| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 16, Number 10, October 2024, pages 491-502
The Effects of Preoperative Serum Carcinoembryonic Antigen, Cancer Antigen 15-3 and Cancer Antigen 125 on the Prognosis of Breast Cancer Patients With Different Molecular Subtypes
Yipala Yilihamua, Lei Wangb, Tao Maa, Ting Zhaoc, Yan Wangd, g, Gang Sune, f, g
aCountry College of Public Health, Xinjiang Medical University, Urumqi, Xinjiang, China
bDepartment of Medical Engineering and Technology, Xinjiang Medical University, Urumqi, Xinjiang, China
cDepartment of Medical Record Management, The Affiliated Cancer Hospital of Xinjiang Medical University, Urumqi, Xinjiang, China
dDepartment of Tumor Control and Research, The Affiliated Cancer Hospital of Xinjiang Medical University, Urumqi, Xinjiang, China
eDepartment of Breast and Thyroid Surgery, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, Xinjiang, China
fKey Laboratory of Oncology of Xinjiang Uygur Autonomous Region, Urumqi, Xinjiang, China
gCorresponding Author: Yan Wang, Department of Tumor Control and Research, The Affiliated Cancer Hospital of Xinjiang Medical University, Urumqi, Xinjiang, China; Gang Sun, Department of Breast and Thyroid Surgery, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, Xinjiang, China
Manuscript submitted June 13, 2024, accepted September 25, 2024, published online October 16, 2024
Short title: Effect of STMs on Prognosis of Breast Cancer
doi: https://doi.org/10.14740/jocmr5237
| Abstract | ▴Top |
Background: The aim of the study was to investigate the relationship between serum carcinoembryonic antigen (CEA), cancer antigen 15-3 (CA15-3), and cancer antigen 125 (CA125) levels and traditional clinicopathological factors in patients with early invasive breast cancer in Xinjiang, and the influence of those serum markers on the prognosis of patients with different molecular subtypes.
Methods: We conducted a retrospective study based on the clinical data of 2,940 invasive breast cancer patients who were diagnosed and treated at the Affiliated Cancer Hospital of Xinjiang Medical University from 2015 to 2019. Firstly, in this study, preoperative serum CEA, CA15-3, and CA125 levels were divided into elevated and normal groups based on the optimal cut-off values. Secondly, Chi-square test was used to analyze the correlation between the elevated and normal groups of CEA, CA15-3, and CA125 and traditional clinicopathological factors. Finally, Cox regression model was also used to evaluate the effect of preoperative CEA, CA15-3, and CA125 elevated groups on the prognosis of patients with different molecular subtypes compared with normal groups.
Results: The optimal cut-off values for preoperative CEA, CA15-3, and CA125 were 4.32 ng/mL, 23.10 U/mL and 29.80 U/mL, respectively. The elevated group of preoperative CEA, CA15-3, and CA125 patients usually had larger tumors (tumor size: T2-4), later clinical staging (TNM stage: II-III), and higher histological grading (histological grade: II-III). Univariate analysis showed that the overall survival (OS) of preoperative CEA, CA15-3, and CA125 patients in the elevated group was lower than that in the normal group (P < 0.0001), the 5-year OS was 76.63% vs. 95.35%, 74.34% vs. 95.60%, and 83.73% vs. 94.71%, respectively. Multivariate analysis revealed that for the luminal A, compared with the normal group, the hazard ratios (HRs) of preoperative CEA, CA15-3, and CA125 elevated groups were 6.475 (95% confidence interval (CI): 1.850 - 22.66), 5.192 (95% CI: 1.153 - 23.38), and 7.294 (95% CI: 1.152 - 46.18), respectively. However, for the luminal B, elevated levels of CEA, CA15-3, and CA125 were not independent prognostic factors for OS. For the human epidermal growth factor receptor-2 (HER2)-enriched, the HR of preoperative CA15-3 elevated group was 3.155 (95% CI: 1.325 - 7.509). Additionally, for the triple-negative breast cancer, the HR of preoperative CEA elevated group was 2.390 (95% CI: 1.247 - 4.583).
Conclusions: High levels of CEA, CA15-3, and CA125 were positively correlated with increased tumor load. Preoperative CEA, CA15-3, and CA125 levels may have different prognostic effects on patients with different molecular subtypes. Particularly, preoperative elevated levels of CEA have a significant adverse impact on the prognosis of luminal A and triple-negative patients, while preoperative elevated levels of CA15-3 have an adverse effect on the prognosis of luminal A and HER-positive patients.
Keywords: Breast cancer; Serum tumor markers; Molecular subtype; Prognosis
| Introduction | ▴Top |
Breast cancer is the first of the three most common cancers among women [1]. According to the 2020 Global Cancer Statistics Report [2], breast cancer has become the one with the largest number of new cases, accounting for 11.7% of new cancers. The incidence and mortality of breast cancer in China have been steadily increasing each year; in 2020, there were 416,000 new cases and 117,000 deaths of breast cancer [3]. Breast cancer is highly heterogeneous at the molecular level. According to the estrogen receptor (ER), progesterone receptor (PR), and Ki-67 index detected by immunohistochemistry (IHC), breast cancer can be divided into four molecular subtypes (i.e., luminal A, luminal B, human epidermal growth factor receptor-2 (HER2)-enriched (HER2+), and triple-negative breast cancer (TNBC)) [4], whose survival outcomes and prognosis are different [5].
Besides some traditional pathological factors (such as tumor size, TNM stage, lymph node status, hormone receptor status, and HER2) [6], serum tumor markers (STMs) also play an important role in the screening, early diagnosis, and treatment of many malignant tumors, and have become a powerful tool for follow-up and monitoring efficacy of many tumor patients [7]. STM is a protein or enzyme produced by tumor cells or induced by them and secreted into the bloodstream that can be detected immunologically, biologically, and chemically. STM is almost not expressed in the blood of healthy people but is abnormally high expressed in tumor patients [8]. There are 13 common STMs, among which carcinoembryonic antigen (CEA), cancer antigen 15-3 (CA15-3), and cancer antigen 125 (CA125) are the most widely used in breast cancer. CEA, a cell adhesion molecule and a member of immunoglobulin family, is the specific antigen to be firstly studied and one of markers for breast cancer, adenocarcinoma, colorectal cancer, and other cancers [9]. CA15-3 is a protein antigen containing carbohydrates and a product of the MUC-1 gene. Previous studies have proved that patients with high CA15-3 level have poor prognosis [10, 11]. CA125, also known as mucin 16 or MUC16, is a glycoprotein that is highly expressed in 80% of ovarian cancer patients [12].
There is a positive correlation between preoperative serum levels of CEA, CA15-3, and CA125 and increased tumor load. For example, it was shown that patients with high levels of CEA, CA15-3, and CA125 have larger tumor volume, lymph node metastasis, and late TNM stage [8]. CA15-3 level of patients with tumor volume in stage T3 was higher than that with T1 and T2. CA15-3 level of patients with lymph node status ≥ N1 was significantly higher than that of patients with N0 [13]. However, most studies on the prognosis of breast cancer patients with preoperative STMs have primarily focused on overall levels, and also prognostic value of preoperative serum CEA and CA15-3 levels in breast cancer patients were only discussed in literature [14-16]. In addition, to our knowledge, there are few studies on STMs of breast cancer patients in Xinjiang [17]. Therefore, in this study, the clinical data of 2,940 breast cancer patients who were diagnosed and treated at the Affiliated Cancer Hospital of Xinjiang Medical University were collected, the Chi-square test was used to analyze the correlation between the levels of preoperative serum CEA, CA15-3, and CA125 and traditional clinicopathological factors. In addition, univariate and multivariate Cox regression models were used to investigate the influence of those levels on the prognosis of breast cancer patients with different molecular subtypes.
| Materials and Methods | ▴Top |
Subjects
We collected the clinical data of patients with operable invasive breast cancer who were diagnosed and treated at the Affiliated Cancer Hospital of Xinjiang Medical University from January 1, 2015 to December 31, 2019. Inclusion criteria for the study population were as follows: 1) female patients; 2) patients with invasive breast cancer; 3) patients with preoperative detection of STMs CEA, CA15-3, and CA125 levels; 4) patients receiving adjuvant chemotherapy, radiotherapy, and endocrine therapy according to international guidelines after operation; and 5) patients with complete clinical data (Fig. 1). Exclusion criteria were as follows: 1) male patients; 2) stage IV patients with distant metastases at diagnosis; 3) patients receiving neoadjuvant therapy; and 4) patients with incomplete clinical data (STMs, molecular subtypes, TNM stage, histological grade, tumor size, lymph node status, hormone receptor status, postoperative treatment plan, etc.). Therefore, a total of 2,940 patients were included in the study according to the inclusion and exclusion criteria.
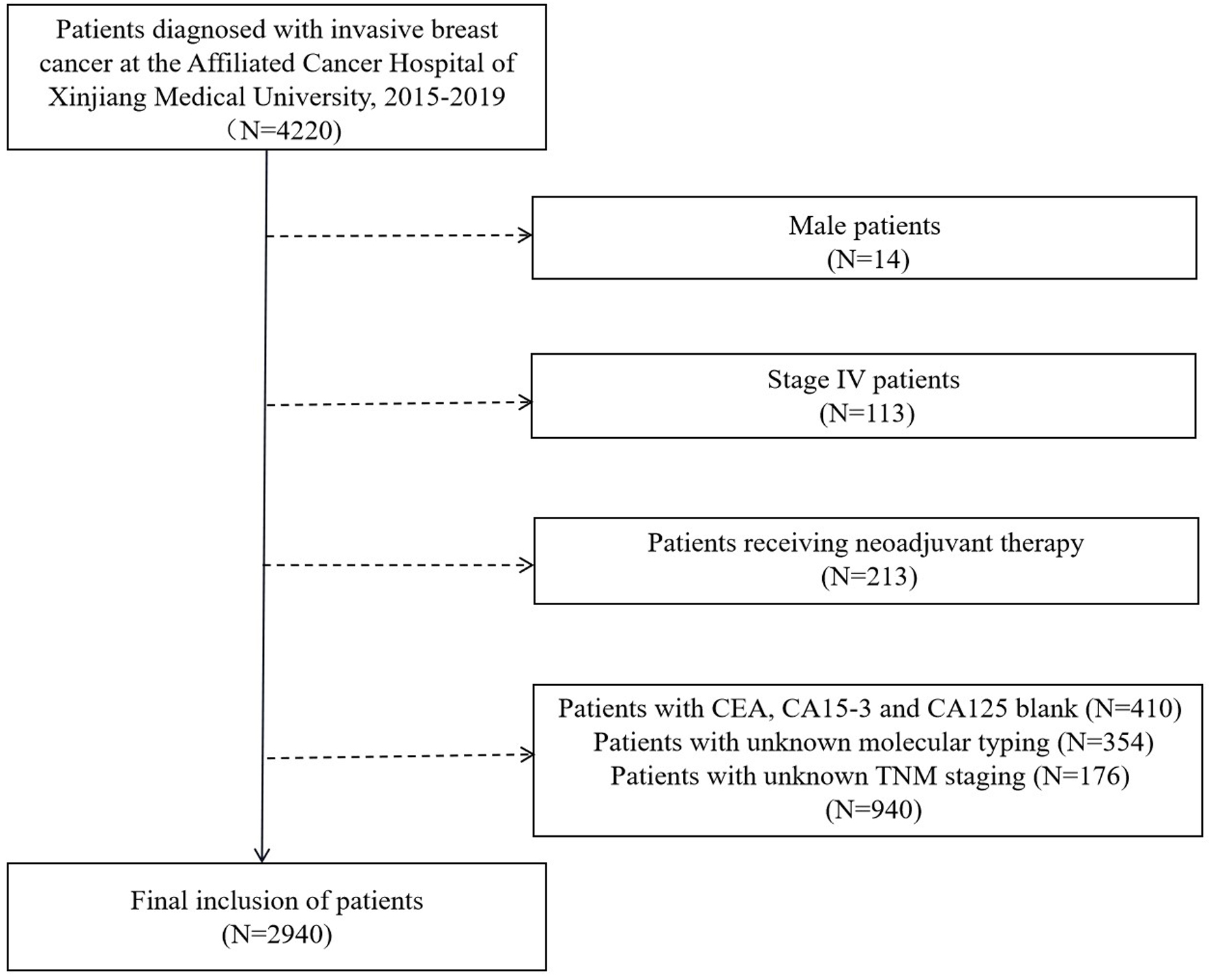 Click for large image | Figure 1. Flow chart for determining the research object. CEA: carcinoembryonic antigen; CA15-3: cancer antigen 15-3; CA125: cancer antigen 125. |
Ethical issues and informed consent
This study protocol strictly adheres to the ethical guidelines of the Helsinki Declaration (sixth revision, 2008), and has obtained written informed consent of the included subjects or their guardians. Moreover, the design and methods of this study have been approved by the Ethics Committee of the Cancer Hospital affiliated with Xinjiang Medical University (approval No. K-2023001).
Measurement of STM level
The preoperative peripheral blood samples collected by patient was 3 mL. The serum sample was centrifuged (3,000 rpm, 10 min) and stored in a refrigerator at -80 °C. The serum CEA, CA15-3, and CA125 levels were detected by automated chemiluminescent immunoassay system (Abbott i2000, USA). The surv_cutpoint function [18] was used to determine the optimal cut-off values of preoperative serum CEA, CA15-3, and CA125, and the optimal cut-off values were 4.32 ng/mL, 23.10 U/mL, and 29.80 U/mL, respectively. An elevated level is defined as the one which higher than the optimal cut-off value, and the normal level is defined as the one which is lower than or equal to the optimal cut-off value.
Stratification of molecular subtypes
The status of hormone receptors ER, PR, and Ki-67 was determined by IHC staining method. The HER2 status was determined based on IHC or fluorescence in situ hybridization (FISH) test. ER positivity and PR positivity referred to the presence of ≥ 1% nuclear-stained malignant cells. HER2 positivity referred to an IHC score of 3+, or when IHC score was 2+, the FISH test was positive if the amplification was positive, otherwise it was negative. Breast cancer was classified into four subtypes according to the expression of the ER, PR, HER2, and Ki-67 status [19]: 1) luminal A subtype: ER-positive and PR-positive (> 20%), HER2-negative, Ki-67 < 14%; 2) luminal B subtype: HER2- (ER and/or PR-positive (≤ 20%), HER2-negative, Ki-67 ≥ 14%) and HER2+ (ER and/or PR-positive, HER2-positive, Ki-67 at any level); 3) HER2+ subtype: ER-negative, PR-negative and HER2-positive; and 4) basal-like subtype: also known as TNBC, ER-negative, PR-negative and HER2-negative.
Statistical analysis
The clinicopathological characteristics of the patients were descriptively analyzed, the quantitative data were described by the number and proportion of cases, and the categorical data were described by the median and range because they did not meet the normality. The χ2 test was used to analyze the correlation between the elevated and normal groups of CEA, CA15-3, and CA125 and traditional clinicopathological factors. The overall survival (OS) of patients was defined as the time from surgery to death. The Kaplan-Meier method was applied to estimate the OS of the elevated and normal groups for preoperative CEA, CA15-3, and CA125, and log-rank was used to test whether there was a difference between the OS of two groups. Independent prognostic factors and their hazard ratios (HRs) of OS were obtained by univariate and multivariate Cox regression models, and the prognostic effects of CA15-3, CA125, and CEA levels on the overall breast cancer patients and patients with different molecular subtypes were also analyzed. P < 0.05 considered that the difference was statistically significant. All data in this study were statistically analyzed using R software (version 4.3.0).
| Results | ▴Top |
Study population characteristics
As shown in Figure 1, a total of 4,220 patients with invasive breast cancer who were diagnosed and treated at the Affiliated Cancer Hospital of Xinjiang Medical University from January 1, 2015 to December 31, 2019 were included, and 2,940 patients were finally determined in the study according to the inclusion and exclusion criteria. The basic clinicopathological features of these patients are shown in Table 1. The median age at diagnosis for patients was 52.5 (range 27.0 - 97.1) years, and the median follow-up time was 26.9 (range 0.3 - 72) months. Luminal B was the main molecular subtypes (57.7%), followed by TNBC (15.4%), luminal A (15.2%), and HER2+ (11.7%). The number of patients who received chemotherapy was the most (1,372 cases, 50.7%), and that of patients who received radiotherapy was the least (453 cases, 16.7%). A total of 185 (6.3%) patients died in the total population. The median levels of preoperative CEA, CA15-3, and CA125 were 1.68 (0.5 - 1449.1) µg/L, 9.7 (2.3 - 800) U/mL, and 13.8 (1.9 - 1,000) U/mL, respectively, and there were 298, 297, and 340 patients in the elevated group of serum CEA, CA15-3, and CA125, respectively. As shown in Figure 2, preoperative levels of CEA, CA15-3, and CA125 in the elevated group were significantly higher than those in the normal group (Z = 28.34, 28.30, and 30.30, P < 0.001, < 0.001, and < 0.001).
 Click to view | Table 1. General Characteristics of the Study Population |
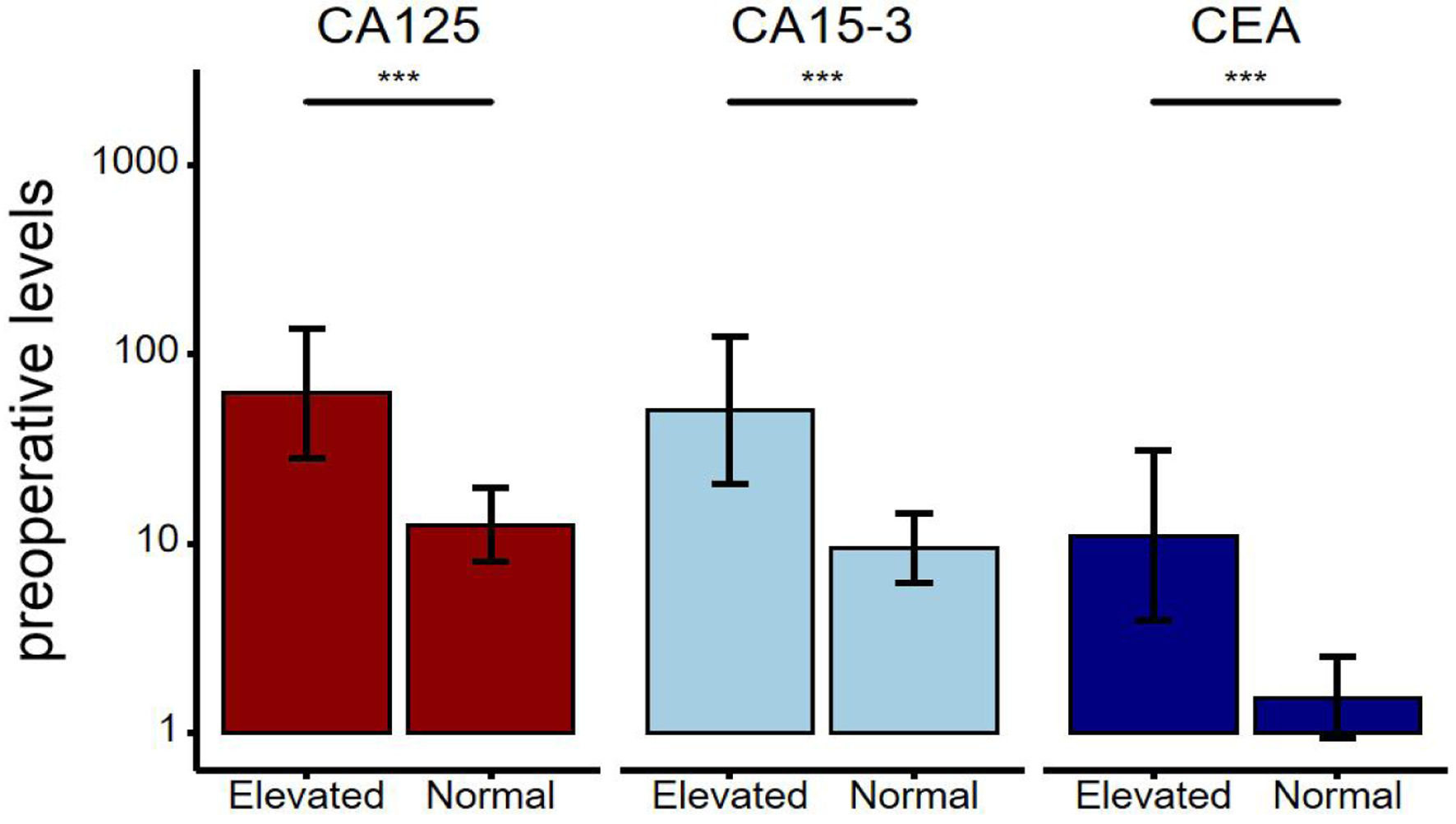 Click for large image | Figure 2. The distribution and comparison of preoperative serum levels of CA125, CA15-3, and CEA in breast cancer patients in the elevated group and the normal group. The line on the bar is an error bar. *P < 0.001 indicates a significant difference. CEA: carcinoembryonic antigen; CA15-3: cancer antigen 15-3; CA125: cancer antigen 125. |
Relationship between preoperative tumor markers and clinicopathological factors
As shown in Table 2, preoperative CEA, CA15-3, and CA125 levels were all related to tumor size, lymph node status, TNM stage, PR status, molecular subtype, histological grade, and chemotherapy (P < 0.05). Especially, patients in the elevated group of CEA and CA15-3 were more inclined to be at the T2-4 stages of tumor size (CEA, 70.8%; CA15-3, 85.8%), and usually accompanied by significant lymph node metastasis (N2-3 vs. N0: CEA, 39.9% vs. 29.9%; CA15-3, 44.1% vs. 21.9%). Patients with elevated levels of CEA, CA15-3, and CA125 were more likely to have later clinical stage and higher histological grades. In the CEA elevated group, the proportion of ER-positive and HER2-negative patients was significant, accounting for 66.4% and 65.1% respectively, and the molecular subtype was dominated by luminal B (56.0%), followed by HER2+, and the other two subtypes accounted for a similar proportion. While in the CA15-3 elevated group, the proportion of HER2-negative patients was significant (70.4%), and CA15-3 levels were not related to age and ER status. In the CA125 elevated group, ER-positive patients were the most common (58.2%), and CA125 levels were not related to HER2+ status, radiation therapy, or endocrine therapy. The molecular subtypes of CA15-3 and CA125 patients in the elevated group were mostly luminal B and TNBC, while luminal A was rare.
 Click to view | Table 2. Correlation Between Preoperative Serum CEA, CA15-3, and CA125 Levels and Clinical Pathological Factors |
Effect of preoperative tumor markers on survival prognosis of breast cancer patients
As shown in Table 3 and Figure 3, it was found from the results of univariate and Kaplan-Meier analyses that in both overall breast cancer patients and patients with different molecular subtypes, patients in the CEA and CA15-3 elevated group had poorer OS compared to those in the normal group (P < 0.001). There was no OS difference between the elevated and normal CA125 groups in luminal A and HER2+ patients (P = 0.087, 0.076), but the elevated CA125 group had worse OS in both overall and other subtypes breast cancer patients (P < 0.05). The 5-year OS of patients in the elevated CEA, CA15-3, and CA125 group versus the normal group was 76.63% vs. 95.35%, 74.34% vs. 95.60%, and 83.73% vs. 94.71%, respectively. By the overall analysis of breast cancer patients and adjusting factors (such as TNM stage, tumor size, ER status, PR status, HER2 status, molecular subtypes, histological grading, chemotherapy, radiotherapy, and endocrine therapy), multivariate analysis was further performed to identify variables that were significant predictors of OS. As shown in Table 4, high levels of CEA and CA15-3, larger tumors, lymph node metastases, no chemotherapy, and endocrine therapy were significantly related to the poor OS of patients. In additional, the results of separate analysis for the molecular subtypes (Table 4 and Fig. 4) showed that high levels of CEA, CA15-3, and CA125 would increase the risk of death from breast cancer (in luminal A patients, after adjusting the tumor size, lymph node status, TNM stage). Moreover, the HRs of patients in the elevated group were 6.475 (95% CI: 1.850 - 22.66), 5.192 (95% CI: 1.153 - 23.38), and 7.294 (95% CI: 1.152 - 46.18), respectively, compared to the normal CEA, CA15-3, and CA125 groups. However, in luminal B patients (after adjusting for tumor size, lymph node status, TNM stage, PR status, chemotherapy, and endocrine therapy), it was found that the high levels of all three tumor markers did not affect the OS of the patients, but only the traditional clinicopathological factors (such as tumor size and chemotherapy) were independent prognostic factors in luminal B patients (P < 0.05). Moreover, in HER2+ patients (after adjusting for tumor size, lymph node status, TNM stage, and chemotherapy), it was shown that CA15-3 elevated groups would increase the patients’ poor OS compared with the normal group (HR (95% CI): 3.155 (1.325 - 7.509)). At last, it would demonstrate that only high levels of CEA and no chemotherapy reduced the OS of TNBC patients (after adjusting for tumor size, lymph node status, TNM stage, and chemotherapy), and the HR in the preoperative CEA elevated group was 2.390 (95% CI: 1.247 - 4.583).
 Click to view | Table 3. Univariate Cox Regression Analysis of Preoperative Serum CEA, CA15-3, and CA125 and Overall Survival of Breast Cancer |
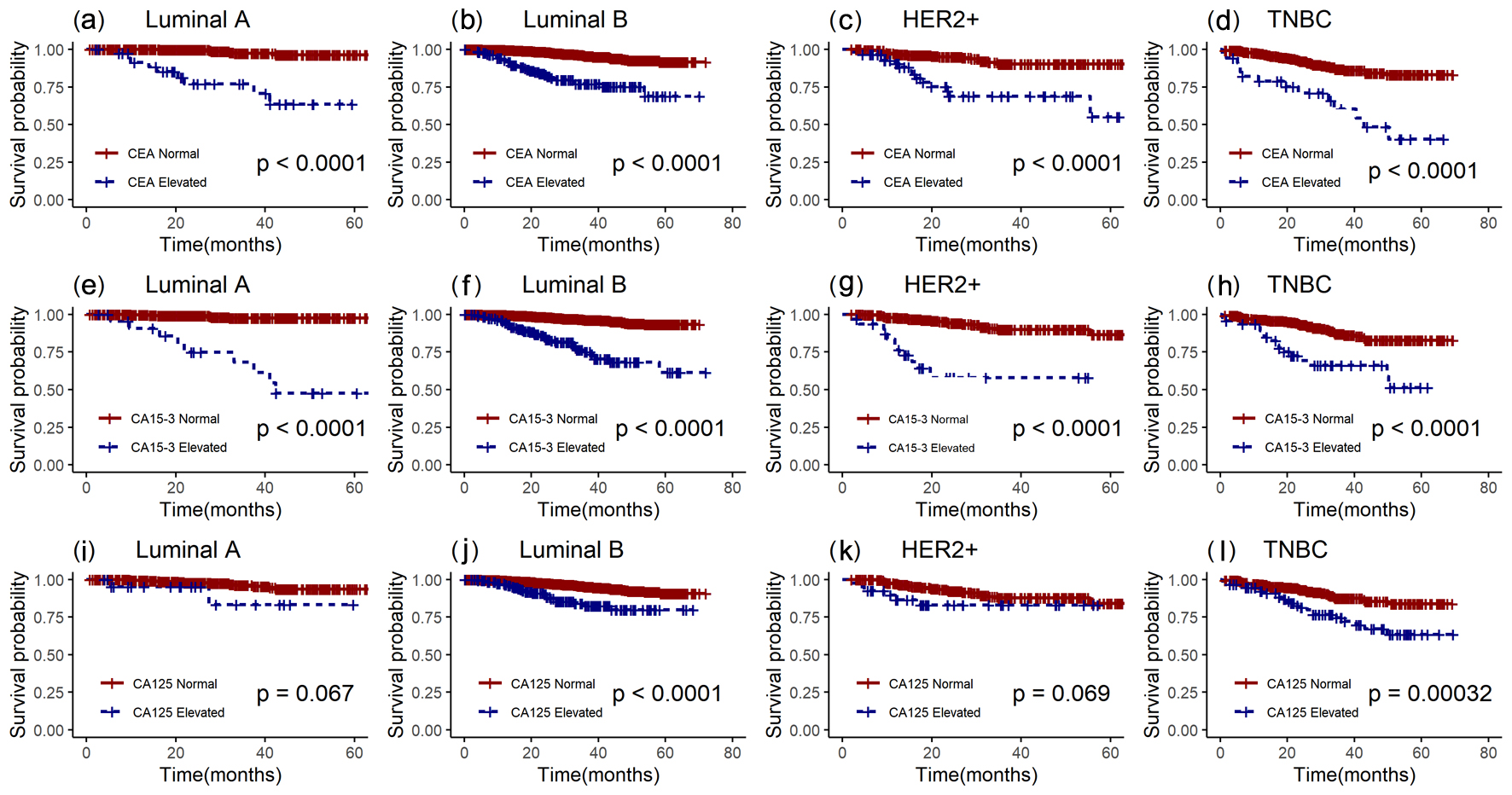 Click for large image | Figure 3. Kaplan-Meier survival curves of preoperative serum CEA, CA15-3, and CA125 in patients with luminal A subtype, luminal B subtype, HER2+ subtype, and TNBC. a, e, i: luminal A; b, f, j: luminal B; c, g, k: HER2+; d, h, l: TNBC. The red lines represent the elevated group, and the blue lines represent the normal group. CEA: carcinoembryonic antigen; CA15-3: cancer antigen 15-3; CA125: cancer antigen 125; HER2+: human epidermal growth factor receptor-2-enriched; TNBC: triple-negative breast cancer. |
 Click to view | Table 4. Multivariate Cox Regression Analysis of the Association Between Preoperative Serum CEA, CA15-3, and CA125 and Traditional Clinicopathologic Factors and Overall Survival of Breast Cancer Patients |
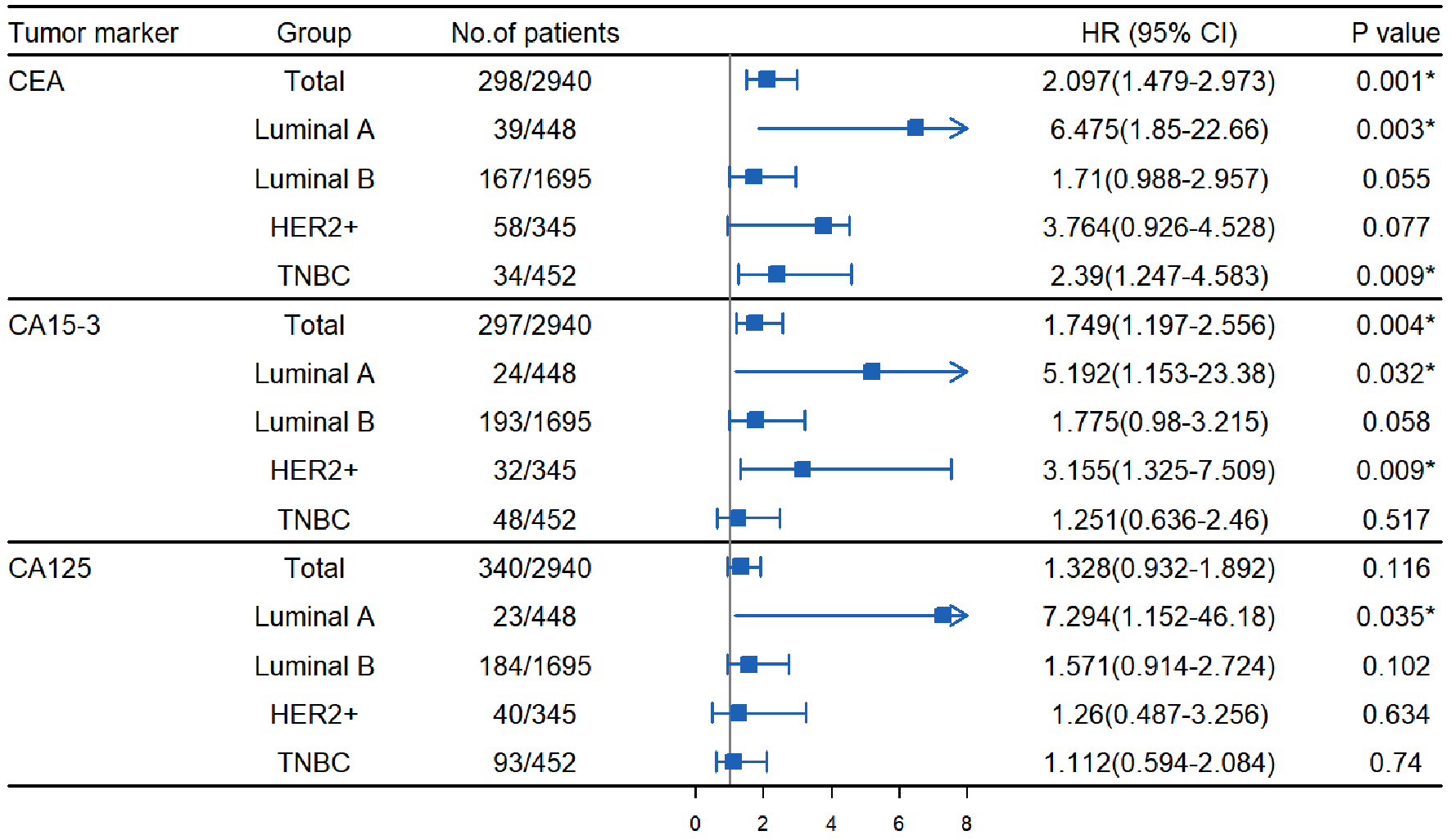 Click for large image | Figure 4. Multivariate Cox regression analysis of the effects of preoperative serum CEA, CA15-3, and CA125 on survival of patients with different molecular subtypes of breast cancer. CEA: carcinoembryonic antigen; CA15-3: cancer antigen 15-3; CA125: cancer antigen 125; 95% CI: 95% confidence interval; HR: hazard ratio; HER2+: human epidermal growth factor receptor-2-enriched; TNBC: triple-negative breast cancer. *P < 0.05 versus normal group. |
| Discussion | ▴Top |
Preoperative STMs (CEA, CA15-3, CA125, etc.) are quantifiable biochemical indicators associated with malignant tumors, generated during the carcinogenesis of breast cancer cells due to the abnormal expression of oncogenes and their products. STMs have the advantages of safety, convenience, easy dynamic monitoring, objective and inexpensive, which could be used for diagnostic and prognostic of breast cancer patients [20].
The optimal cut-off values for preoperative CEA, CA15-3, and CA125 levels were determined to be 4.32 ng/mL, 23.10 U/mL, and 29.80 U/mL, respectively. These cut-off values were similar to those determined by Samy et al [21] through receiver-operating characteristic (ROC) curve analysis. In this study, there were 298 patients (10.1%), 297 patients (10.1%), and 340 patients (11.6%) in the elevated groups for preoperative CEA, CA15-3, and CA125, respectively. It was shown that patients in the elevated group of CEA, CA15-3, and CA125 had larger tumor volume, later TNM stage, and higher histological grade, which is consistent with the results in literature [22-25], which may be because of increased levels of CEA, CA15-3, and CA125, which means that the tumor has begun to form blood vessels, and there are tiny metastases, and tumor antigens may also exist in patients’ body at the time of diagnosis, resulting in patients’ poor clinical outcome [26]. Therefore, it is recommended that regular monitoring of STMs would be implemented to dynamically understand patients’ condition and timely facilitate intervention.
Secondly, in all breast cancers, the OS of patients with elevated CEA, CA15-3, and CA125 was significantly lower than that of normal patients (P < 0.0001). The 5-year OS values of patients in the elevated and normal groups were 76.63% vs. 95.35%, 74.34% vs. 95.60%, and 83.73% vs. 94.71%, respectively. Shao et al [27] also found that patients with elevated tumor markers had lower OS compared to those with normal levels. In addition, the present study showed that preoperative CEA and CA15-3 levels were independent prognostic factors for all breast cancer patients, which is different from the results of previous studies. For example, Clinton et al [28] found that CA15-3 level was not an independent prognostic factor, while Wu et al [14] reported that CEA level affected patients’ disease-free survival, but did not have a significant impact on OS (P > 0.05).
Finally, preoperative CEA, CA15-3, and CA125 levels may have different prognostic effects on patients with different molecular subtypes. In luminal A type, high levels of CEA, CA15-3, and CA125 could increase the risk of death of breast cancer patients, which is similar to the conclusions in literature [29, 30]. Luminal A type (ER-positive and PR-positive (> 20%), HER2-negative, Ki-67 < 14%) breast cancer is usually strongly associated with hormone receptor positivity, as a result, the hormone fluctuations or abnormalities would directly affect the activity of tumor cells, leading to the increase of CEA and CA15-3 levels. This study also showed that some clinicopathological factors (such as tumor size and absence of chemotherapy) were independent prognostic factors for luminal B patients. In addition, it was confirmed that CA15-3 is an independent prognostic factor for HER2+ breast cancer patients, which was similar with results in literature [30, 31], and it would probably be attributed to the diversity of gene expression, activity of cell signal pathway, and different responses to drugs [32].
There are some limitations in this paper. At first, the follow-up time in this study was short (a median follow-up of only 26.9 months), which might lead to a certain bias of results. In the future work, we will increase the frequency of follow-up (every 3 - 4 months) and enhance the follow-up for individuals lost to follow-up. Moreover, some demographic characteristics of patients (such as age of menarche, menopause status, body mass index, etc.) will be collected to analyze. Secondly, there was missing information about TNM stage, serum CA15-3, CEA, and CA125. In order to improve the efficiency of statistical inference, we could use methods such as mean imputation, regression imputation, or K-nearest neighbor imputation to infer and fill in missing values based on patterns of known data. Finally, according to the clinicopathological characteristics (age, ER, PR, HER2, Ki-67, etc.) of patients with known molecular subtypes, we can apply random forest, decision tree, support vector, and other methods to predict the unknown molecular subtypes of other patients, by dividing patients with known molecular subtypes into training and test sets. Finally, CA27-29 is a member of a family of mucin-based breast cancer tumor markers [33]. Related studies [34, 35] have shown that the dynamic change of CA27-29 level can effectively reflect the occurrence and development of breast cancer, and has important significance in the early auxiliary diagnosis of breast cancer. This marker has been included as a diagnostic criterion for breast cancer by the American Society of Clinical Oncology. However, we only collected preoperative serum CEA, CA15-3, and CA125 levels of breast cancer patients in Xinjiang. Therefore, follow-up studies will consider this marker to further explore its role in breast cancer prognosis.
Conclusions
In summary, preoperative high levels of CEA, CA15-3, and CA125 were closely associated with aggressive clinicopathologic features (tumor size, lymph node status, and TNM stage) in early invasive breast cancer patients without metastasis. Preoperative CEA, CA15-3, and CA125 levels may have different prognostic effects on patients with different molecular subtypes. Particularly, preoperative elevated levels of CEA have a significant adverse impact on the prognosis of luminal A and TNBC patients, while preoperative elevated levels of CA15-3 have an adverse effect on the prognosis of luminal A and HER+ patients.
Acknowledgments
None to declare.
Financial Disclosure
This research was supported by the National Natural Science Foundation of China (12061079, 82060520), the Project of Topnotch Talents of Technological Youth of Xinjiang (2022TSYCCX0108), the Natural Science Foundation of Xinjiang (2022D01C287), and the Tianshan Cedar Talent Training Project of Science and Technology Department of Xinjiang Uygur Autonomous Region (2020XS14), which provided important support for the smooth development of the research.
Conflict of Interest
The authors claim they have no conflict of interest.
Informed Consent
All subjects included in the study provided informed consent.
Author Contributions
Yipala Yilihamu and Lei Wang created the idea for the paper. Ting Zhao and Tao Ma are responsible for data collection and collation. Yipala Yilihamu and Yan Wang performed the analysis and wrote the manuscript. Lei Wang prepared the figures. Gao Sun edited and revised the manuscript. All authors read and approved the final manuscript.
Data Availability
The Affiliated Cancer Hospital of Xinjiang Medical University provided data to support the results of this study, but due to the confidentiality of the data, these data are not suitable for public disclosure. If you need to access the dataset, please contact Ting Zhao at zhaoting0557@163.com.
Abbreviations
CA15-3: cancer antigen 15-3; CA125: cancer antigen 125; CEA: carcinoembryonic antigen; CI: confidence interval; ER: estrogen receptor; HER2+: human epidermal growth factor receptor-2-enriched; HRs: hazard ratios; IHC: immunohistochemistry; OS: overall survival; PR: progesterone receptor; STMs: serum tumor markers; TNBC: triple-negative breast cancer
| References | ▴Top |
- Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145-164.
doi pubmed - Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, Vignat J, et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 2022;66:15-23.
doi pubmed - Anaya-Ruiz M, Cebada J, Delgado-Lopez G, Sanchez-Vazquez ML, Perez-Santos JL. miR-153 silencing induces apoptosis in the MDA-MB-231 breast cancer cell line. Asian Pac J Cancer Prev. 2013;14(5):2983-2986.
pubmed - Liu J, Wang H, Deng Y, Cao P, Li X. [Expression of EZH2 in breast cancer and its clinicopathological significance]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2013;42(4):443-449.
doi pubmed - Campbell HE, Gray AM, Harris AL, Briggs AH, Taylor MA. Estimation and external validation of a new prognostic model for predicting recurrence-free survival for early breast cancer patients in the UK. Br J Cancer. 2010;103(6):776-786.
doi pubmed - Johnson H, Taylor S, Peat S, Booker J, Yorke J. Evaluation of the safety and effectiveness of prostate-specific antigen (PSA) monitoring in primary care after discharge from hospital-based follow-up following prostate cancer treatment. Eur J Cancer Care (Engl). 2021;30(4):e13389.
doi pubmed - Guo JH. A study on the correlation between serum tumor markers, molecular subtypes, and clinicopathological characteristics in patients with stage I-III invasive breast cancer before treatment. Hebei Medicine. 2021;27(11):1782-1790. (in Chinese)
- Kabel AM, Al-Shehri AH, Madani BS, Al-Shafie SI, Amasha SA. Tumor markers of breast cancer: role in early diagnosis, monitoring response to therapy and determination of prognosis. Science and Education Publishing Co Ltd. 2016;4:80-87.
- Kabel AM. Tumor markers of breast cancer: new prospectives. J Oncol Sci. 2017;3:5-11.
- Kumpulainen EJ, Keskikuru RJ, Johansson RT. Serum tumor marker CA 15.3 and stage are the two most powerful predictors of survival in primary breast cancer. Breast Cancer Res Treat. 2002;76(2):95-102.
doi pubmed - Reinartz S, Failer S, Schuell T, Wagner U. CA125 (MUC16) gene silencing suppresses growth properties of ovarian and breast cancer cells. Eur J Cancer. 2012;48(10):1558-1569.
doi pubmed - Lian M, Zhang C, Zhang D, Chen P, Yang H, Yang Y, Chen S, et al. The association of five preoperative serum tumor markers and pathological features in patients with breast cancer. J Clin Lab Anal. 2019;33(5):e22875.
doi pubmed - Wu SG, He ZY, Zhou J, Sun JY, Li FY, Lin Q, Guo L, et al. Serum levels of CEA and CA15-3 in different molecular subtypes and prognostic value in Chinese breast cancer. Breast. 2014;23(1):88-93.
doi pubmed - Gupta SK, Kumar V, Anees A, Goel A. The study of prognostic significance of CA 15-3 in breast cancer. Int Surg J. 2018;5(2):580-583.
- Valic A, Milas I, Mayer L, Setic M, Matijevic V, Stanec M. Prognostic significance of CA 15-3 tumor marker in breast cancer patients. Libri Oncol. 2017;45:1-8.
- Gao HB, Gong P, Li N, Guo N, Fei J, Huang W, Wang ZH. Significance of tumor serum markers in the diagnosis of breast cancer. Advances in modern biomedicine. 2019;9:4531-4533. (in Chinese)
- Royston P, Sauerbrei W. Multivariable model-building: a pragmatic approach to regression analysis based on fractional polynomials for modelling continuous variables. John Wiley & Sons. 2008.
- Tan PH, Ellis I, Allison K, Brogi E, Fox SB, Lakhani S, Lazar AJ, et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology. 2020;77(2):181-185.
doi pubmed - Duffy MJ. Tumor markers in clinical practice: a review focusing on common solid cancers. Med Princ Pract. 2013;22(1):4-11.
doi pubmed - Samy N, Ragab HM, El Maksoud NA, Shaalan M. Prognostic significance of serum Her2/neu, BCL2, CA15-3 and CEA in breast cancer patients: a short follow-up. Cancer Biomark. 2010;6(2):63-72.
doi pubmed - Lee JS, Park S, Park JM, Cho JH, Kim SI, Park BW. Elevated levels of preoperative CA 15-3 and CEA serum levels have independently poor prognostic significance in breast cancer. Ann Oncol. 2013;24(5):1225-1231.
doi pubmed - Nam SE, Lim W, Jeong J, Lee S, Choi J, Park H, Jung YS, et al. The prognostic significance of preoperative tumor marker (CEA, CA15-3) elevation in breast cancer patients: data from the Korean Breast Cancer Society Registry. Breast Cancer Res Treat. 2019;177(3):669-678.
doi pubmed - Hashim ZM. The significance of CA15-3 in breast cancer patients and its relationship to HER-2 receptor status. Int J Immunopathol Pharmacol. 2014;27(1):45-51.
doi pubmed - Fang C, Cao Y, Liu X, Zeng XT, Li Y. Serum CA125 is a predictive marker for breast cancer outcomes and correlates with molecular subtypes. Oncotarget. 2017;8(38):63963-63970.
doi pubmed - Gasparini G, Toi M, Gion M, Verderio P, Dittadi R, Hanatani M, Matsubara I, et al. Prognostic significance of vascular endothelial growth factor protein in node-negative breast carcinoma. J Natl Cancer Inst. 1997;89(2):139-147.
doi pubmed - Shao Y, Sun X, He Y, Liu C, Liu H. Elevated levels of serum tumor markers CEA and CA15-3 are prognostic parameters for different molecular subtypes of breast cancer. PLoS One. 2015;10(7):e0133830.
doi pubmed - Clinton SR, Beason KL, Bryant S, Johnson JT, Jackson M, Wilson C, Holifield K, et al. A comparative study of four serological tumor markers for the detection of breast cancer. Biomed Sci Instrum. 2003;39:408-414.
pubmed - Li J, Liu L, Feng Z, Wang X, Huang Y, Dai H, Zhang L, et al. Tumor markers CA15-3, CA125, CEA and breast cancer survival by molecular subtype: a cohort study. Breast Cancer. 2020;27(4):621-630.
doi pubmed - Yerushalmi R, Tyldesley S, Kennecke H, Speers C, Woods R, Knight B, Gelmon KA. Tumor markers in metastatic breast cancer subtypes: frequency of elevation and correlation with outcome. Ann Oncol. 2012;23(2):338-345.
doi pubmed - Molina R, Filella X, Alicarte J, Zanon G, Pahisa J, Munoz M, Farrus B, et al. Prospective evaluation of CEA and CA 15.3 in patients with locoregional breast cancer. Anticancer Res. 2003;23(2A):1035-1041.
pubmed - Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869-10874.
doi pubmed - Gion M, Mione R, Leon AE, Luftner D, Molina R, Possinger K, Robertson JF. CA27.29: a valuable marker for breast cancer management. A confirmatory multicentric study on 603 cases. Eur J Cancer. 2001;37(3):355-363.
doi pubmed - Noonan SA, Patil T, Gao D, King GG, Thibault JR, Lu X, Bunn PA, et al. Baseline and on-treatment characteristics of serum tumor markers in Stage IV oncogene-addicted adenocarcinoma of the lung. J Thorac Oncol. 2018;13(1):134-138.
doi pubmed - Hao Y, Ren G, Yang W, Zheng W, Wu Y, Li W, Li X, et al. Combination diagnosis with elastography strain ratio and molecular markers effectively improves the diagnosis rate of small breast cancer and lymph node metastasis. Quant Imaging Med Surg. 2020;10(3):678-691.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.