer Res. 2012;14(6):R144.
doi pubmed - Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62(1):145-158.
doi pubmed - Barke LD, Freivogel ME. Breast cancer risk assessment models and high-risk screening. Radiol Clin North Am. 2017;55(3):457-474.
doi pubmed - Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148(5):337-347.
doi pubmed - Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879-1886.
doi pubmed - Costantino JP, Gail MH, Pee D, Anderson S, Redmond CK, Benichou J, Wieand HS. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91(18):1541-1548.
doi pubmed - McCarthy AM, Guan Z, Welch M, Griffin ME, Sippo DA, Deng Z, Coopey SB, et al. Performance of breast cancer risk-assessment models in a large mammography cohort. J Natl Cancer Inst. 2020;112(5):489-497.
doi pubmed - Terry MB, Liao Y, Whittemore AS, Leoce N, Buchsbaum R, Zeinomar N, Dite GS, et al. 10-year performance of four models of breast cancer risk: a validation study. Lancet Oncol. 2019;20(4):504-517.
doi pubmed - Pace LE, Keating NL. New recommendations for breast cancer screening-in pursuit of health equity. JAMA Netw Open. 2024;7(4):e2411638.
doi pubmed - US Preventive Services Task Force, Nicholson WK, Silverstein M, Wong JB, Barry MJ, Chelmow D, Coker TR, et al. Screening for breast cancer: US preventive services task force recommendation statement. JAMA. 2024;331(22):1918-1930.
doi pubmed - Trentham-Dietz A, Chapman CH, Jayasekera J, Lowry KP, Heckman-Stoddard BM, Hampton JM, Caswell-Jin JL, et al. Collaborative modeling to compare different breast cancer screening strategies: a decision analysis for the US preventive services task force. JAMA. 2024;331(22):1947-1960.
doi pubmed - Henderson JT, Webber EM, Weyrich M, Miller M, Melnikow J. In: Screening for breast cancer: a comparative effectiveness review for the U.S. preventive services task force. Rockville (MD), 2024.
pubmed - Jatoi I, Sung H, Jemal A. The emergence of the racial disparity in U.S. breast-cancer mortality. N Engl J Med. 2022;386(25):2349-2352.
doi pubmed - Hall IJ, Tangka FKL, Sabatino SA, Thompson TD, Graubard BI, Breen N. Patterns and trends in cancer screening in the United States. Prev Chronic Dis. 2018;15:E97.
doi pubmed - Kasper G, Momen M, Sorice KA, Mayhand KN, Handorf EA, Gonzalez ET, Devlin A, et al. Effect of neighborhood and individual-level socioeconomic factors on breast cancer screening adherence in a multi-ethnic study. BMC Public Health. 2024;24(1):63.
doi pubmed - Silber JH, Rosenbaum PR, Ross RN, Reiter JG, Niknam BA, Hill AS, Bongiorno DM, et al. Disparities in breast cancer survival by socioeconomic status despite Medicare and Medicaid Insurance. Milbank Q. 2018;96(4):706-754.
doi pubmed - Malone J, Snguon S, Dean LT, Adams MA, Poteat T. Breast cancer screening and care among black sexual minority women: a scoping review of the literature from 1990 to 2017. J Womens Health (Larchmt). 2019;28(12):1650-1660.
doi pubmed - Andersen AN, Schioler V. Influence of breast-feeding pattern on pituitary-ovarian axis of women in an industrialized community. Am J Obstet Gynecol. 1982;143(6):673-677.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.