| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 2, February 2025, pages 97-105
Ischemic Preconditioning Negatively Affects Thrombogenic Clotting Profile in Cerebral Small Vessel Occlusion Stroke Patients
Line Boel Norregaarda, f , Nicolai Ryttera, f
, Laura Cathrine Christoffersenb
, Lasse Gliemanna
, Christian Stevns Hansenc
, Matthew Lawrenced, Philip Adrian Evansd, e
, Christina Kruuseb
, Ylva Hellstena, g
aDepartment of Nutrition, Exercise and Sports, University of Copenhagen, Copenhagen, Denmark
bNeurovascular Research Unit, Department of Neurology, Copenhagen University Hospital-Herlev Gentofte, Copenhagen, Denmark
cSteno Diabetes Centre Copenhagen, Gentofte, Denmark
dWelsh Centre for Emergency Medicine Research, Morriston Hospital, SBU Health Board, Swansea, UK
eSwansea University Medical School, Swansea, UK
fThese authors contributed equally to the work.
gCorresponding Author: Ylva Hellsten, Department of Nutrition, Exercise and Sports, University of Copenhagen, DK-2100 Copenhagen, Denmark
Manuscript submitted September 13, 2024, accepted January 13, 2025, published online February 13, 2025
Short title: IPC and Thrombogenic Risk in Stroke Patients
doi: https://doi.org/10.14740/jocmr6086
| Abstract | ▴Top |
Background: The study evaluated the effect of an acute and a 2-week daily repetitive ischemic preconditioning (IPC) on conduit artery vascular function and thrombogenic clotting profile, in patients with a recent ischemic stroke.
Methods: Fourteen patients, aged 71 ± 8 years, with a cerebral small vessel occlusion stroke were included in a randomized, controlled, open-label cross-over study. Treatment consisted of 2 weeks of daily IPC, four 5-min rounds of upper-arm occlusion, interspersed by 5 min rest periods. Control was without treatment. Brachial artery flow-mediated dilation (FMD) was determined at baseline and after the control and treatment periods. Before and after each period, the patients underwent an acute bout of IPC. Blood samples were obtained for thrombogenic clotting profile at baseline and after the acute IPC bout, both before and after the control and treatment periods.
Results: The period of daily IPC increased brachial artery diameter but did not influence FMD. Acutely, IPC was found to induce an increase in fractal dimension, indicating a denser clot microstructure, and a reduction in plasma levels of plasminogen activator inhibitor 1 (PAI-1). There was no effect of daily IPC on the basal thrombogenic clotting profile, or on the change in clotting profile induced by acute IPC.
Conclusions: Collectively, the data show that acute IPC leads to a prothrombotic clotting profile, despite antiplatelet therapy. Moreover, 2 weeks of daily treatment with IPC does not influence conduit artery vascular function or thrombogenicity in stroke patients.
Keywords: Cerebral small vessel occlusion stroke; Vascular function; Preconditioning; Clotting profile
| Introduction | ▴Top |
Ischemic preconditioning (IPC) is a procedure where blood flow to a limb is repeatedly occluded for a brief period of time, with each period followed by a reperfusion phase. Just one session of the procedure has been shown to enhance both microvascular [1] and conduit artery [2, 3] function in humans and when repeated regularly over a period, IPC has been shown to improve vascular endothelial function [4-7]. Application of the IPC procedure in different clinical populations has resulted in promising effects [8-10] and a period of daily IPC may therefore be an effective strategy to improve vascular function and reduce the susceptibility to cerebral infarct in individuals at risk. Preclinical studies have initial positive results; however, these have not been fully confirmed in humans yet [11].
Cerebral small vessel occlusion (lacunar) stroke is caused by thrombosis in arterioles subcortically or deep brain regions, which may lead to significant disability [2, 3]. Lacunar stroke is prevalent in lifestyle-related diseases such as hypertension and diabetes [12] and represents approximately 20% of all cerebral strokes with a relatively high risk of a recurrent stroke [13, 14]. Home-based and easy-to-use interventions which reduce the risk of recurrent stroke are therefore warranted.
The underlying pathology of lacunar infarct includes thickening of the arterial media presence of intimal plaques [15] and endothelial dysfunction [12, 16]. The vascular endothelium prevents thrombosis by inhibiting platelet reactivity through the production of nitric oxide (NO) and prostacyclin [17]. In dysfunctional endothelium, the formation of NO and prostacyclin is reduced [18, 19] whereby the inhibition of platelet reactivity is attenuated and the risk of arterial thrombosis enhanced, in particular with additional presence of atherosclerotic plaques.
The susceptibility to arterial thrombosis is commonly assessed by measurements of plasma biomarkers such as fibrinogen and plasminogen activator inhibitor 1 (PAI-1) or by determination of platelet reactivity [20]. However, as hemostasis is a highly complex process involving many steps, such measurements provide only partial information. A new useful global hemostatic biomarker of coagulability has been developed [21, 22] which estimates the density of the microstructure of a blood clot when formed. The method involves viscoelastic measurements of whole blood to provide a measurement of the gel point or formation of the incipient blood clot. From a gel point measurement, it can be quantified how the incipient blood clot, which consists mainly of activated platelets and fibrin, is organized by calculating its corresponding fractal dimension [23]. The outcome is diagnostically useful as high values of fractal dimension indicate a denser clot structure which is more serious as it is difficult to degrade by fibrinolysis [22, 24, 25]. The influence of IPC on fractal dimension has not previously been assessed.
The present study evaluated the effect of an acute bout of IPC as well as a 2-week period of daily IPC on conduit artery vascular function and thrombogenic clotting profile in patients who had suffered from cerebral small vessel occlusion stroke. The hypothesis was that IPC would improve conduit artery function, assessed as flow-mediated dilation (FMD), and the thrombogenic clotting profile.
| Materials and Methods | ▴Top |
Participants
A total of 14 patients diagnosed with cerebral small vessel occlusion stroke were included in the study. Inclusion criteria were: > 18 years of age; clinical symptoms and corresponding computed tomography (CT)/magnetic resonance (MR) scan with lacunar infarct, defined as either: 1) lacunar stroke according to TOAST classification [26], diagnosed within the past 5 years, or 2) isolated neurologic outcomes (e.g., motoric, sensoric or language) for over 24 h; no cortical or cerebellar dysfunction, MR-verified acute lacunar supratentorial or infratentorial infarct according to STRIVE criteria [27] (< 2 cm in diameter in acute phase, 1.5 cm in chronic phase); no clinically significant carotid stenosis or cardioembolic cause of infarct. Exclusion criteria were: chronic diseases not directly related to stroke, e.g. cardiac disease, cancer and immune deficiency; current treatment with peroral steroids, smoking, currently or within past 10 years, alcohol or drug abuse (Table 1). All subjects received antiplatelet treatment, clopidogrel 75 mg/day. One patient received dual anti-platelet treatment with clopidogrel 75 mg and acetylsalicylic acid 75 mg/day 3 months prior to study day. Four patients had received acetylsalicylic acid 75 mg/day for 5 days while admitted to the hospital.
 Click to view | Table 1. Characteristics of 14 Stroke Patients at Baseline |
The study was approved by the Ethics Committee of the Capital Region of Copenhagen (H-16048498) and all procedures were carried out in accordance with the Declaration of Helsinki. The study was registered at ClinicalTrials.gov (NCT03635177) prior to first inclusion.
Study design
The study adopted an open-label repeated-measures cross-over design where all participants were subjected to a 2-week treatment period of daily IPC and a 2-week control period. Participants were randomized to the order of treatment or control and each period was separated by at least a 3-week washout period. The randomization was achieved by administration of sealed opaque envelopes containing the allocation of treatment made by personnel not involved in the study.
Before and after the two intervention periods, all patients attended an experimental day that included determination of conduit artery vascular function and assessment of thrombogenic clotting profile. The determination of vascular endothelial function was accomplished by the FMD technique in the brachial artery and performed after at least 30 min rest in a supine position. Blood samples were collected at baseline and 5 min after a 4 × 5 min session of acute IPC. The blood samples were used for analysis of full blood count, plasma coagulation biomarkers as well as fractal dimension: viscoelastic measurement of coagulating whole blood, indicating clot microstructure.
Interventional treatment
The participants conducted IPC at home once daily for 14 days by use of an automated blood pressure cuff positioned on the upper non-dominant arm (autoRIC™, Cellaegis Devices, Toronto, Ontario, Canada). Each session consisted of four rounds of 5-min periods of occlusion, separated by 5 min of non-occluded reperfusion. All participants were carefully instructed in the use of the device prior to initiation of the treatment period, and continuous guidance was offered as well.
Due to the obvious intervention treatment, the study was conducted as open-label and the control period served as a time-control without treatment.
FMD
FMD was conducted by an experienced researcher and the procedure was conducted according to guidelines [28]. FMD was expressed as the percentage change in diameter of the brachial artery (FMD%). The change was calculated as the difference between baseline and maximal diameter measured after 5 min of blood flow occlusion. Occlusion was achieved by inflation of a pressure cuff (E20 Rapid Cuff Inflator and AG101 Cuff Inflator Air Source, Hokanson®, Bellevue, WA, USA) placed on the arm. Analysis of the vascular 2D images was conducted in a blinded manner by use of validated software (Brachial Analyzer for Research, Version 6.8.7; Medical Imaging Applications, Iowa, USA). Brachial diameter and velocity traces were analyzed by use of automated edge detection. Obtained values were applied in the calculation of the following variables: FMD%: (Maximal diameter - Baseline diameter)/Baseline diameter) × 100. Blood flow (mL/min): πr2 × Average blood velocity (cm/s) × 60. Shear rate: 4 × (Blood mean velocity (cm/s)/Diameter (cm)).
Viscoelastic measurement of fresh whole blood
Rheometric determination of gel point
The viscoelastic measurement is based on rheometric analysis of the gel point [29], from which fractal dimension (df) of an incipient blood clot can be determined [22]. The rheology technique used to attain the gel point has been validated for use with human blood in previous studies [22, 30, 31]. In brief, blood was drawn slowly from the antecubital vein into syringes and transferred to tubes not containing any anticoagulant. Within 60 s, 7 mL of blood was placed in a double concentric measuring geometry mounted on a controlled stress rheometer (Discovery Series Hybrid Rheometers (DHR-2); TA Instruments, New Castle, USA), which was held at a constant temperature of 37 °C. The viscoelastic analysis was performed using small amplitude oscillatory shear measurements at varying frequencies (2, 0.93, 0.43 and 0.2 Hz) with an applied peak stress amplitude of 0.03 Pa sequentially with time. These sequential measurements of the four frequencies over time allow for the determination of the gel point [32].
Fractal dimension and clot microstructure
The gel point obtained by viscoelastic measurement of whole blood marks the transition of the blood from a viscoelastic liquid to a viscoelastic solid, and thereby identifies the formation of an incipient blood clot. From the gel point measurement, it can be quantified how the incipient blood clot, which consists mainly of activated platelets and fibrin, is organized by calculating its corresponding fractal dimension [23].
The relationship between fractal dimension and the microstructure and fibrin mass of an incipient blood clot has been validated by electron microscopy and computational analysis previously [30, 33, 34].
Analysis of blood samples
Metabolic and health-related biomarkers
All measures of glucose metabolism, cholesterol, triglyceride and hepatic enzymes were performed as routine clinical tests at Department of Clinical Biochemistry, Copenhagen University Hospital-Herlev Gentofte, Denmark.
Full blood count and plasma coagulation biomarkers
The full blood counts, including thrombocytes, erythrocytes and leukocytes, were measured in a fresh sample of whole blood at Department of Clinical Biochemistry, Copenhagen University Hospital-Herlev Gentofte. The plasma coagulation biomarkers were measured in aliquots of blood drawn into 3.2% sodium citrate vacutainers (Greiner Bio-One GmbH, Austria). Fibrinogen (ab108842, Abcam, Cambridge, UK), PAI-1 (ab269373, Abcam, Cambridge, UK) and D-dimer (ab260076, Abcam, Cambridge, UK) were analyzed by use of ELISA according to manufacturer’s protocols.
Statistical analysis
In all studies, a priori sample size determination was performed for the primary outcome, FMD [35]. The required sample size was calculated to be 13, based on an expected FMD mean of 10%, mean FMD difference with the intervention of 2% and a standard deviation (SD) of 2.5%. Data are presented as mean ± SD. Statistical analyses were performed with R (version 3.4.1; R Foundation for Statistical Computing, Vienna, Austria) using the interface RStudio (version 1.1.463; RStudio Team, Boston, USA). A linear mixed-model approach was used to detect differences with the intervention. Subjects were specified as a repeated factor and identifier of random variation. For df, subjects and time were used as identifier of random variation. Residual and Q-Q plots confirmed homogeneity and normal distribution, respectively. Post-hoc analysis was used to detect all differences. The reported P-values are non-adjusted. Graphical visualization was performed in Prism (version 8.3.0; GraphPad Software, San Diego, USA).
| Results | ▴Top |
Participant characteristics
Of the 14 included patients, three were females and the mean age was 71 ± 8 years. Cardiovascular risk factors and comorbidities are presented in Table 1.
Brachial artery FMD
There was an increase in baseline diameter with IPC treatment compared with the 2-week control period (P = 0.0307; Fig. 1a). There was no change in peak diameter (P = 0.9014; Fig. 1b) or brachial artery FMD (P = 0.3467; Fig. 1c) with 2 weeks of IPC treatment compared with the 2-week control period.
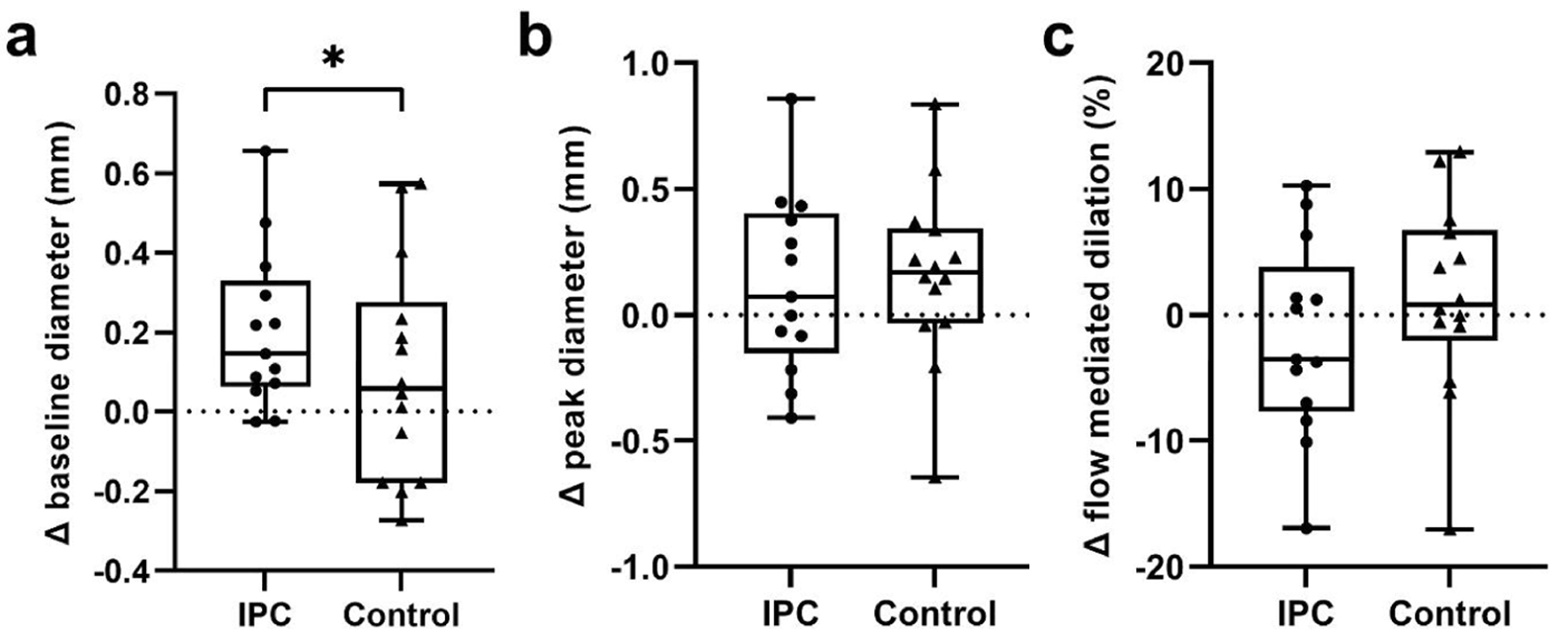 Click for large image | Figure 1. (a) Change in baseline diameter, (b) peak diameter and (c) flow-mediated dilation of the brachial artery with a 2-week period of daily IPC or a control period in cerebral small vessel occlusion stroke patients. *Significant difference between control and IPC; P < 0.05. IPC: ischemic preconditioning. |
Clot microstructure - fractal dimension
The 2 weeks of IPC did not influence the level of df, compared to the 2-week control period (P = 0.0975; Fig. 2a). Analysis of pooled data from the interventions revealed that an acute session of IPC increased df in the venous samples (from 1.64 ± 0.046 to 1.66 ± 0.055, P = 0.0463; Fig. 2b).
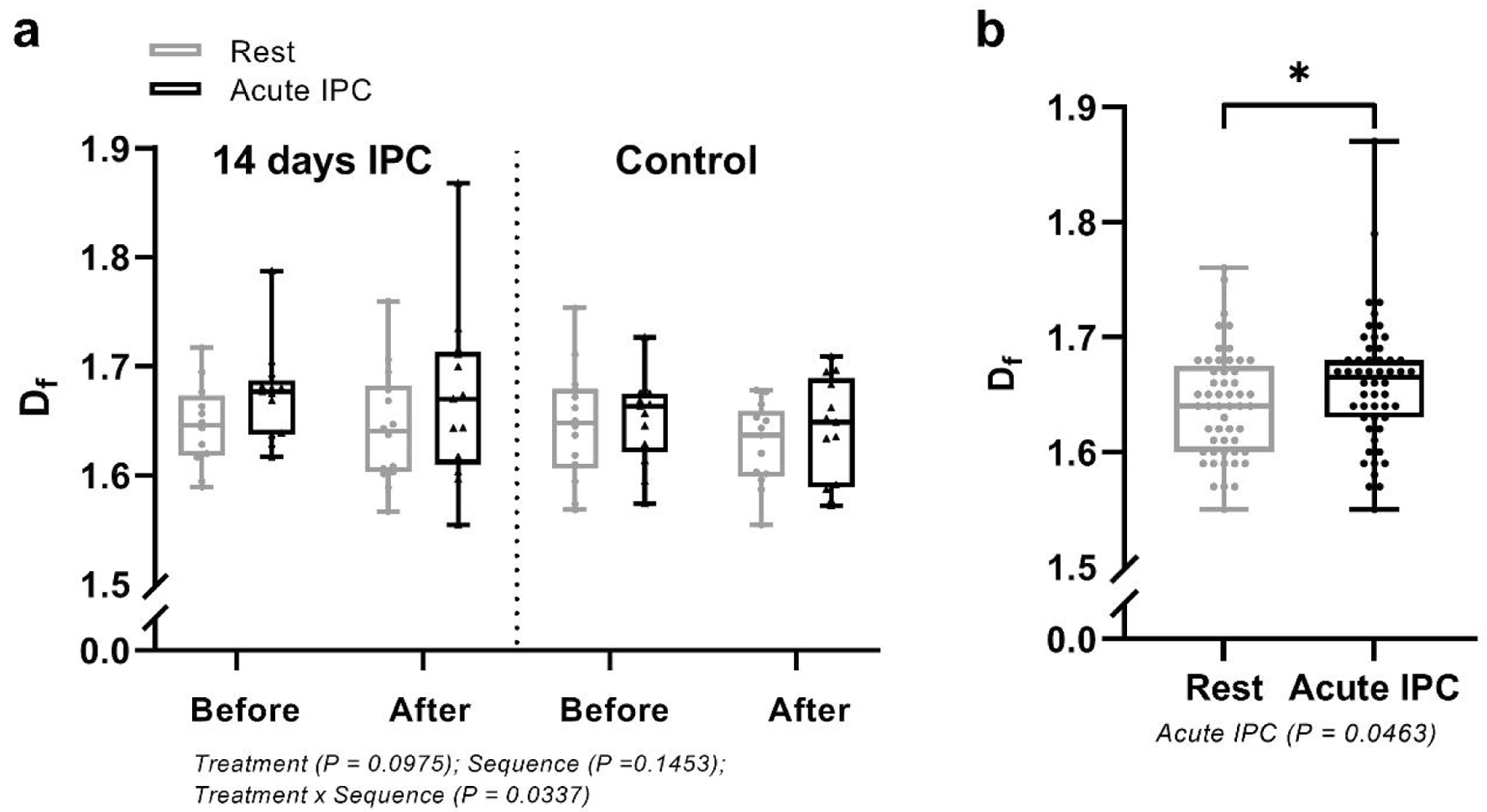 Click for large image | Figure 2. Fractal dimension (df) in cerebral small vessel occlusion stroke patients (a) before and after an acute bout of IPC conducted before and after a 2-week period of daily IPC or a 2-week control period and (b) before and after an acute bout of IPC. Data combined from the acute interventions. *Significant difference between control and IPC; P < 0.05. IPC: ischemic preconditioning. |
Plasma coagulation markers and full blood count
The 2-week intervention period did not influence the full blood count (Table 2) or the concentration of D-dimer (P = 0.9970; Fig. 3a), PAI-1 (P = 0.3080; Fig. 3c), or fibrinogen (0.8423; Fig. 3e). An acute session of IPC reduced the plasma PAI-1 concentration (P = 0.0125; Fig. 3d), when pooling data from both intervention periods, whereas there was no effect on D-dimer (P = 0.103; Fig. 3b) or fibrinogen (P = 0.308; Fig. 3f). No differences were observed in any parameter of the full blood count (Table 2).
 Click to view | Table 2. Full Blood Count Before and After 2 Weeks of IPC Treatment and Control |
 Click for large image | Figure 3. Plasma indicators of thrombogenesis: (a, b) D-dimer, (c, d) PAI-1 and (e, f) fibrinogen, before and after an acute bout of IPC conducted before and after a 2-week period of daily IPC, or a 2-week control period, in cerebral small vessel occlusion stroke patients. *Significant difference between control and IPC; P < 0.05. IPC: ischemic preconditioning. |
| Discussion | ▴Top |
The main finding of the present study was that, in contrast to our hypothesis, a period of daily IPC in patients who had suffered a lacunar infarct, had no beneficial influence on conduit artery vascular function, assessed by FMD, or on the thrombogenic clotting profile, assessed by fractal dimension and plasma coagulation biomarkers. Instead, an acute session of IPC resulted in an increase in fractal dimension suggesting that the procedure may increase the thrombogenicity acutely, and thereby susceptibility to stroke in this population.
In this study, we evaluated if 2 weeks of daily IPC in cerebral small vessel occlusion stroke patients would improve vascular function and in parallel improve the thrombogenic clotting profile. FMD, which indicates NO-dependent endothelial function, was used to assess conduit artery function. As NO inhibits platelet reactivity, we reasoned that improved FMD would reflect a more pronounced NO bioavailability which also would benefit the clotting profile. In contrast to our hypothesis, daily IPC did not have an effect either on FMD or on the clotting profile in patients with recent small vessel occlusion stroke. The lack of effect of IPC on conduit artery function contrasts that of a recent study, in which an improved brachial artery FMD response was observed in prior stroke patients after a 2-week treatment period with IPC [36]. However, there were numerous differences between the studies; as in the previous study [36], the patients had experienced either a cerebral small vessel occlusion stroke or hemorrhage and patients were included up to 8 years past the last stroke. In the current study, most of the patients had experienced a stroke within the past 6 months and only patients with previous cerebral small vessel occlusion stroke were included. Moreover, in the previous study [36], the IPC procedure was conducted on the leg three times per week as opposed to on the arm every day. We chose arm treatment as we considered it to be practical and more feasible for the patients in real-life. Also, 1 week of daily IPC of the arm was shown to improve FMD in healthy and diabetic individuals [4, 37]. Thus, the discrepancy in disease ethiology may be a more likely cause of the disparity in results between the studies.
In contrast to our hypothesis that IPC would be beneficial for the clotting profile, we made the important observation that the treatment resulted in an immediate more pro-thrombotic clotting profile in cerebral small vessel occlusion stroke patients. This was evidenced as an increase in fractal dimension indicating a denser blood clot structure as discussed below. This observation is particularly striking considering that all patients were on antiplatelet therapy by clopidogrel. The finding of a potential pro-thrombotic effect of IPC could be important clinically and should be held up against the current lack of evidence of a beneficial effect of a period of daily treatment on stroke outcome in humans [11].
For the assessment of the thrombogenic clotting profile, we combined plasma coagulation biomarkers with a novel global biomarker of hemostasis, which involves the assessment of the viscoelastic properties of fresh whole blood. This measurement, expressed as fractal dimension, provides an indication of the integrated hemostatic property and is an immediate indication of the risk of cardiovascular events [25]. A high numerical value of fractal dimension is equivalent to a strong and dense clot with a high number of complex structured fibrin branches, which is more difficult to dissolve biologically or through pharmacotherapeutics [22, 25]. In a Welsh cohort of 149 individuals, the fractal dimension was found to be higher in stroke patients, treated either by aspirin or dual antiplatelet therapy compared to healthy control participants [38], indicating a greater propensity for the formation of dense clots in stroke patients. The current patient population, consisting of patients who had experienced a stroke within the past 6 months and who all were undergoing treatment with clopidogrel, accordingly [30] displayed fractal dimension values of between 1.59 and 1.72 at baseline, which is somewhat lower than the stroke patients in the Welsh cohort [38], probably due to a generally better lifestyle in the Danish population.
The acute session of IPC elevated the fractal dimension by, on average 0.02 units. Although numerically this appears as a small change, the functional implication is substantial; based on computational modeling of a simplified branching network, this difference can be calculated to correspond to a 20-40% increase in clot mass. In parallel with the increase in fractal dimension, there was a significant decrease in the fibrinolysis inhibitor, PAI-1, indicating improved fibrinolysis. In a previous study, it was noted that the effect of acute IPC resulted in a reduction in PAI-1 [39]. However, contrary to our findings using rotational thromboelastography, they found no change in clot mass or strength nor increase in thrombogenicity. In our study, we show a clear and marked increase in clot elasticity indicative of a prothromobotic tendency. This value may be higher than normally anticipated as all the patients were on clopidogrel treatment which has been shown previously to contribute to a looser and weakening effect on clot architecture with a corresponding reduction in df [30]. The rise in df is probably due to vascular endothelial damage during ischemia reperfusion; however, the finding of a difference between an elevation of df and reduction in PAI-1 may be an interesting mechanistic effect which requires further explanation. It is of note that the acute effect of the IPC procedure on the change in thrombogenicity was similar before and after the 2 weeks of daily treatment, suggesting that habituation to IPC had no effect on the thrombogenic response.
Study limitations
Although the current study was sufficiently powered for the primary outcome of FMD, it was a pilot study with a limited number of participants and larger studies would be required to verify the findings. However, given the observed negative influence of an acute session of IPC on thrombogenicity, it may be questioned whether a full study would be justified.
Previous studies have shown that only 1 week of daily IPC, including in diabetic individuals, improved FMD; however, it cannot be excluded that a longer intervention period would be required for an effect to be detectable in stroke patients. In terms of thrombogenicity assessed by fractal dimension, it is unclear whether a longer period could have had an effect.
Conclusion
Two weeks of daily IPC performed on the arm in cerebral small vessel occlusion stroke patients did not improve either brachial artery FMD or basal thrombogenic clotting profile. Instead, the results show that one acute session of IPC can increase thrombogenicity in stroke patients, despite antiplatelet therapy treatment. This somewhat controversial observation suggests that caution should be exercised when subjecting stroke patients to IPC. This finding also raises the question of whether IPC to a greater extent may affect thrombogenicity in patients at risk who are not being treated by antiplatelet therapy.
Learning points
Two weeks of daily IPC increased brachial artery diameter, but did not change brachial artery FMD in patients with a recent ischemic stroke.
Acute IPC was found to increase clot microstructural density.
The novelty of this study is using a global biomarker of hemostasis to identify the density of the clot microstructure when investigating the effect of IPC on the clotting profile in ischemic stroke patients.
The findings of the present study have negative implications for the use of IPC in the treatment of ischemic stroke patients.
Acknowledgments
None to declare.
Financial Disclosure
The study was funded by Independent Research Fund, Denmark, Medical and Health Sciences.
Conflict of Interest
The authors declare no conflict of interest associated with this study.
Informed Consent
Before enrollment, written and informed consent was obtained from all patients.
Author Contributions
YH and CK conceived the experiments. YH, CK, LC, NR, ML and PAE designed the experiments. LBN, NR, LC and LG collected the data. ML, CSH and PAE assisted with data collection. All authors interpreted the data. LBN, NR, CK and YH analyzed data and drafted the manuscript. All authors revised and approved the final version of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request and must be in accordance with the current guidelines of the General Data Protection Regulation (GDPR).
Abbreviations
df: fractal dimension; FMD: flow-mediated dilation; IPC: ischemic preconditioning; NO: nitric oxide; PAI-1: plasminogen activator inhibitor 1
| References | ▴Top |
- Rytter N, Carter H, Piil P, Sorensen H, Ehlers T, Holmegaard F, Tuxen C, et al. Ischemic preconditioning improves microvascular endothelial function in remote vasculature by enhanced prostacyclin production. J Am Heart Assoc. 2020;9(15):e016017.
doi pubmed - Enko K, Nakamura K, Yunoki K, Miyoshi T, Akagi S, Yoshida M, Toh N, et al. Intermittent arm ischemia induces vasodilatation of the contralateral upper limb. J Physiol Sci. 2011;61(6):507-513.
doi pubmed - Moro L, Pedone C, Mondi A, Nunziata E, Antonelli Incalzi R. Effect of local and remote ischemic preconditioning on endothelial function in young people and healthy or hypertensive elderly people. Atherosclerosis. 2011;219(2):750-752.
doi pubmed - Jones H, Hopkins N, Bailey TG, Green DJ, Cable NT, Thijssen DH. Seven-day remote ischemic preconditioning improves local and systemic endothelial function and microcirculation in healthy humans. Am J Hypertens. 2014;27(7):918-925.
doi pubmed - Jones H, Nyakayiru J, Bailey TG, Green DJ, Cable NT, Sprung VS, Hopkins ND, et al. Impact of eight weeks of repeated ischaemic preconditioning on brachial artery and cutaneous microcirculatory function in healthy males. Eur J Prev Cardiol. 2015;22(8):1083-1087.
doi pubmed - Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374(9700):1557-1565.
doi pubmed - Kimura M, Ueda K, Goto C, Jitsuiki D, Nishioka K, Umemura T, Noma K, et al. Repetition of ischemic preconditioning augments endothelium-dependent vasodilation in humans: role of endothelium-derived nitric oxide and endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2007;27(6):1403-1410.
doi pubmed - Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375(9716):727-734.
doi pubmed - Ekeloef S, Homilius M, Stilling M, Ekeloef P, Koyuncu S, Munster AB, Meyhoff CS, et al. The effect of remote ischaemic preconditioning on myocardial injury in emergency hip fracture surgery (PIXIE trial): phase II randomised clinical trial. BMJ. 2019;367:l6395.
doi pubmed - Lim SY, Hausenloy DJ. Remote ischemic conditioning: from bench to bedside. Front Physiol. 2012;3:27.
doi pubmed - Hansen LF, Nielsen NSK, Christoffersen LC, Kruuse C. Translational challenges of remote ischemic conditioning in ischemic stroke - a systematic review. Ann Clin Transl Neurol. 2021;8(8):1720-1729.
doi pubmed - Mast H, Thompson JL, Lee SH, Mohr JP, Sacco RL. Hypertension and diabetes mellitus as determinants of multiple lacunar infarcts. Stroke. 1995;26(1):30-33.
doi pubmed - Hart RG, Pearce LA, Bakheet MF, Benavente OR, Conwit RA, McClure LA, Talbert RL, et al. Predictors of stroke recurrence in patients with recent lacunar stroke and response to interventions according to risk status: secondary prevention of small subcortical strokes trial. J Stroke Cerebrovasc Dis. 2014;23(4):618-624.
doi pubmed - Jackson C, Sudlow C. Comparing risks of death and recurrent vascular events between lacunar and non-lacunar infarction. Brain. 2005;128(Pt 11):2507-2517.
doi pubmed - Caplan LR. Lacunar infarction and small vessel disease: pathology and pathophysiology. J Stroke. 2015;17(1):2-6.
doi pubmed - Corban MT, Lerman LO, Lerman A. Endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2019;39(7):1272-1274.
doi pubmed - Moro MA, Russel RJ, Cellek S, Lizasoain I, Su Y, Darley-Usmar VM, Radomski MW, et al. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc Natl Acad Sci U S A. 1996;93(4):1480-1485.
doi pubmed - Hellsten Y, Jensen L, Thaning P, Nyberg M, Mortensen S. Impaired formation of vasodilators in peripheral tissue in essential hypertension is normalized by exercise training: role of adenosine and prostacyclin. J Hypertens. 2012;30(10):2007-2014.
doi pubmed - Nyberg M, Egelund J, Mandrup CM, Nielsen MB, Mogensen AS, Stallknecht B, Bangsbo J, et al. Early postmenopausal phase is associated with reduced prostacyclin-induced vasodilation that is reversed by exercise training: the Copenhagen women study. Hypertension. 2016;68(4):1011-1020.
doi pubmed - Kanji R, Kubica J, Navarese EP, Gorog DA. Endogenous fibrinolysis-Relevance to clinical thrombosis risk assessment. Eur J Clin Invest. 2021;51(4):e13471.
doi pubmed - Evans PA, Hawkins K, Lawrence M, Barrow MS, Williams PR, Williams RL. Studies of whole blood coagulation by oscillatory shear, thromboelastography and free oscillation rheometry. Clin Hemorheol Microcirc. 2008;38(4):267-277.
pubmed - Evans PA, Hawkins K, Morris RH, Thirumalai N, Munro R, Wakeman L, Lawrence MJ, et al. Gel point and fractal microstructure of incipient blood clots are significant new markers of hemostasis for healthy and anticoagulated blood. Blood. 2010;116(17):3341-3346.
doi pubmed - Muthukumar M, Winter HH. Fractal dimension of a crosslinking polymer at the gel point. Macromolecules. 1986;19(4):1284-1285.
- Collet JP, Park D, Lesty C, Soria J, Soria C, Montalescot G, Weisel JW. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arterioscler Thromb Vasc Biol. 2000;20(5):1354-1361.
doi pubmed - Sabra A, Lawrence MJ, Curtis D, Hawkins K, Williams PR, Evans PA. In vitro clot model to evaluate fibrin-thrombin effects on fractal dimension of incipient blood clot. Clin Hemorheol Microcirc. 2019;-1:147-153.
doi pubmed - Adams HP, Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE, 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35-41.
doi pubmed - Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822-838.
doi pubmed - Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257-265.
doi pubmed - Winter HH. Gel point. Encyclopedia of Polymer Science and Technology. p. 1-15.
- Knowles RB, Lawrence MJ, Ferreira PM, Hayman MA, D'Silva LA, Stanford SN, Sabra A, et al. Platelet reactivity influences clot structure as assessed by fractal analysis of viscoelastic properties. Platelets. 2018;29(2):162-170.
doi pubmed - Lawrence MJ, Davies G, Nyberg M, Whitley J, Evans V, Williams R, Hellsten Y, et al. The effect of tyramine infusion and exercise on blood flow, coagulation and clot microstructure in healthy individuals. Thromb Res. 2018;170:32-37.
doi pubmed - Evans PA, Hawkins KM, Lawrence MJ, Williams PR, Williams RL. Rheometrical studies of blood clot formation by oscillatory shear, thromboelastography, sonoclot analysis and free oscillation rheometry. AIP Conference Proceedings. 2008;1027(1):597-599.
- Davies NA, Llwyd O, Brugniaux JV, Davies GR, Marley CJ, Hodson D, Lawrence MJ, et al. Effects of exercise intensity on clot microstructure and mechanical properties in healthy individuals. Thromb Res. 2016;143:130-136.
doi pubmed - Lawrence MJ, Sabra A, Mills G, Pillai SG, Abdullah W, Hawkins K, Morris RH, et al. A new biomarker quantifies differences in clot microstructure in patients with venous thromboembolism. Br J Haematol. 2015;168(4):571-575.
doi pubmed - Altman DG. Statistics and ethics in medical research: III How large a sample? Br Med J. 1980;281(6251):1336-1338.
doi pubmed - Hyngstrom AS, Nguyen JN, Wright MT, Tarima SS, Schmit BD, Gutterman DD, Durand MJ. Two weeks of remote ischemic conditioning improves brachial artery flow mediated dilation in chronic stroke survivors. J Appl Physiol (1985). 2020;129(6):1348-1354.
doi pubmed - Maxwell JD, Carter HH, Hellsten Y, Miller GD, Sprung VS, Cuthbertson DJ, Thijssen DHJ, et al. Seven-day remote ischaemic preconditioning improves endothelial function in patients with type 2 diabetes mellitus: a randomised pilot study. Eur J Endocrinol. 2019;181(6):659-669.
doi pubmed - Stanford SN, Sabra A, D'Silva L, Lawrence M, Morris RH, Storton S, Brown MR, et al. The changes in clot microstructure in patients with ischaemic stroke and the effects of therapeutic intervention: a prospective observational study. BMC Neurol. 2015;15:35.
doi pubmed - Kristiansen J, Grove EL, Rise N, Neergaard-Petersen S, Wurtz M, Kristensen SD, Hvas AM. Effect of remote ischaemic conditioning on coagulation and fibrinolysis. Thromb Res. 2016;141:129-135.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.