| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 000, Number 000, November 2024, pages 000-000
Association Between the Development of Sensorineural Hearing Loss and Blood NAD+ Levels
Hideaki Sakataa, f , Ken Hayashia, Ryo Matsuyamab, c, Tomoyo Omatac, Masanobu Kanoub, d, Kei Yamanab, d, Sho Kanzakie
aKawagoe Ear Institute, Division of Otorhinolaryngology, Kawagoe Mine Medical Center, Kawagoe City, Saitama 350-1122, Japan
bNutraceutical Group, New Business Development Unit, Teijin Limited, Hino, Tokyo, Japan
cDiscovery DMPK Research Group, Toxicology & DMPK Research Department, Teijin Institute for Bio-Medical Research, Teijin Pharma Limited, Hino, Tokyo, Japan
dNOMON Co. Ltd, Kasumigaseki, Chiyoda Ku, Tokyo, Japan
eDivision of Auditory Disorders, National Institute of Sensory Organ, National Hospital Organization of Tokyo Medical Center, Meguro Ku, Tokyo, Japan
fCorresponding Author: Hideaki Sakata, Kawagoe Ear Institute, Division of Otorhinolaryngology, Kawagoe Mine Medical Center, Kawagoe City, Saitama 350-1122, Japan
Manuscript submitted October 1, 2024, accepted November 5, 2024, published online November 11, 2024
Short title: Sensorineural Hearing Loss and NAD+ Levels
doi: https://doi.org/10.14740/jocmr6083
| Abstract | ▴Top |
Background: Hearing loss prevalence increases with age, affecting over 25% of the global population aged 60 years or older. The aim of the study was to investigate the association between the development of sensorineural hearing loss (SNHL) and the blood levels of nicotinamide adenine dinucleotide (NAD+).
Methods: A single-center, observational study was conducted at Kawagoe Otology Institute in Japan. A total of 80 patients were included and allocated to four groups of 20 patients each: patients aged 50 - 79 years with or without unilateral sudden sensorineural hearing loss (SSNHL), and patients aged ≥ 80 years with or without bilateral age-related hearing loss (ARHL). The distribution of whole-blood NAD+ levels was investigated. We also measured oxidative stress markers (diacron-reactive oxygen metabolites (dROMs) and biological antioxidant potential (BAP)) and examined the relationship between the development of SNHL and whole-blood NAD+ levels, dROMs, and BAP.
Results: Comparison of NAD+ levels with and without hearing loss in the same age group by analysis of covariance showed a significantly lower NAD+ level in those with hearing loss than those without in the ≥ 80 age group (P = 0.047), whereas there was no difference between the two groups in the 50 - 79 age group (P = 0.232). All 80 patients, without consideration of age or type of hearing loss, were subjected to multivariate analysis to explore factors contributing to the development of hearing loss. With each 1 µM increase in the NAD+ level, the probability of developing SNHL decreased to 0.9-fold (P = 0.047), and each 1 U.CARR increase in dROMs was associated with a 1.01-fold increase in the risk of developing SNHL (P = 0.014). Whole-blood NAD+ levels in ARHL patients were significantly lower than those in non-ARHL patients. There was no association between whole-blood NAD+ and dROMs or BAP levels. This study has some limitations, including a sample size that was not large enough to detect a significant difference and an imbalance in the male-to-female ratio.
Conclusions: Decreased amount of NAD+ in the body and increased dROMs levels were associated with increased risk of developing SNHL, and the development of ARHL was especially highly associated with a decreased amount of NAD+ in the body.
Keywords: Sensorineural hearing loss; Nicotinamide adenine dinucleotide; Sirtuin 1; Mitochondrion; Aging
| Introduction | ▴Top |
It is estimated that 430 million people worldwide, or more than 5% of the total population, need hearing rehabilitation for hearing loss, and that by 2050, more than 2.5 billion people will be suffering from some form of hearing loss. The prevalence of hearing loss increases with age, with more than 25% of the current global population aged 60 years or older being affected [1].
Sensorineural hearing loss (SNHL) is a type of hearing loss caused by abnormalities in the organs of the auditory sensory system, including cells and nervous systems in the inner ear through the cerebral sensory centers, and consists primarily of unilateral sudden sensorineural hearing loss (SSNHL) and bilateral age-related hearing loss (ARHL) [2].
The etiology of SSNHL involves many factors, including infectious, autoimmune, traumatic, and vascular causes, and damage to auditory hair cells in the inner ear and other cochlear structures is considered to lead to hearing loss [3].
ARHL is considered to be caused by chronic degeneration or dysfunction of the cochlear hair cells and the cochlea itself [4, 5]. Studies have also documented that ARHL can be induced by environmental factors, genetic predisposition, comorbidities such as hypertension and diabetes, as well as many other factors such as aging, direct mechanical stimulation from exposure to noise, and oxidative stress in the mitochondria [6].
SSNHL and ARHL share a common pathogenic mechanism: damage to the outer hair cell center. ARHL is thought to be caused by involved excessive production of reactive oxygen species (ROS) and is significantly influenced by oxidative stress [7].
Excessive oxidative stress, a factor in aging, promotes the expression of mitochondrial pro-apoptotic genes and decreases sirtuin (SIRT) activity [8]. The degeneration of hairy cells is believed to involve SIRT1 in the nucleus and SIRT3 in the mitochondria [9]. Recent studies have suggested that a decrease in the amount of nicotinamide adenine dinucleotide (NAD+) in the body is closely related to decreased activity of NAD+-dependent deacetylase sirtuins [10]. With respect to hearing loss, evidence has suggested that mitochondrial dysfunction may cause damage to hair cells and, ultimately, the development of SNHL.
Although many reports have shown that NAD+ levels in the body decrease with age [11, 12], there have been no reports on the distribution of blood NAD+ levels in patients with SNHL.
The objectives of this study were to measure blood NAD+ levels in patients with and without SSNHL/ARHL, and to determine the distribution of NAD+ levels and factors where the age and the presence or absence of SNHL have an impact on NAD+ levels. Evidence has also suggested that decreased intracellular NAD+ levels lead to oxidative stress and impaired deoxyribonucleic acid (DNA) damage repair [13]. Therefore, we simultaneously measured diacron-reactive oxygen metabolites (d-ROMs) as a measure of oxidative stress and biological antioxidant potential (BAP) as a measure of antioxidant capacity, and examined their relationship to hearing loss.
| Materials and Methods | ▴Top |
Study design and patients
This single-center, observational study included those patients who presented to Kawagoe Otology Institute between July 2022 and April 2023, and met any of the inclusion criteria 1) to 4) plus 5) and 6) and none of the exclusion criteria as listed below, and provided written informed consent. Testing was conducted in the order of the participants’ visits, and the study was concluded when data for 20 participants in each group were obtained within the study period.
Inclusion criteria were: 1) patients aged 50 - 79 years without bilateral hearing loss; 2) patients aged 50 - 79 years with unilateral SSNHL (average hearing level of 40 dB or more) without acute vertigo and not progressive (at least 1 month after onset); 3) patients aged ≥ 80 years without bilateral hearing loss; 4) patients aged ≥ 80 years with bilateral SNHL; 5) patients who can fast in the morning and can visit the clinic in the morning clinic in the morning for blood tests of NAD+, d-ROM, BAP, etc. (to eliminate the influence of diurnal variation and food); and 6) patients who consented to participate in the study.
Hearing levels were calculated as the mean of six frequencies as follows: (500 Hz + 1000 Hz × 2 + 2000 Hz × 2 + 4000 Hz)/6.
Hearing loss in this study was defined as a hearing threshold greater than or equal to 25 dB on the means of six frequencies.
Exclusion criteria were: 1) hearing loss due to non-age-related causes, such as trauma congenital conditions (genetic hearing loss), functional hearing loss, conductive hearing loss, or SSNHL under treatment (these types of hearing loss are not relevant to the purpose of this study); and 2) patients receiving nicotinamide mononucleotide (it affects blood NAD+ levels).
Ethical approval and participation consent
This study was registered with the Clinical Trials Registry (UMIN000048229) [14] and was undertaken in accordance with the Declaration of Helsinki and the ethical guidelines provided by the Ministry of Health, Labour and Welfare. This study was approved by the clinical research ethical review board of Shido, Inc. (#S20220622). The measurement of NAD+ was approved by the Human Ethics Committee of the Teijin Institute for Bio-Medical Research, Teijin Pharma Limited, Tokyo, Japan (approval number: HB22-005-M1). All participants provided written informed consent before participation.
Investigation items
We investigated age, sex, comorbidities, BAP, d-ROMs, serum zinc level (Zn), herpes simplex virus (HSV) antibody titer, vesicular stomatitis virus (VSV) antibody titer, NAD+, and pure-tone hearing acuity (SSNHL patients: on the side with hearing loss, others: on the side with poorer test result).
Measurement methods
A portion of the blood collected for routine laboratory testing was submitted to Teijin Limited (Hino, Tokyo, Japan) and Teijin Pharma Limited (Hino, Tokyo, Japan) for measurement of whole-blood NAD+ levels using the method established by Matsuyama et al [15]. Blood d-ROMs and BAP levels as measures of oxidative stress were measured in plasma with an in-house automated reactive oxygen species/free radicals analyzer (FRAS4, WISMERLL Co., Ltd, Grosseto, Italy). Blood samples for cases of SSNHL were collected within 1 month of onset. For SSNHL cases, testing was conducted when hearing levels had stabilized within 1 month. Hearing acuity was measured with an audiometer (Harp Plus, Starkey Eden Prairie, USA).
Evaluation items
The primary endpoints were the difference in whole-blood NAD+ levels between those aged 50 - 79 years without hearing loss and those aged 50 - 79 years with SSNHL (unilateral, severe), and the difference in whole-blood NAD+ levels between those aged ≥ 80 years without bilateral hearing loss and those aged ≥ 80 years with bilateral SNHL. In addition, correlations among factors associated with the development of hearing loss and laboratory test values were analyzed.
Statistical analysis
For the summary statistics of patient demographics, the mean ± standard deviation (SD) was used for frequency (number), percentage, and continuous variables for which normality was not rejected by the Shapiro-Wilk test, and the median (first and third quartiles) for variables that were non-normally distributed. For the comparison of patient demographics, Fisher’s exact test was used for nominal variables and Student’s t-test and Wilcoxon rank-sum test for continuous variables. In the comparison of NAD+ levels with and without hearing loss in the same age group, we used analysis of covariance. Wald’s Chi-squared test was used for the multivariate analysis of factors associated with the development of hearing loss. Pearson’s test or Spearman’s test was used for correlation testing. R analysis version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) [16] was used for analysis. All statistical tests were two-tailed with a significance level of 5%.
| Results | ▴Top |
In this study, patient demographics were analyzed for four groups of 20 patients each: patients aged 50 - 79 years with or without SSNHL, and patients aged ≥ 80 years with or without ARHL. No significant differences were observed in any of the variables, except for hypertension aged 50 - 79 years and the significantly older age of the patients aged 50-79 years with SSNHL (Table 1).
 Click to view | Table 1. Patient Characteristics |
The distribution of NAD+ levels in each group, the primary endpoint, is shown in Figure 1. The median NAD+ level (first and third quartiles) was 31.25 (27.47 - 37.98) for those aged 50 - 79 years without hearing loss, 30.21 (24.44 - 32.19) for those aged 50 - 79 years with SSNHL, 27.69 (25.34- 29.50) for those aged ≥ 80 years without ARHL, and 25.91 (22.69 - 28.13) for those aged ≥ 80 years with ARHL.
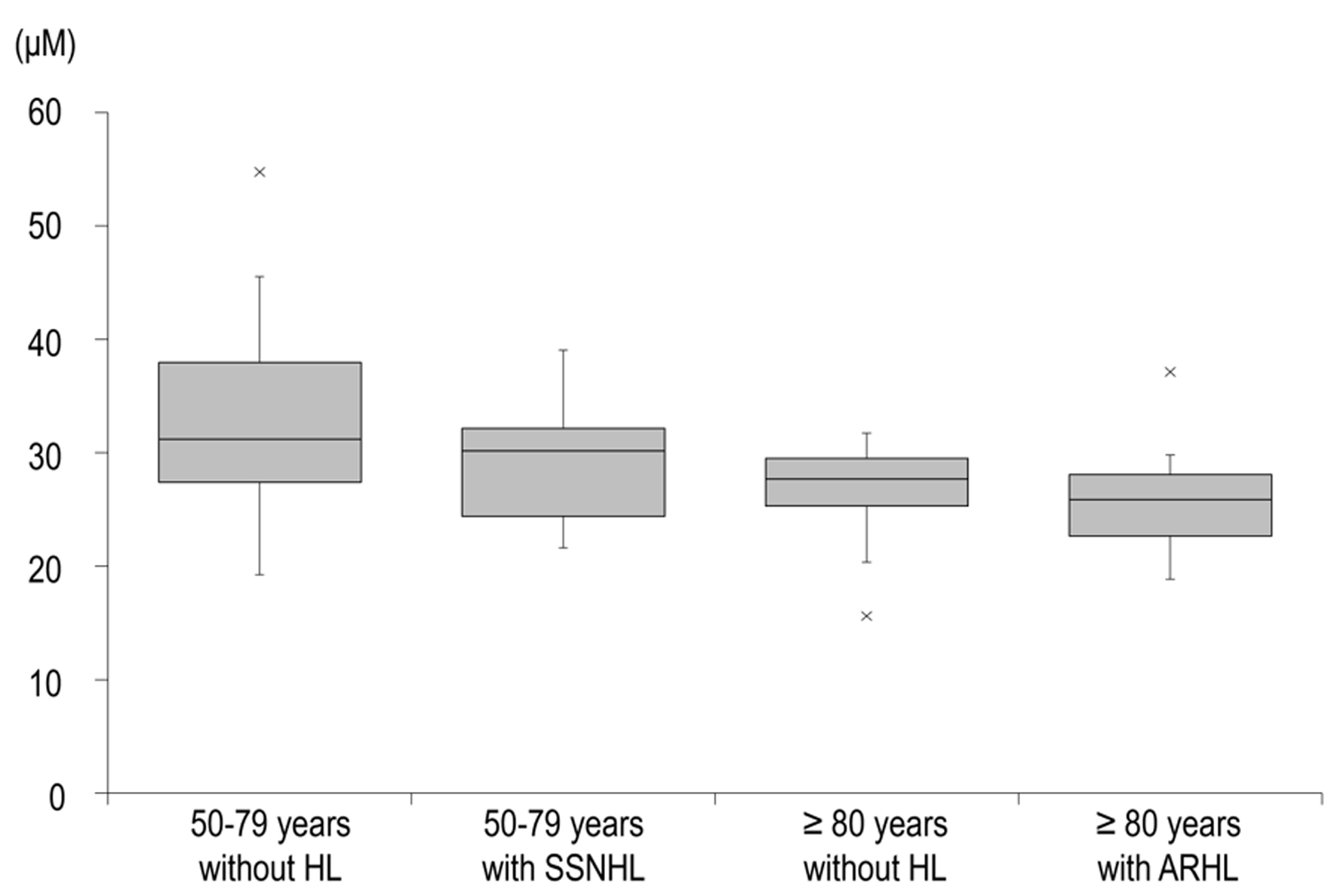 Click for large image | Figure 1. Distribution of NAD+ levels in each group. The boxes indicate the IQR, the black band in the middle of each box indicates the median, and the upper and lower whiskers indicate the upper and lower limits of the 1.5-fold range of the IQR, respectively. Data are median (interquartile range). ARHL: age-related hearing loss; HL: hearing loss; IQR: interquartile range; NAD+: nicotinamide adenine dinucleotide; SSNHL: sudden sensorineural hearing loss. |
Comparison of NAD+ levels with and without hearing loss in the same age group by analysis of covariance showed a significantly lower NAD+ level in those with hearing loss than those without in the ≥ 80 age group (P = 0.047), whereas there was no difference between the two groups in the 50 - 79 age group (P = 0.232; Table 2).
 Click to view | Table 2. Comparison of NAD+ Levels With and Without Hearing Loss (ANCOVA) |
Next, all 80 patients, without consideration of age or type of hearing loss, were subjected to multivariate analysis to explore factors contributing to the development of hearing loss. The results showed that for each 1 µM increase in the NAD+ level, the probability of developing SNHL decreased to 0.9-fold (P = 0.047); that males were 6.82-fold more likely than females to have SNHL (P = 0.012); and that for each 1 U.CARR increase in the d-ROMs level, the probability of developing SNHL increased 1.01-fold (P = 0.014). The significant factors associated with hearing loss were low NAD+ levels and high d-ROM values (Table 3).
 Click to view | Table 3. Factor Analysis for SNHL |
We also investigated the association of normal NAD+ levels with d-ROMs levels, a measure of oxidative stress that showed significant differences between patients with and without hearing loss in a multivariate analysis of all patients, and with BAP levels, a measure of antioxidant capacity, using Spearman’s test, finding no association for either variable (Supplementary Materials 1 and 2, jocmr.elmerjournals.com). Similarly, no correlation was found when the analysis was limited to patients with hearing loss, nor was there any correlation between d-ROMs and BAP levels (Supplementary Materials 3-5, jocmr.elmerjournals.com).
The audiometry results of each group, as presented in audiograms, are shown in Figure 2. The mean hearing levels of those aged 50 - 79 with and without SSNHL and those aged ≥ 80 years with and without ARHL were 66.2 ± 21.4, 12.5 ± 4.7, 56.0 ± 6.4, and 24.7 ± 6.2 dB, respectively.
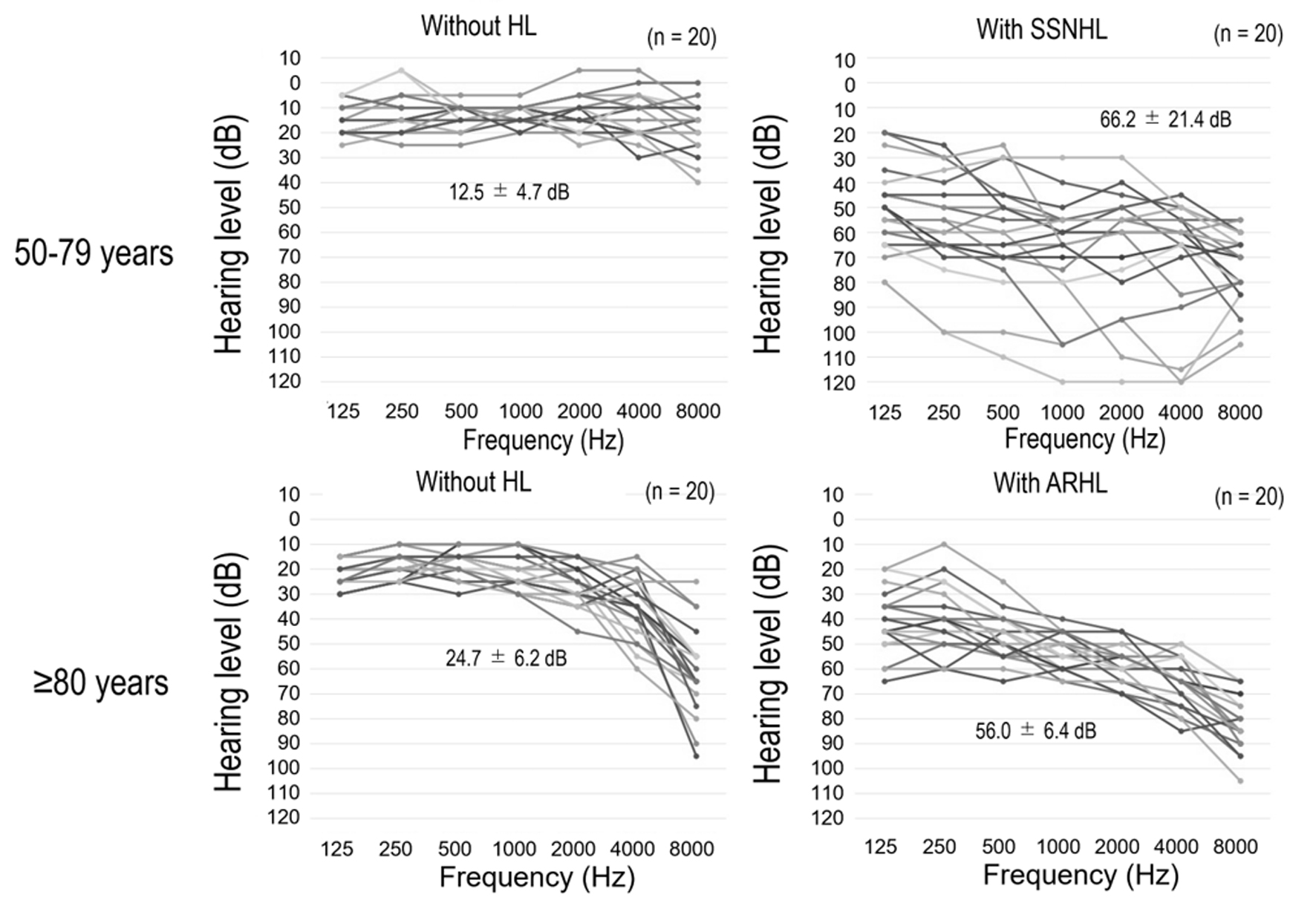 Click for large image | Figure 2. Audiograms for each group. All data are from the side of the ear with the poorer test result, and the values are the means of six frequencies. ARHL: age-related hearing loss; HL: hearing loss; SSNHL: sudden sensorineural hearing loss. |
| Discussion | ▴Top |
Association of whole-blood NAD+ levels with SSNHL and ARHL
Many studies have shown that NAD+ contributes to SIRT proteins, which are considered longevity genes, essential for genome stability and gene transcriptional regulation. A decrease in NAD+ leads to accelerated aging [17, 18], and NAD+ levels naturally decline with age [11, 12]. One common pathogenic mechanism of SSNHL and ARHL is damage to hair cells. Brown et al reported that supplementation with nicotinamide riboside, an NAD+ precursor, suppressed hair cell degeneration, suggesting NAD+ involvement in protecting hair cells [19].
However, no reports have examined the relationship between SSNHL/ARHL development and NAD+ levels in humans. This study investigated NAD+ level distribution in individuals with and without SSNHL/ARHL.
In a study of healthy men over 65, Nakagawa-Nagahama et al found a negative correlation between hearing thresholds and blood nicotinic acid levels, an NAD+ precursor. However, they found no correlation with NAD+, which they attributed to challenges in measuring NAD+ due to its instability in blood [20]. In this study, we used the dried blood spot (DBS) method to measure NAD+ stably [15], yielding whole-blood NAD+ levels much higher than those in Nakagawa-Nagahama’s report. NAD+ levels by patient demographics showed no difference between those with and without SSNHL/ARHL. However, an analysis by age group showed a significant difference in NAD+ levels between patients with and without ARHL in the ≥ 80 age group. Multivariate analysis of all SNHL patients showed that higher NAD+ levels correlated with lower SNHL incidence.
NAD+ levels are known to decline with age. In this study, NAD+ levels were significantly lower in the ≥ 80 age group than in the 50 - 79 age group, regardless of SNHL presence (Table 1), which aligns with previous studies. This suggests that lower NAD+ levels may contribute to hearing loss in SSNHL/ARHL patients. Although NAD+ levels for all patients tended to converge at ages over 55 (Supplementary Material 5, jocmr.elmerjournals.com), covariate analysis showed a significant difference between non-ARHL and ARHL groups, indicating that NAD+ changes in older patients may impact functions such as hearing.
The three main causes of NAD+ decline - increased consumption, decreased synthesis, and increased degradation [21] - are closely linked to aging. When DNA damage occurs with age, NAD+ is consumed in PARP-mediated DNA repair [22], while chronic inflammation from aging also reduces NAD+ synthesis [23].
Decreased NAD+ levels have been shown to decrease the activity of SIRT proteins, such as SIRT1 and SIRT3, leading to impaired mitochondrial function [10, 24]. The reason why low NAD+ increases the incidence of SSNHL and ARHL will be closely related to their disease causes. It is reported that SSNHL is caused by the damage to hair cells in the cochlea and auditory nerve, autoimmunity, viral infection, and thrombus formation due to poor inner ear circulation [3, 25]. ARHL is thought to be caused by involved excessive production of reactive oxygen species (ROS) and is significantly influenced by oxidative stress [7], which promotes the expression of mitochondrial pro-apoptotic genes and decreases SIRT activity [8]. Given that mitochondrial disorder is identified as an etiology of ARHL, it is possible that NAD+ decline may indirectly contribute to the development of ARHL. The results of the present study also support this hypothesis, indicating that blood NAD+ levels have the potential to be an early diagnostic biomarker for ARHL. Someya et al have demonstrated that SIRT3 mediates the alleviation of oxidative damage and prevention of ARHL in calorie-restricted mice [26], while Okur et al have found that supplementation with NAD+ precursors slows the progression of ARHL in mice [27], suggesting that increasing the abundance of NAD+ in the body may prevent the development and progression of ARHL. Recently Morifuji reported that nicotinamide mononucleotide intake could increase blood NAD+ levels, maintain walking speed, and improve sleep quality in older adults, which might support the efficacy of increasing the abundance of NAD+ in the body [28].
Studies have shown that disorders of sensory organs, including the auditory organs, cause overactivity of the upper network of the multisensory integration region (i.e., the brain hub region; the entorhinal-hippocampus system), which, combined with age-related reduction in energy supply, results in a massive consumption of intraneuronal adenosine triphosphate (ATP) [29, 30]. A study has also documented an approximately 8% decrease in the number of human mitochondria every 10 years [31]. The finding of the present study that NAD+ levels were significantly reduced in patients over 80 years of age with bilateral SNHL suggests that hearing loss in the older patients is closely related to abnormal mitochondrial energy metabolism in the multisensory integration region. It is thus speculated that the high ATP consumption in neurons of the entorhinal cortex and hippocampus may promote the neurodegenerative process in these regions that occurs preclinically in dementia. Thus, blood NAD+ levels may be a potential biomarker for assessing the impact of hearing loss on cognitive decline in the older patients.
Association between whole-blood NAD+ levels and oxidative stress-related markers
Previous studies in animals and humans have documented that antioxidants such as N-acetylcysteine, sodium thiosulfate, d-methionine, alpha-lipoic acid, and coenzyme Q10 have hearing protection or rescue effects [32], supporting the association between oxidative stress markers and the development of SNHL.
Studies have also shown that NAD+ depletion can affect ROS generation [33] and that d-ROMs levels are associated with age [34], while others have described that d-ROMs and BAP levels are not associated with age [11]. In the present study, there were no correlations between whole-blood NAD+ levels and d-ROMs and BAP levels, indicating no direct association between them. In this study, we used oxidized ferrous iron as a marker for ROS; however, since there are various markers for ROS, such as hydroxyl radicals, we believe that the lack of significant relationships observed with this marker is understandable.
Limitations
Because it was not known to what extent NAD+ levels in SSNHL/ARHL patients differed from those in individuals without hearing loss, a statistical sample size calculation was not able to perform. It is therefore possible that the sample size was not large enough to obtain a significant difference. It is also undeniable that there was an imbalance in the male/female ratio. The findings cannot be fully generalized due to the small sample size and the small number of men. We expect further investigation such as a larger cohort study in the future. Additionally, we used correlation analysis and this does not a causal relationship between NAD+ levels and hearing loss.
Conclusion
Based on the results of this study, when not considering the classification of hearing loss, the significant factors associated with hearing loss, regardless of SSNHL or ARHL, were low NAD+ levels, high d-ROM values, and being male. Additionally, covariance analysis indicated that NAD+ levels were significantly lower in ARHL patients compared to non-ARHL patients, suggesting that age-related NAD+ decline may be one of the causes of hearing loss. Furthermore, NAD+ levels in SSNHL patients tended to be lower, showing similar results to those observed in ARHL. No significant correlation was found between NAD+ levels and d-ROMs and BAP as oxidative stress indicators in this study.
| Supplementary Material | ▴Top |
Suppl 1. Correlation between NAD+ and d-ROMs levels in all patients.
Suppl 2. Correlation between NAD+ and BAP levels in all patients.
Suppl 3. Correlation between NAD+ and d-ROMs levels in patients with hearing loss.
Suppl 4. Correlation between NAD+ and BAP levels in patients with hearing loss.
Suppl 5. Distribution of NAD+ levels in each group.
Acknowledgments
None to declare.
Financial Disclosure
This study was self-funded.
Conflict of Interest
HS, KH and SK declare no competing interests. RM, MK and KY are employees of Teijin Limited, RM and TO are employees of Teijin Pharma Limited, MK and KY are employees of NOMON Co. Ltd.
Informed Consent
All participants provided written informed consent before participation.
Author Contributions
HS conceived and designed the experiments, analyzed the data, wrote the first draft of the manuscript, and contributed to acquisition and interpretation of data and reviewed/edited the manuscript. KH contributed to acquisition and interpretation of data. RM, TO, MK and KY made significant contributions to the design of the work. SK supervised the study and analyzed the data. HS and other authors substantially contributed to the revision of the manuscript drafts. All authors have approved the submitted version of the manuscript and agreed to be accountable for any part of the work.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
ARHL: age-related hearing loss; ATP: adenosine triphosphate; BAP: biological antioxidant potential; DBS: dried blood spot; DNA: deoxyribonucleic acid; d-ROMs: diacron-reactive oxygen metabolites; HSV: herpes simplex virus; NAD+: nicotinamide adenine dinucleotide; ROS: reactive oxygen species; SD: standard deviation; SIRT: sirtuin; SNHL: sensorineural hearing loss; SSNHL: sudden sensorineural hearing loss; VSV: vesicular stomatitis virus; Zn: serum zinc level
| References | ▴Top |
- WHO. Fact sheets, Deafness and hearing loss 2023. https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss. Accessed May 11, 2023.
- Tanna RJ, Lin JW, De Jesus O. Sensorineural Hearing Loss. In: StatPearls. Treasure Island (FL). 2024.
pubmed - Kuhn M, Heman-Ackah SE, Shaikh JA, Roehm PC. Sudden sensorineural hearing loss: a review of diagnosis, treatment, and prognosis. Trends Amplif. 2011;15(3):91-105.
doi pubmed - Wang J, Puel JL. Presbycusis: an update on cochlear mechanisms and therapies. J Clin Med. 2020;9(1):218.
doi pubmed - Wu PZ, O'Malley JT, de Gruttola V, Liberman MC. Age-related hearing loss is dominated by damage to inner ear sensory cells, not the cellular battery that powers them. J Neurosci. 2020;40(33):6357-6366.
doi pubmed - Bowl MR, Dawson SJ. Age-related hearing loss. Cold Spring Harb Perspect Med. 2019;9(8):a033217.
doi pubmed - Zhang L, Du Z, He L, Liang W, Liu K, Gong S. ROS-induced oxidative damage and mitochondrial dysfunction mediated by inhibition of SIRT3 in cultured cochlear cells. Neural Plast. 2022;2022:5567174.
doi pubmed - Verdin E, Hirschey MD, Finley LW, Haigis MC. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci. 2010;35(12):669-675.
doi pubmed - Xiong H, Dai M, Ou Y, Pang J, Yang H, Huang Q, Chen S, et al. SIRT1 expression in the cochlea and auditory cortex of a mouse model of age-related hearing loss. Exp Gerontol. 2014;51:8-14.
doi pubmed - Imai SI, Guarente L. It takes two to tango: NAD(+) and sirtuins in aging/longevity control. NPJ Aging Mech Dis. 2016;2:16017.
doi pubmed - Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One. 2012;7(7):e42357.
doi pubmed - Peluso A, Damgaard MV, Mori MAS, Treebak JT. Age-dependent decline of NAD(+)-universal truth or confounded consensus? Nutrients. 2021;14(1):101.
doi pubmed - Xie N, Zhang L, Gao W, Huang C, Huber PE, Zhou X, Li C, et al. NAD(+) metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct Target Ther. 2020;5(1):227.
doi pubmed - https://www.umin.ac.jp/.
- Matsuyama R, Omata T, Kageyama M, Nakajima R, Kanou M, Yamana K. Stabilization and quantitative measurement of nicotinamide adenine dinucleotide in human whole blood using dried blood spot sampling. Anal Bioanal Chem. 2023;415(5):775-785.
doi pubmed - https://www.R-project.org/.
- Camacho-Pereira J, Tarrago MG, Chini CCS, Nin V, Escande C, Warner GM, Puranik AS, et al. CD38 dictates age-related nad decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 2016;23(6):1127-1139.
doi pubmed - Braidy N, Berg J, Clement J, Khorshidi F, Poljak A, Jayasena T, Grant R, et al. Role of nicotinamide adenine dinucleotide and related precursors as therapeutic targets for age-related degenerative diseases: rationale, biochemistry, pharmacokinetics, and outcomes. Antioxid Redox Signal. 2019;30(2):251-294.
doi pubmed - Brown KD, Maqsood S, Huang JY, Pan Y, Harkcom W, Li W, Sauve A, et al. Activation of SIRT3 by the NAD(+) precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 2014;20(6):1059-1068.
doi pubmed - Nakagawa-Nagahama Y, Igarashi M, Miura M, Kashiwabara K, Yaku K, Fukamizu Y, Sato T, et al. Blood levels of nicotinic acid negatively correlate with hearing ability in healthy older men. BMC Geriatr. 2023;23(1):97.
doi pubmed - Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD(+) metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22(2):119-141.
doi pubmed - McReynolds MR, Chellappa K, Baur JA. Age-related NAD(+) decline. Exp Gerontol. 2020;134:110888.
doi pubmed - Conlon N, Ford D. A systems-approach to NAD+ restoration. Biochem Pharmacol. 2022;198:114946.
doi pubmed - Scher MB, Vaquero A, Reinberg D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev. 2007;21(8):920-928.
doi pubmed - Ishak MN, Nik-Abdul-Ghani NM, Mohamad I. Sudden bilateral sensorineural hearing loss secondary to cerebral venous thrombosis. Iran J Otorhinolaryngol. 2018;30(97):113-116.
pubmed - Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143(5):802-812.
doi pubmed - Okur MN, Sahbaz BD, Kimura R, Manor U, Patel J, Park JH, Andrade L, et al. Long-term NAD+ supplementation prevents the progression of age-related hearing loss in mice. Aging Cell. 2023;22(9):e13909.
doi pubmed - Morifuji M, Higashi S, Ebihara S, Nagata M. Ingestion of beta-nicotinamide mononucleotide increased blood NAD levels, maintained walking speed, and improved sleep quality in older adults in a double-blind randomized, placebo-controlled study. Geroscience. 2024;46(5):4671-4688.
doi pubmed - Fulcher BD, Fornito A. A transcriptional signature of hub connectivity in the mouse connectome. Proc Natl Acad Sci U S A. 2016;113(5):1435-1440.
doi pubmed - Gregory S, Long JD, Kloppel S, Razi A, Scheller E, Minkova L, Papoutsi M, et al. Operationalizing compensation over time in neurodegenerative disease. Brain. 2017;140(4):1158-1165.
doi pubmed - Watanabe H, Bagarinao E, Maesawa S, Hara K, Kawabata K, Ogura A, Ohdake R, et al. Characteristics of neural network changes in normal aging and early dementia. Front Aging Neurosci. 2021;13:747359.
doi pubmed - Pak JH, Kim Y, Yi J, Chung JW. Antioxidant therapy against oxidative damage of the inner ear: protection and preconditioning. Antioxidants (Basel). 2020;9(11):1076.
doi pubmed - Massudi H, Grant R, Guillemin GJ, Braidy N. NAD+ metabolism and oxidative stress: the golden nucleotide on a crown of thorns. Redox Rep. 2012;17(1):28-46.
doi pubmed - Fukui T, Yamauchi K, Maruyama M, Yasuda T, Kohno M, Abe Y. Significance of measuring oxidative stress in lifestyle-related diseases from the viewpoint of correlation between d-ROMs and BAP in Japanese subjects. Hypertens Res. 2011;34(9):1041-1045.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.