| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 16, Number 11, December 2024, pages 536-546
Downregulation of miR-25-3p and Its Impact on PTAFR and IGF2BP3 Expression in Type 2 Diabetes Mellitus: Implications for Biomarker Discovery and Disease Pathogenesis
Yanisa Rattanapana, b, Kallayarat Nongwaa, Chanoknan Supanponga, Chanasorn Satsadeedata, Thaveesak Sai-ongc, Nateelak Kooltheata, b, Takol Chareonsirisuthiguld, e
aMedical Technology, School of Allied Health Sciences, Walailak University, Nakhon Si Thammarat, Thailand
bHematology and Transfusion Science Research Center, Walailak University, Nakhon Si Thammarat, Thailand
cSchool of Public Health, Walailak University, Nakhon Si Thammarat, Thailand
dDepartment of Pathology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
eCorresponding Author: Takol Chareonsirisuthigul, Department of Pathology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Manuscript submitted October 9, 2024, accepted November 25, 2024, published online November 30, 2024
Short title: miR-25-3p Impact in T2DM
doi: https://doi.org/10.14740/jocmr6099
| Abstract | ▴Top |
Background: This study is designed to investigate the differential microRNA (miRNA) expression profiles in individuals with and without type 2 diabetes mellitus (T2DM). The focus is on miRNAs that play a crucial role in the onset and progression of T2DM, particularly in glucose metabolism, inflammation, platelet reactivity, and endothelial dysfunction.
Methods: Twenty samples were categorized into groups of T2DM and non-T2DM, and miRNA profiling was conducted using microarray analysis. The expression levels of the candidate miR-25-3p, as well as its target genes platelet-activating factor receptor (PTAFR) and insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3), were validated using quantitative polymerase chain reaction (qPCR).
Results: The present study revealed a significant reduction in the level of miR-25-3p in the T2DM group compared to the non-T2DM group. This suggests higher levels of PTAFR and IGF2BP3 in individuals with T2DM, indicating a potential biomarker for the condition.
Conclusions: The downregulation of miR-25-3p, which is associated with increased PTAFR levels, may contribute to heightened platelet reactivity and inflammation, worsening endothelial dysfunction, and potentially influencing vascular complications in diabetes. Additionally, the upregulation of IGF2BP3 is correlated with insulin resistance and β-cell dysfunction, which may contribute to elevated hyperglycemia and hyperinsulinemia, further aggravating the progression of diabetes. These findings highlight the potential of miR-25-3p and IGF2BP3 as biomarkers for T2DM and suggest their possible relevance for improving diagnosis and treatment strategies.
Keywords: miR-25-3p; Type 2 diabetes mellitus; PTAFR; IGF2BP3; Biomarkers
| Introduction | ▴Top |
Type 2 diabetes mellitus (T2DM) is characterized by elevated blood glucose levels resulting from cellular resistance to insulin or dysfunction of pancreatic β cells, leading to decreased insulin production and secretion. Insufficient insulin activity impedes cellular glucose uptake, consequently raising blood sugar levels. In patients with T2DM, insulin production may be inadequate, or the body may exhibit insulin resistance, further exacerbating hyperglycemia. Common signs and symptoms of T2DM include increased thirst and appetite, unintended weight loss, blurred vision, frequent urination, and peripheral neuropathy manifesting numbness or tingling in the hands or feet. Additionally, there may be slow wound healing, ketones in the urine, and potential acidosis. In males, issues related to sex hormone regulation and sexual performance are observed, while females may experience vaginal candidiasis, abnormal itching, and increased vaginal discharge [1]. Management of T2DM encompasses dietary regulation, physical activity, self-monitoring of blood glucose levels, and, when necessary, oral hypoglycemic agents and insulin therapy. Approximately 40% of individuals with T2DM require insulin injections. The diagnostic process aims to confirm the presence of T2DM and facilitate effective treatment and prevention strategies. This process necessitates integrating clinical symptomatology with laboratory test results to ensure accurate diagnosis and optimal patient care [2, 3].
Platelet-activating factor receptor (PTAFR) pathway plays a key role in astrocyte activation and inflammation in Alzheimer’s disease (AD), leading to increased Aβ accumulation and reactive oxygen species (ROS) production. Polar lipids (PLs) from salmon and yogurt inhibit PTAFR-mediated activation in astrocytes, reducing inflammation and improving neuronal health. These findings suggest that PL-rich foods may help reduce AD risk by targeting neuroinflammation [4]. PTAFR is upregulated in AD patients’ brain tissue, blood, and cerebrospinal fluid, contributing to inflammation via the IL10-STAT3 pathway, especially in microglia. Elevated PTAFR levels are also seen in APP/PS1 mice, and PTAFR is a potential target for anti-AD therapies, highlighting its role as a biomarker for early diagnosis and treatment [5].
Insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3), a ribonucleic acid (RNA)-binding protein, plays a crucial role in MLL-AF4 leukemia by regulating leukemogenesis. Targeting IGF2BP3 using CRISPR-Cas9 and inhibiting the menin-MLL interaction with small molecules enhanced the therapeutic effect, leading to increased differentiation and improved survival in leukemia models. IGF2BP3 depletion showed more potent antileukemic effects than menin-MLL inhibition alone, suggesting it as a promising therapeutic target in leukemia [6]. IGF2BP3 is upregulated in colorectal cancer (CRC) and associated with poor prognosis. It promotes CRC tumorigenesis and progression by stabilizing epidermal growth factor receptor (EGFR) mRNA and activating the EGFR pathway in an m6A-dependent manner. Additionally, IGF2BP3 enhances resistance to EGFR-targeted therapies, making it a functional oncogene in CRC. Targeting IGF2BP3 and m6A modification offers potential therapeutic strategies for CRC patients [7].
MicroRNA (miRNA, miR) is a small RNA molecule consisting of approximately 20 - 25 nucleotides, with the specific nucleotide sequence varying depending on the type of miRNA. These single-stranded RNA molecules, produced by genes responsible for their synthesis or by non-coding regions of the genome (introns), play a crucial role in regulating gene expression. They bind to target RNA at the 3' untranslated region (UTR), which does not code for proteins [8]. This binding results in the inhibition of protein production or the degradation of the target RNA, responsible for approximately 30% of protein production within the entire genome [9]. What is fascinating is that a single type of miRNA can bind specifically to multiple types of RNA, underscoring their versatility in gene regulation and the complexity of diabetes research [10, 11].
The mechanism by which miRNAs inhibit protein production or cause the degradation of target mRNA relies on the binding ability of the complementary bases on the miRNA to the target mRNA’s 3' UTR [12]. When there is perfect or near-perfect complementarity between the miRNA and the target mRNA, the mRNA is degraded, a mechanism commonly observed in plants. Conversely, when the complementarity is partial, protein production is inhibited by blocking the translation process of the target mRNA. In this case, the miRNA-mRNA complex is stored in a cellular region called the processing body (P-body) in the cytoplasm, which contains enzymes capable of degrading mRNA. However, the inhibition of protein production does not always occur through mRNA degradation [13]. Under appropriate stimulation, mRNA stored in the P-body can be released back into the cytoplasm and translated, indicating that this mechanism is reversible [14]. This reversible mechanism of action has been observed in animals but not in plants.
Currently, miRNAs are recognized for their involvement in various fundamental cellular processes, including embryonic development, metabolic processes, cell differentiation, programmed cell death (apoptosis), and insulin secretion [15]. The versatility and complexity of miRNAs in gene regulation are intriguing. Additionally, miRNAs are implicated in numerous human diseases such as AD, Parkinson’s disease, muscle disorders, and cancer. Studies have identified a significant role of miRNAs in the disease process, specifically concerning diabetes. In T2DM, abnormal miRNA expressions have been linked to the regulation of glucose metabolism, inflammation, platelet reactivity, and endothelial dysfunction [16]. For instance, the downregulation of miR-25-3p, as observed in this study, is associated with increased levels of PTAFR and IGF2BP3, which in turn may intensify platelet reactivity and inflammation, worsen endothelial dysfunction, and contribute to vascular complications in diabetes. These findings are consistent with prior research linking miR-25-3p to platelet function and endothelial health [17, 18]. Additionally, the upregulation of IGF2BP3 has been implicated in promoting insulin resistance and β-cell dysfunction, leading to heightened hyperglycemia and hyperinsulinemia, thus exacerbating diabetes progression. These conclusions are supported by these experimental data and are in line with previous studies that highlighted the role of IGF2BP3 in glucose metabolism and insulin resistance [19, 20].
miRNAs are considered excellent biomarkers due to their specific structure and stability in cells, the bloodstream, and bodily fluids. Typically, exosomes encapsulate miRNAs, but some are bound to proteins, such as argonaute-2 (AGO-2) or lipoproteins. miRNAs can exit the cell through secretion, exocytosis, and cell death (apoptosis). Importantly, miRNA assays in serum or plasma remain stable for 48 h at room temperature and can be preserved at -70 °C, providing a reliable method for disease diagnosis [21]. This stability, along with the potential for miRNAs to serve as biomarkers for indicating abnormal conditions, offers reassurance about the reliability of miRNA assays and their potential for disease diagnosis.
miRNAs are integral to physiological development and the pathogenesis of various diseases, including T2DM. In individuals with T2DM, miRNAs produced and released by cells exhibit stability and resistance to enzyme degradation, allowing them to be detected in serum or plasma. These stable molecules, present in biological fluids like plasma, retain their integrity, making them potential biomarkers for identifying abnormal conditions. Therefore, the objective of this study is to investigate and compare miRNA biomarkers in plasma samples from patients with T2DM and healthy controls. The findings may contribute to a better understanding of miRNA’s role in T2DM and their potential use as diagnostic tools for this condition.
| Materials and Methods | ▴Top |
Sample collection
Twenty leftover ethylenediaminetetraacetic acid (EDTA) plasma blood samples were collected, consisting of 10 samples from patients with T2DM and 10 samples from non-T2DM individuals. The inclusion criteria for the T2DM patients were defined by fasting blood sugar (FBS) levels of ≥ 126 mg/dL and hemoglobin A1c (HbA1c) levels of ≥ 6.5. Patients with other forms of diabetes, such as type 1 diabetes mellitus (T1DM) and gestational diabetes, were excluded from the study. Although the presence of comorbidities such as hypertension and dyslipidemia was not systematically assessed in this study, these factors should be considered in future investigations, and statistical adjustments for comorbidities may be necessary to ensure accurate interpretation. This study was approved by the Human Research Ethics Committee at Walailak University, Thailand (WUEC-23-235-01).
miRNA extraction
miRNA extraction was performed on all 20 blood samples using the HiPure Serum miRNA Kit (Magen, China), following the manufacturer’s instructions. The quantification and purity of the extracted miRNA were assessed using the NanoDrop™ One/OneC Microvolume UV-Vis Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
Microarray analysis
All samples were analyzed using the microarray technique with the GeneChip™ miRNA 4.0 Assay (Applied Biosystems, USA), following the manufacturer’s instructions. The microarray results from the two sample groups were processed using the Transcriptome Analysis Console (TAC) Software (Thermo Fisher Scientific, Waltham, MA, USA) to identify candidate miRNA genes and their target genes for quantitative analysis.
RNA secondary structure of miR-25-3p
The RNA secondary structure of miR-25-3p was analyzed to gain insights into its functional stability and interaction with target mRNAs. The secondary structure prediction was performed using miRTarBase [22] that modelled the folding of the miRNA into its characteristic stem-loop configuration.
Comprehensive overview of miRNA expression profiles across different tissues
Expression levels of miR-25-3p in vein tissue were obtained from the Human miRNA Tissue Atlas, which offers a comprehensive overview of miRNA expression profiles across different tissues using miRTarBase [22]. The atlas was accessed to evaluate the tissue-specific expression patterns of miR-25-3p, providing contextual information relevant to its role in vascular health and systemic inflammation in T2DM.
Complementary DNA (cDNA) synthesis
According to the manufacturer’s instructions, miRNA samples were converted to cDNA using the ExcelRT™ Reverse Transcription Kit (SMOBIO, Taiwan). All cDNA products were subsequently subjected to real-time polymerase chain reaction (PCR). The first-strand cDNA synthesis involved incubation at 42 °C for 50 min, termination at 85 °C for 5 min, and storage at -20 °C before the PCR reaction. The reactions were conducted using the Veriti™ 96-Well Thermal Cycler (Applied Biosystems™, Thermo Fisher Scientific, Waltham, MA, USA).
Real-time PCR
Quantitative analysis of the candidate miRNA gene (miR-25-3p) and its target genes (PTAFR and IGF2BP3) was performed using RNA oligo primers (Macrogen, Korea). Plasma samples were used to extract miRNA, and the expression levels of PTAFR and IGF2BP3 were analyzed using quantitative PCR (qPCR). The reactions were conducted with the ExcelTaq™ 2X Q-PCR Master Mix (SYBR, ROX) (SMOBIO, Taiwan) according to the manufacturer’s instructions. The qPCR conditions included an initial denaturation and enzyme activation step at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 60 s. The reactions were performed using the StepOne™ Real-Time PCR System (Applied Biosystems™, Thermo Fisher Scientific, Waltham, MA, USA).
Data analysis
The mean CT values were determined from duplicate experiments. Comparative gene expression data were analyzed using the comparative CT (2-ΔΔCT) method. U6 snRNA served as an internal control for the miRNA qPCR assay. Data were summarized by calculating the mean and standard deviation (SD). The means of the two groups were compared using Student’s t-test. Statistical analysis was conducted using SPSS version 20.0 (IBM, Armonk, NY, USA), with statistical significance defined at P < 0.05.
| Results | ▴Top |
The patients in this study were divided into two groups. Patients with T2DM were assigned sample IDs DM 1 through DM 10, while patients without T2DM were assigned sample IDs NDM 11 through NDM 20. In the T2DM group, there were three male patients (30%); in the non-T2DM group, there were five male patients (50%). The distribution of male and female participants was noted for transparency; however, the analysis of miRNA expression was not stratified by sex in this study. Future investigations with larger sample sizes and subgroup analyses will be necessary to explore potential sex-based differences in miRNA expression. The average age of the T2DM group was 54.8 years, compared to 45 years in the non-T2DM group. The average FBS level was 184.1 mg/dL in the T2DM group and 87.2 mg/dL in the non-T2DM group. The average HbA1c level was 10.76% in the T2DM group and 5.27% in the non-T2DM group (Table 1).
 Click to view | Table 1. Characteristics of Patients With Type 2 Diabetes Mellitus (DM) and Patients Without Type 2 Diabetes Mellitus (NDM) |
Principal component analysis (PCA) mapping from microarray analysis demonstrated differential miRNA expression, with DM samples represented by blue dots and NDM samples represented by red dots (Fig. 1).
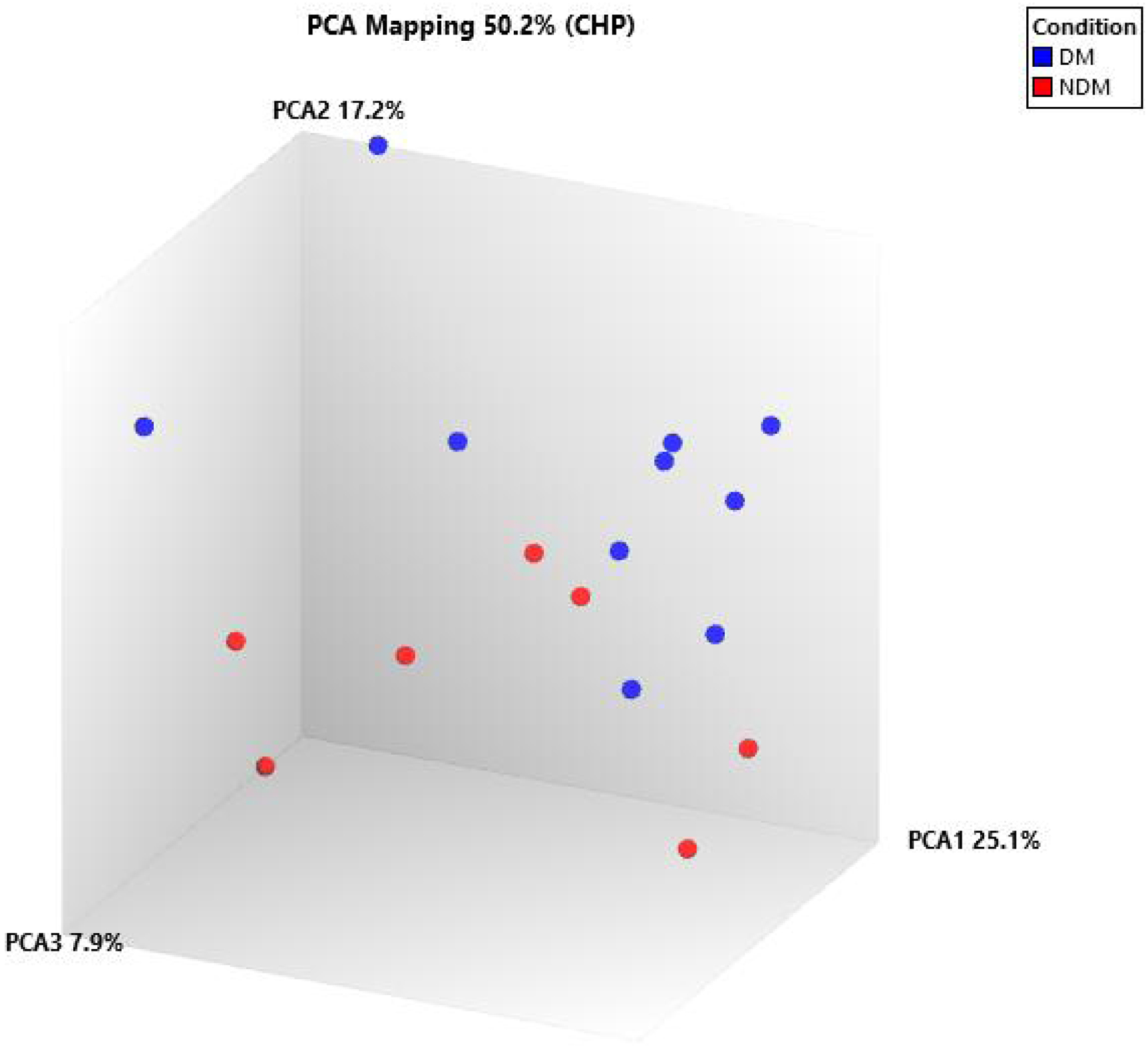 Click for large image | Figure 1. Principal component analysis (PCA) mapping showed the differential miRNA expression between DM and NDM. DM: diabetes mellitus; miRNA: microRNA; NDM: non-diabetes mellitus. |
The hierarchical clustering analysis of microarray data has unveiled significant differential miRNA expression patterns between DM (blue columns) and NDM (red columns). This discovery, depicted in Figure 2, provides a deeper understanding of the molecular mechanisms underlying diabetes.
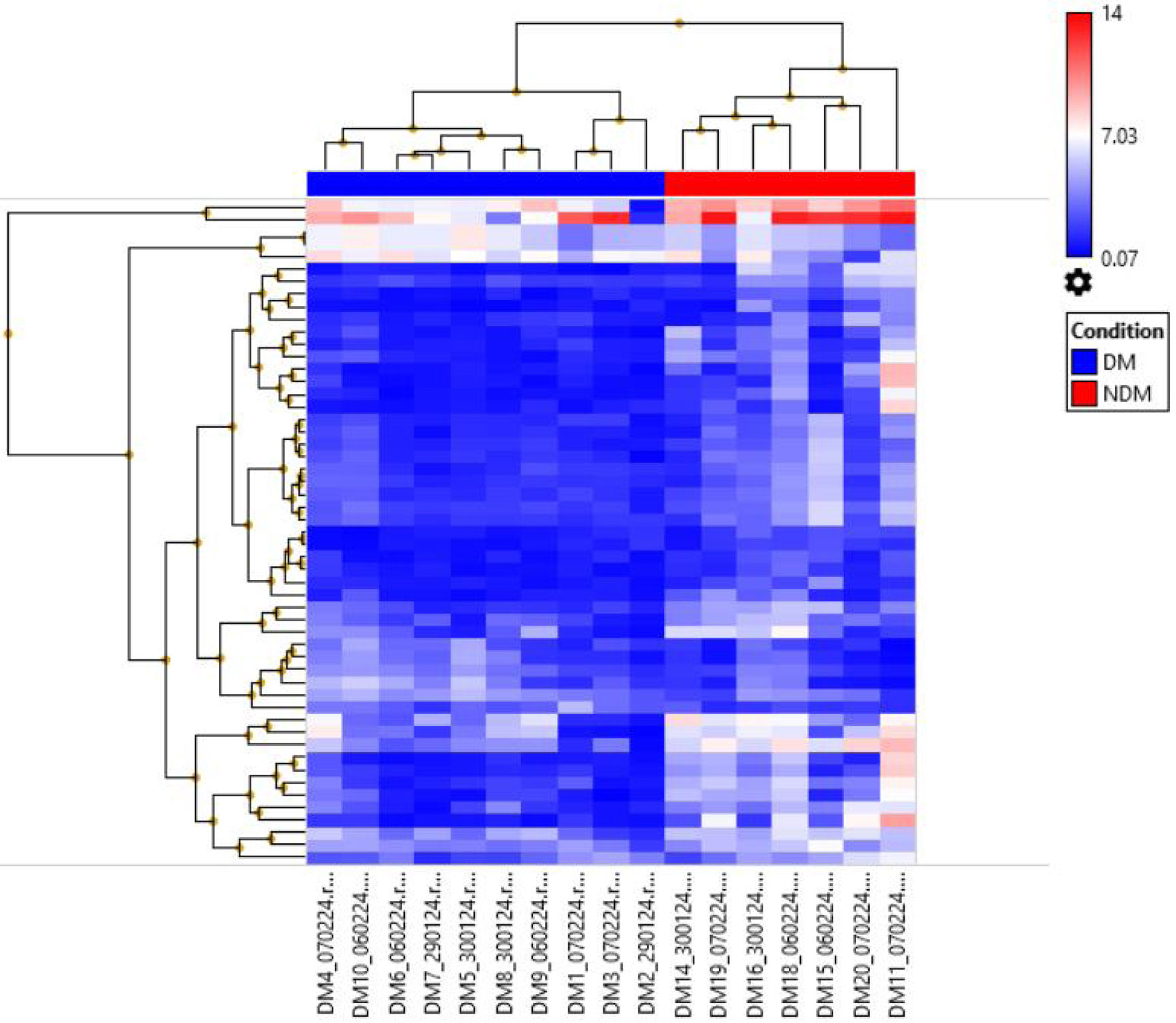 Click for large image | Figure 2. Hierarchical clustering showed the differential miRNA expression between DM and NDM. DM: diabetes mellitus; miRNA: microRNA; NDM: non-diabetes mellitus. |
Based on the microarray analysis results, 53 miRNAs exhibited differential expression between the DM and NDM groups. The selection of potential miRNAs implicated in the pathogenesis of diabetes was informed by in-house and third-party resources integrated into miRTargetLink 2.0 (Table 2) [23].
 Click to view | Table 2. In-House and Third-Party Resources Are Included in miRTargetLink 2.0 to Select the Potential miRNA Expression Involved in the Pathogenesis of Diabetes |
This study has leveraged the collaborative power of miRTarBase and miRDB to identify potential miRNAs implicated in the pathogenesis of diabetes. This comprehensive analysis, detailed in Table 3, underscores the robustness of this findings.
 Click to view | Table 3. Ten Downregulated and Six Upregulated miRNAs Are Involved in the Pathogenesis of Diabetes |
miRNA and their corresponding targets were selected based on the following criteria: 1) involvement of target genes in complications associated with DM; 2) validation of target genes across multiple miRNA targets; and 3) target ranking of ≥ 90. miR-25-3p, PTAFR, and IGF2BP3 were selected and validated using qPCR analysis for individual validation.
The RNA secondary structure of miR-25-3p, characterized by its base-pairing interactions that form a stem-loop or hairpin structure, is crucial for its stability, processing, and function. This structure plays a vital role in the maturation of miR-25-3p from its precursor form and directly influences its ability to bind effectively to target mRNAs, such as PTAFR and IGF2BP3. Proper folding of the miR-25-3p molecule is essential for its gene regulatory functions, as any alterations in its secondary structure could impair its interaction with the 3' UTR of target mRNAs, ultimately affecting the expression levels of these genes and contributing to disease pathogenesis (Fig. 3).
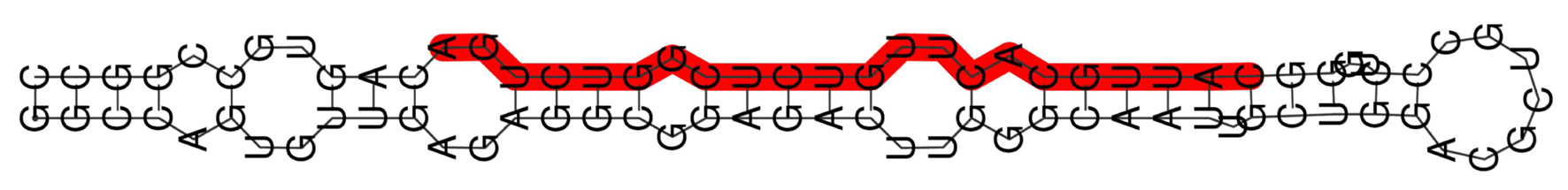 Click for large image | Figure 3. RNA secondary structure of miR-25-3p. |
Furthermore, the Human miRNA Tissue Atlas provides comprehensive insights into the expression profiles of various miRNAs across different tissues and organs. For miR-25-3p, this atlas reveals its expression levels specifically in the vein tissue, offering crucial context for understanding its role in vascular and systemic health (Fig. 4).
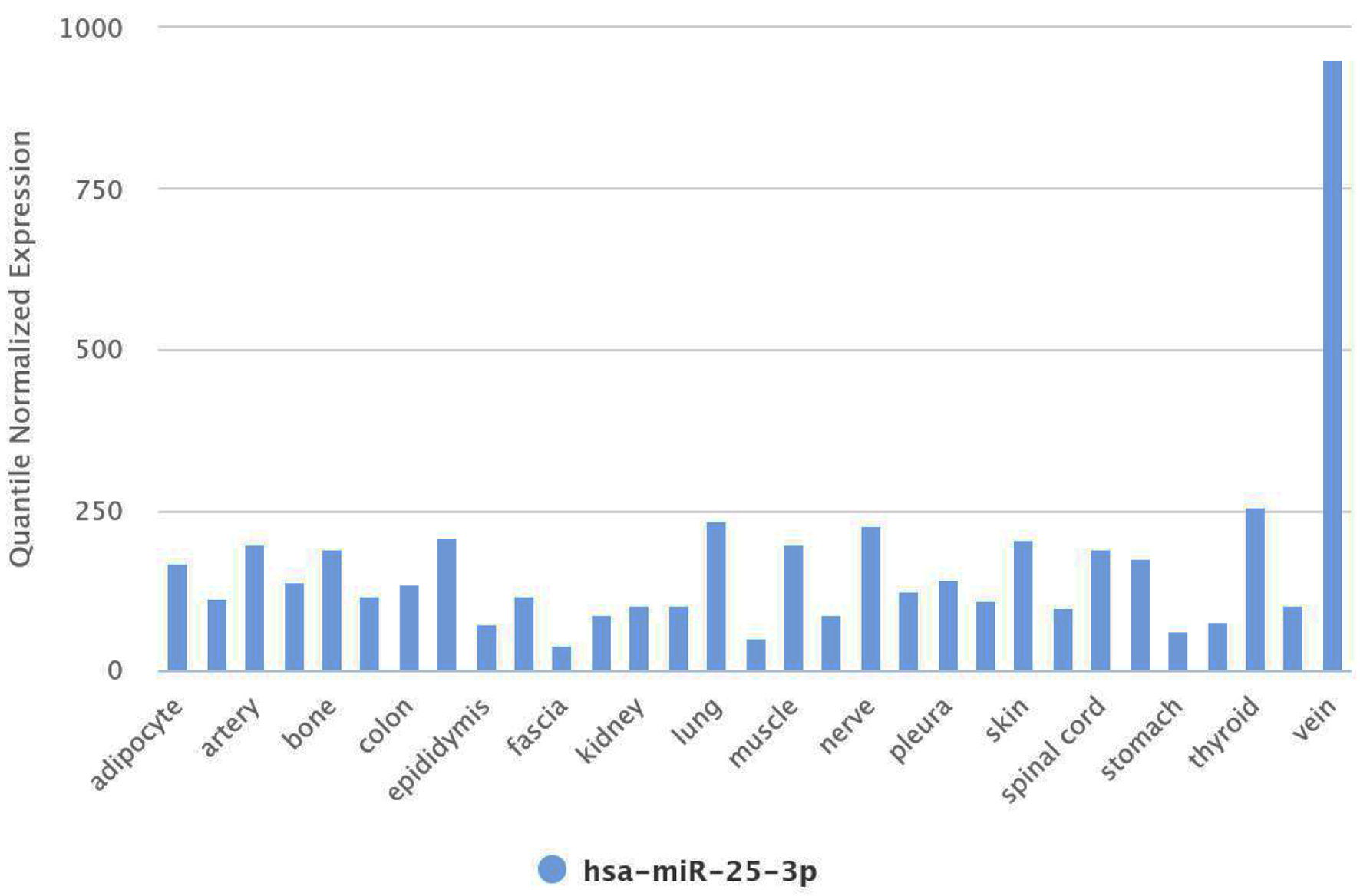 Click for large image | Figure 4. miR-25-3p expression in vein tissue from the human miRNA tissue atlas. miRNA: microRNA. |
miR-25-3p exhibited downregulation in the DM group compared to the NDM group. The x-axis depicts the sample groups comprising DM and NDM, while the y-axis represents the relative expression of miR-25-3p measured by qPCR analysis (Fig. 5).
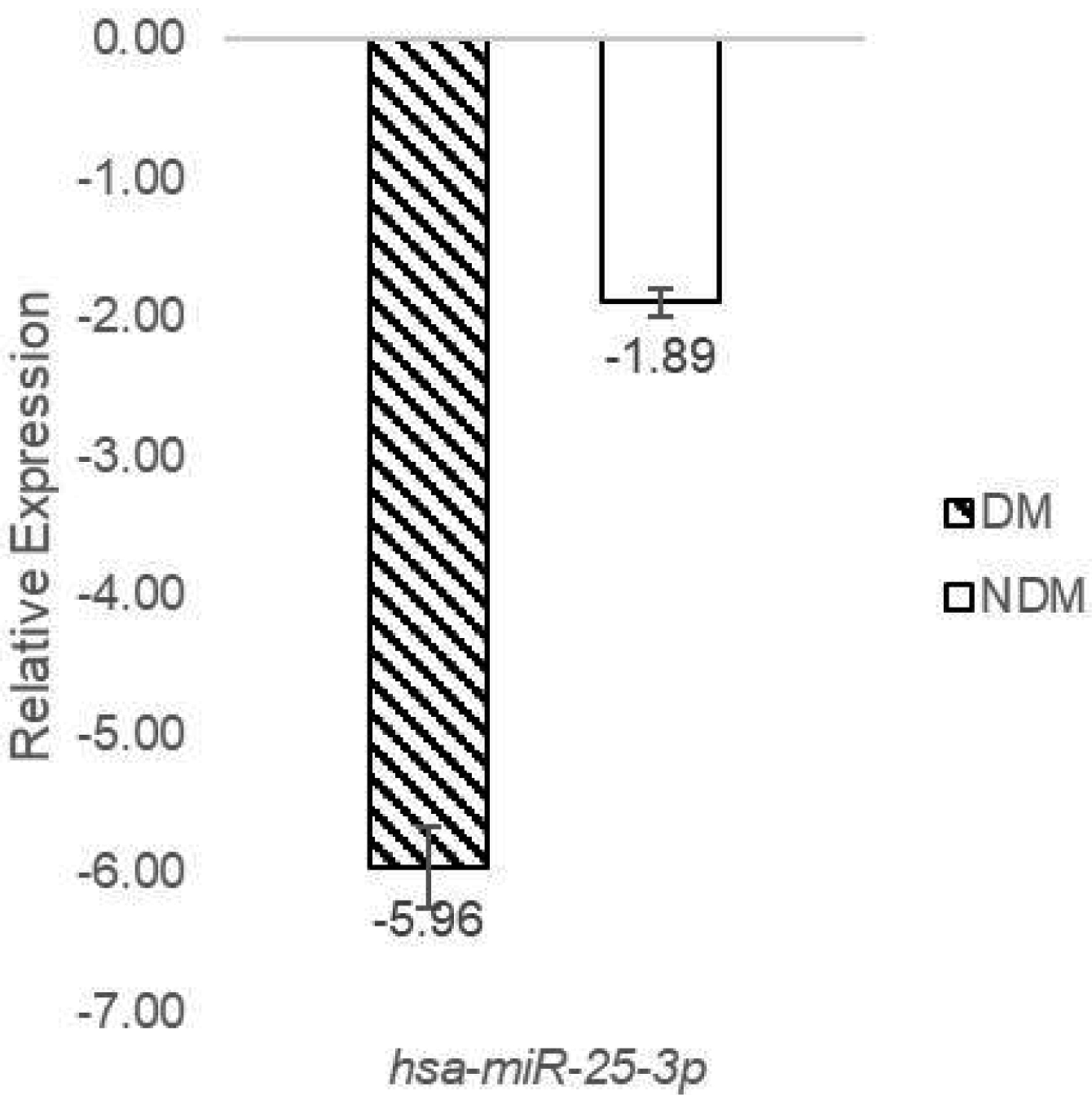 Click for large image | Figure 5. miR-25-3p downregulated in DM patients. DM: diabetes mellitus. |
PTAFR and IGF2BP3 exhibited upregulation in the DM group compared to the NDM group. The x-axis represents the sample groups comprising DM and NDM, while the y-axis represents the relative expression of PTAFR and IGF2BP3 measured by qPCR analysis (Fig. 6).
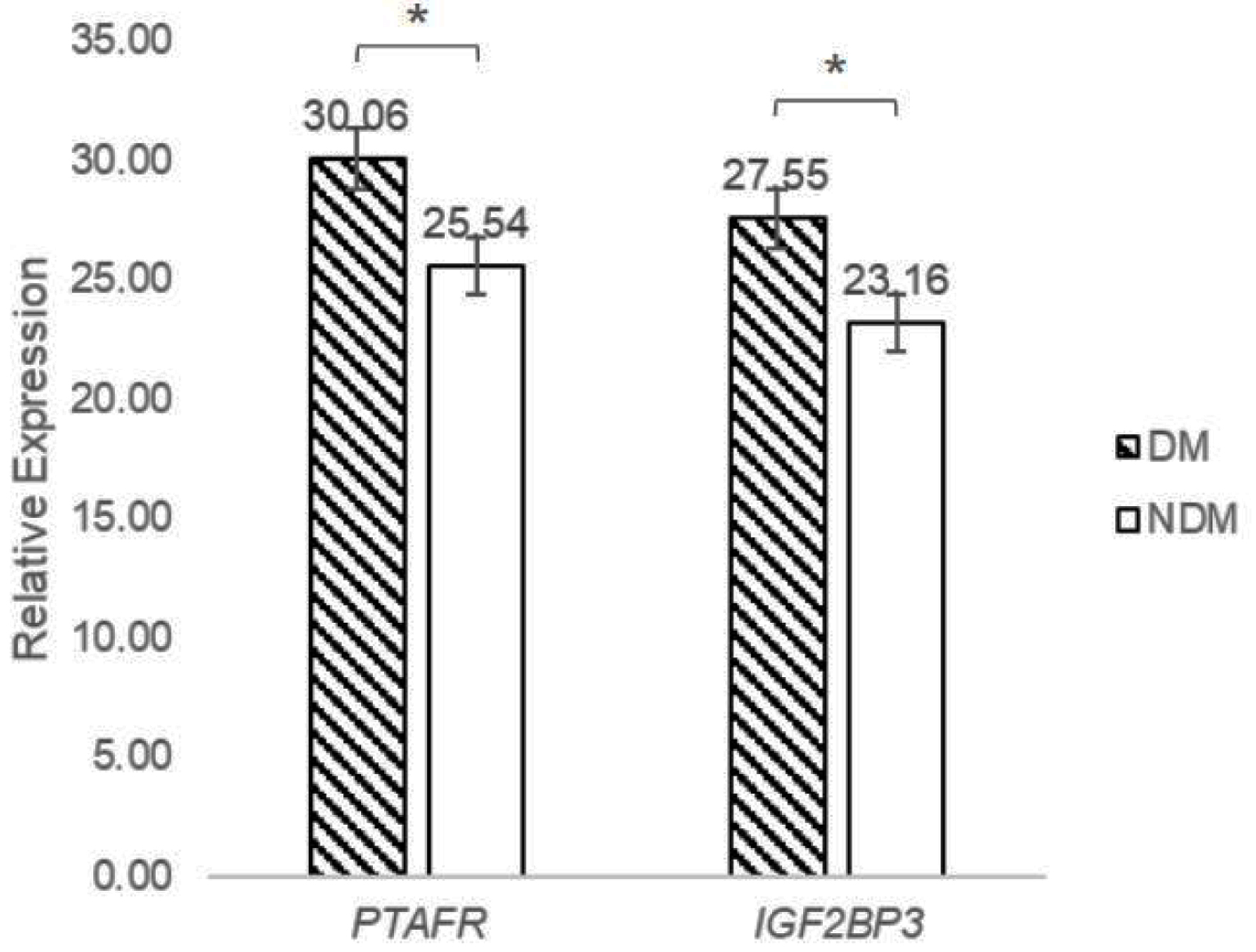 Click for large image | Figure 6. PTAFR and IGF2BP3 upregulated in DM patients. DM: diabetes mellitus; IGF2BP3: insulin-like growth factor 2 mRNA binding protein 3; PTAFR: platelet-activating factor receptor. |
These findings reveal a significant downregulation of miR-25-3p in the T2DM group compared to the non-T2DM group, as demonstrated by qPCR analysis. In contrast, the expression levels of PTAFR and IGF2BP3 were markedly higher in T2DM patients. These results suggest that the downregulation of miR-25-3p is a key factor in the upregulation of PTAFR and IGF2BP3, potentially exacerbating inflammatory responses and insulin resistance, critical mechanisms involved in T2DM progression.
| Discussion | ▴Top |
Patients diagnosed with T2DM were identified based on criteria established by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health. These criteria included fasting plasma glucose levels of 126 mg/dL or higher and HbA1c levels of 6.5% or higher [24]. The DM group comprised 10 patients meeting these criteria, while the NDM group included 10 patients without T2DM. miRNA samples from both groups were analyzed using microarray analysis techniques.
The present study found that miR-25-3p expression was downregulated in DM compared with the NDM group. miR-25-3p, a member of the miR-25 family, has been implicated in regulating metabolic pathways and insulin signaling, making it a significant focus in the study of T2DM. The role of miR-25-3p in T2DM pathophysiology includes insulin resistance and secretion, β-cell apoptosis, inflammation, and oxidative stress.
miR-25-3p is notably expressed in vein tissues, critical in regulating vascular function and homeostasis. In the vein, miR-25-3p modulates several vital biological processes, including endothelial cell function, platelet activation, and inflammatory responses. Its expression levels in the vein reflect its broader involvement in maintaining vascular health and contributing to systemic conditions. However, this analysis of miR-25-3p expression in plasma should be interpreted cautiously, as plasma miRNA levels may not precisely reflect tissue-specific expression, particularly in vascular tissue. Although the reduced levels of miR-25-3p in plasma observed in this study may suggest a systemic dysregulation associated with T2DM, further studies targeting vascular tissues directly would be needed to validate these findings and confirm the decrease of miR-25-3p expression specifically in vascular tissues.
The downregulation of miR-25-3p in T2DM, coupled with its known expression in vascular tissues, raises significant clinical concerns regarding the potential for earlier onset of cardiovascular disease in these patients. This highlights the need for proactive cardiovascular risk assessments and consideration of early primary interventions, such as low-dose aspirin therapy or enhanced monitoring of endothelial function, even in patients without established cardiovascular disease. Such strategies could help mitigate the progression of vascular complications associated with T2DM.
In the context of T2DM, the downregulation of miR-25-3p in vein tissues has been observed. This downregulation is significant because miR-25-3p usually helps regulate genes involved in endothelial function and inflammation. The reduced levels of miR-25-3p in T2DM can lead to disruption in these processes, exacerbating vascular complications commonly associated with diabetes.
The findings from the Human miRNA Tissue Atlas and the subsequent analyses suggest a promising avenue for diabetes management. By targeting miR-25-3p, we may be able to mitigate some of the adverse effects associated with T2DM, offering hope for improved patient outcomes.
miR-25-3p exhibits context-dependent functions across various diseases, including cancer, inflammation, and metabolic disorders. Exosome-derived miR-25-3p stimulates the secretion of pro-inflammatory cytokines from tumor-associated macrophages, thereby promoting liposarcoma progression [25]. In triple-negative breast cancer, miR-25-3p enhances proliferation by directly targeting B-cell translocation gene 2 [26], and it facilitates osteoclast differentiation by regulating the expression of nuclear factor IX [27]. Additionally, miR-25 directly reduces insulin expression, while inhibition of miR-25 using corresponding antagomiRs enhances insulin expression in the INS-1 cell line [28]. Furthermore, miR-25 is linked to residual β-cell function and poor glycemic control in children with new-onset T1DM during disease progression [29]. Conversely, miR-25-3p mitigates oxidized low-density lipoprotein-mediated coronary vascular endothelial cell inflammation by targeting Adam10 in ApoE-/- mouse models of atherosclerosis [17]. Salivary miR-25-3p expression was significantly elevated in obese patients with T2DM compared to those with T2DM alone or healthy individuals. An animal study corroborated the positive effects of downregulated miR-25-3p on mice with periodontitis [30]. Additionally, miR-25-3p has been reported to directly decrease insulin expression by transcriptionally regulating β-cell specific genes [31].
Identifying target genes through post-transcriptional regulation by miRNAs is a crucial strategy for understanding the biological processes in which miRNAs are involved in disease development. The present study determined that two target genes may be implicated in the development of T2DM, including PTAFR and IGF2BP3.
PTAFR is depicted as releasing platelet-derived microparticles (PDMPs). These microparticles express surface markers such as CD41 and CD42, indicating their origin from platelets. The upregulation of PTAFR on platelets enhances their reactivity to platelet-activating factor (PAF), a potent activator of platelet aggregation and inflammation [32]. The interaction of activated platelets and PDMPs with endothelial cells promotes the release of endothelial-derived microparticles (EDMPs), which carry CD144, CD146, and CD31 surface markers. These EDMPs contribute to oxidative stress and increase NADPH oxidase activity, producing ROS. The upregulation of PTAFR enhances this process, as PTAFR signaling can further promote oxidative stress and inflammatory responses. ROS and other inflammatory signals from activated platelets and microparticles cause endothelial dysfunction. Endothelial cells become more permeable and exhibit increased expression of adhesion molecules such as intercellular adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM), facilitating leukocyte adhesion and transmigration [33]. Upregulated PTAFR on these immune cells can enhance their activation and inflammatory responses, further exacerbating endothelial dysfunction. Inflammatory miRNAs and additional microparticles from monocytes (MDMPs) and leukocytes (LDMPs) perpetuate the inflammatory milieu [34]. Vascular smooth muscle cell-derived microparticles (VSMCMPs) are shown contributing to atherogenesis and vascular calcification [35]. Amplified by PTAFR upregulation, the inflammatory and oxidative environment promotes vascular smooth muscle cell proliferation and migration, leading to plaque formation and calcification. The cumulative effect of these processes results in diabetic microvascular complications, atherosclerosis, and atherothrombosis.
IGF2BP3 (insulin resistance) likely plays a role in the development of T2DM by affecting the insulin/insulin-like growth factor 1 receptor (IGF1R) pathway. The function of IGF1R in T2DM development varies across different stages. During the prediabetes phase, disruptions in IGF1R signaling in adipose tissue may induce insulin resistance, potentially progressing to T2DM. Malfunctions in the insulin and IGF1 signaling pathways also contribute to β-cell proliferation and mass decompensation, which disrupts insulin secretion and leads to glucose intolerance in T2DM [36]. Reduced levels of IGF2BP3 may elevate the risk of T2DM by interacting with IGF1 signaling, influencing insulin resistance and β-cell dysfunction. Conversely, increased expression of IGF1R has been observed in association with hyperglycemia and hyperinsulinemia, exacerbating the progression of diabetes mellitus [37]. Elevated circulating levels of miR-25-3p and IGF2BP3 could serve as potential biomarkers for the early detection of T2DM, complementing established diagnostic tools like FBS and HbA1c. These biomarkers may not only help identify early onset but also provide insights into more aggressive disease progression, enabling timely interventions to reduce the risk of severe comorbidities associated with diabetes.
In addition to their potential harmful effects, the modulation of biomarkers such as PTAFR and IGF2BP3 could offer therapeutic benefits for diseases like diabetes. However, excessive or inappropriate regulation of these biomarkers may lead to unintended consequences, including the development of new medical conditions or exacerbating existing ones. Understanding the normal or healthy levels of these biomarkers is critical for their safe manipulation in clinical settings.
The strengths of this study include the use of a well-defined cohort of T2DM and non-T2DM individuals, along with the integration of microarray and qPCR techniques to validate miRNA expression and target gene levels. However, this study has some limitations. This research cohort comprises a relatively small sample size of 20 individuals, and this was designed as an exploratory study to identify differential miRNA expression patterns in T2DM and non-T2DM groups. The findings from this study provide preliminary evidence supporting the potential of miR-25-3p, PTAFR, and IGF2BP3 as biomarkers for T2DM. The authors acknowledge the limitation of the cohort size and have highlighted the need for larger, more diverse cohorts in future studies to validate and expand on these findings. While the study did not perform subgroup analyses to investigate the potential effects of sex, medication use, or comorbidities on miRNA expression. Future studies with larger cohorts and detailed clinical data are warranted to examine how these variables may influence miRNA expression and to confirm that the observed differences are indeed attributable to T2DM. Such analyses will strengthen the understanding of miRNA’s role in T2DM pathophysiology. Furthermore, this study utilized leftover blood specimens collected from patients attending a diabetes clinic. Due to the nature of the specimen source, we had access only to limited clinical information, specifically sex, age, FBS, and HbA1c levels. Unfortunately, data on body weight, height, body mass index, smoking habits, alcohol consumption, comorbidities, diabetic complications, and pharmacotherapy were not available in this dataset. In addition, functional studies are required to elucidate the precise mechanisms by which miR-25-3p regulates PTAFR and IGF2BP3 expression in T2DM.
Conclusions
The downregulation of miR-25-3p observed in plasma, along with the associated changes in PTAFR and IGF2BP3 levels, may suggest a potential role in platelet reactivity, inflammation, and insulin resistance in T2DM. However, as these findings are based on plasma miRNA levels, further studies are required to validate these associations in tissue-specific contexts, particularly in vascular tissues, to confirm their potential contribution to vascular complications in diabetes.
Acknowledgments
None to declare.
Financial Disclosure
The authors received funding from the Thailand Science Research and Innovation Fund No. FF-WU67-18.
Conflict of Interest
The authors disclose no conflict of interest.
Informed Consent
By institutional and ethical guidelines, anonymized leftover blood specimens obtained from routine clinical procedures were utilized. As a result, the study did not require informed consent for its execution.
Author Contributions
YR conceptualized the study, designed the methodology, and conducted the formal analysis. TS, NK, and TC provided resources. TC curated the data. YR, KN, CS (Chanoknan Supanpong), and CS (Chanasorn Satsadeedat) drafted the initial manuscript. TC supervised the study and revised the manuscript. All authors reviewed and approved the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
AGO-2: argonaute-2; cDNA: complementary DNA; DM: diabetes mellitus; EDMPs: endothelial-derived microparticles; EDTA: ethylenediaminetetraacetic acid; FBS: fasting blood sugar; HbA1c: hemoglobin A1c; IGF1R: insulin-like growth factor 1 receptor; IGF2BP3: insulin-like growth factor 2 mRNA binding protein 3; INS: insulin; LDMPs: leukocyte-derived microparticles; miRNA: microRNA; NDM: non-diabetes mellitus; PAF: platelet-activating factor; PAFAH1B1: platelet-activating factor acetylhydrolase 1B regulatory subunit 1; P-body: processing body; PCA: principal component analysis; PCR: polymerase chain reaction; PDMPs: platelet-derived microparticles; PTAFR: platelet-activating factor receptor; qPCR: quantitative polymerase chain reaction; RNA: ribonucleic acid; ROS: reactive oxygen species; SPSS: Statistical Package for the Social Sciences; T1DM: type 1 diabetes mellitus; T2DM: type 2 diabetes mellitus; VSMCMPs: vascular smooth muscle cell-derived microparticles; WUEC: Walailak University Ethics Committee
| References | ▴Top |
- Reinehr T. Type 2 diabetes mellitus in children and adolescents. World J Diabetes. 2013;4(6):270-281.
doi pubmed - Nyenwe EA, Jerkins TW, Umpierrez GE, Kitabchi AE. Management of type 2 diabetes: evolving strategies for the treatment of patients with type 2 diabetes. Metabolism. 2011;60(1):1-23.
doi pubmed - Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, Rosas SE, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753-2786.
doi pubmed - Hans S, Stanton JE, Sauer AK, Shiels K, Saha SK, Lordan R, Tsoupras A, et al. Polar lipids modify Alzheimer's Disease pathology by reducing astrocyte pro-inflammatory signaling through platelet-activating factor receptor (PTAFR) modulation. Lipids Health Dis. 2024;23(1):113.
doi pubmed - Liu J, Jiao L, Zhong X, Yao W, Du K, Lu S, Wu Y, et al. Platelet Activating Factor Receptor Exaggerates Microglia-Mediated Microenvironment by IL10-STAT3 Signaling: A Novel Potential Biomarker and Target for Diagnosis and Treatment of Alzheimer's Disease. Front Aging Neurosci. 2022;14:856628.
doi pubmed - Lin TL, Jaiswal AK, Ritter AJ, Reppas J, Tran TM, Neeb ZT, Katzman S, et al. Targeting IGF2BP3 enhances antileukemic effects of menin-MLL inhibition in MLL-AF4 leukemia. Blood Adv. 2024;8(2):261-275.
doi pubmed - Chen LJ, Liu HY, Xiao ZY, Qiu T, Zhang D, Zhang LJ, Han FY, et al. IGF2BP3 promotes the progression of colorectal cancer and mediates cetuximab resistance by stabilizing EGFR mRNA in an m(6)A-dependent manner. Cell Death Dis. 2023;14(9):581.
doi pubmed - Lee I, Ajay SS, Yook JI, Kim HS, Hong SH, Kim NH, Dhanasekaran SM, et al. New class of microRNA targets containing simultaneous 5'-UTR and 3'-UTR interaction sites. Genome Res. 2009;19(7):1175-1183.
doi pubmed - He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522-531.
doi pubmed - Garofalo M, Condorelli G, Croce CM. MicroRNAs in diseases and drug response. Curr Opin Pharmacol. 2008;8(5):661-667.
doi pubmed - Rajewsky N, Socci ND. Computational identification of microRNA targets. Dev Biol. 2004;267(2):529-535.
doi pubmed - Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9(3):219-230.
doi pubmed - Doench JG, Petersen CP, Sharp PA. siRNAs can function as miRNAs. Genes Dev. 2003;17(4):438-442.
doi pubmed - Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125(6):1111-1124.
doi pubmed - Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132(21):4653-4662.
doi pubmed - Wang XW, Heegaard NH, Orum H. MicroRNAs in liver disease. Gastroenterology. 2012;142(7):1431-1443.
doi pubmed - Yao Y, Sun W, Sun Q, Jing B, Liu S, Liu X, Shen G, et al. Platelet-Derived Exosomal MicroRNA-25-3p inhibits coronary vascular endothelial cell inflammation through Adam10 via the NF-kappaB signaling pathway in ApoE(-/-) mice. Front Immunol. 2019;10:2205.
doi pubmed - Leng Q, Ding J, Dai M, Liu L, Fang Q, Wang DW, Wu L, et al. Insights into platelet-derived MicroRNAs in cardiovascular and oncologic diseases: potential predictor and therapeutic target. Front Cardiovasc Med. 2022;9:879351.
doi pubmed - Wu X, Wang W, Fan S, You L, Li F, Zhang X, Wu H, et al. U-shaped association between serum IGF2BP3 and T2DM: A cross-sectional study in Chinese population. J Diabetes. 2023;15(4):349-361.
doi pubmed - Huang X, He W, Fan S, Li H, Ye G. IGF2BP3-mediated enhanced stability of MYLK represses MSC adipogenesis and alleviates obesity and insulin resistance in HFD mice. Cell Mol Life Sci. 2024;81(1):17.
doi pubmed - Blondal T, Jensby Nielsen S, Baker A, Andreasen D, Mouritzen P, Wrang Teilum M, Dahlsveen IK. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods. 2013;59(1):S1-6.
doi pubmed - https://mirtarbase.cuhk.edu.cn/∼miRTarBase/miRTarBase_2022/php/index.php.
- Kern F, Aparicio-Puerta E, Li Y, Fehlmann T, Kehl T, Wagner V, Ray K, et al. miRTargetLink 2.0-interactive miRNA target gene and target pathway networks. Nucleic Acids Res. 2021;49(W1):W409-W416.
doi pubmed - National Institute of Diabetes and Digestive and Kidney Diseases. Tests and diagnosis of diabetes. Retrieved from: https://www.niddk.nih.gov/health-information/diabetes/overview/tests-diagnosis.
- Casadei L, Calore F, Creighton CJ, Guescini M, Batte K, Iwenofu OH, Zewdu A, et al. Exosome-derived miR-25-3p and miR-92a-3p stimulate liposarcoma progression. Cancer Res. 2017;77(14):3846-3856.
doi pubmed - Chen H, Pan H, Qian Y, Zhou W, Liu X. MiR-25-3p promotes the proliferation of triple negative breast cancer by targeting BTG2. Mol Cancer. 2018;17(1):4.
doi pubmed - Huang Y, Ren K, Yao T, Zhu H, Xu Y, Ye H, Chen Z, et al. MicroRNA-25-3p regulates osteoclasts through nuclear factor I X. Biochem Biophys Res Commun. 2020;522(1):74-80.
doi pubmed - Setyowati Karolina D, Sepramaniam S, Tan HZ, Armugam A, Jeyaseelan K. miR-25 and miR-92a regulate insulin I biosynthesis in rats. RNA Biol. 2013;10(8):1365-1378.
doi pubmed - Nielsen LB, Wang C, Sorensen K, Bang-Berthelsen CH, Hansen L, Andersen ML, Hougaard P, et al. Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls: evidence that miR-25 associates to residual beta-cell function and glycaemic control during disease progression. Exp Diabetes Res. 2012;2012:896362.
doi pubmed - Byun JS, Lee HY, Tian J, Moon JS, Choi J, Lee SH, Kim YG, et al. Effect of salivary exosomal miR-25-3p on periodontitis with insulin resistance. Front Immunol. 2021;12:775046.
doi pubmed - Shen Z, Yu Y, Yang Y, Xiao X, Sun T, Chang X, Tang W, et al. miR-25 and miR-92b regulate insulin biosynthesis and pancreatic beta-cell apoptosis. Endocrine. 2022;76(3):526-535.
doi pubmed - Rustiasari UJ, Roelofs JJ. The role of platelets in diabetic kidney disease. Int J Mol Sci. 2022;23(15):8270.
doi pubmed - Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17(1):121.
doi pubmed - Zahran AM, El-Badawy O, Mohamad IL, Tamer DM, Abdel-Aziz SM, Elsayh KI. Platelet activation and platelet-leukocyte aggregates in type I diabetes mellitus. Clin Appl Thromb Hemost. 2018;24(9_suppl):230S-239S.
doi pubmed - Yang DR, Wang MY, Zhang CL, Wang Y. Endothelial dysfunction in vascular complications of diabetes: a comprehensive review of mechanisms and implications. Front Endocrinol (Lausanne). 2024;15:1359255.
doi pubmed - Rachdaoui N. Insulin: the friend and the foe in the development of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(5):1770.
doi pubmed - Chen B, Li J, Chi D, Sahnoune I, Calin S, Girnita L, Calin GA. Non-coding RNAs in IGF-1R signaling regulation: the underlying pathophysiological link between diabetes and cancer. Cells. 2019;8(12):1638.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.