| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Case Report
Volume 17, Number 1, January 2025, pages 60-65
Left Ventricular Non-Compaction, Atrial Fibrillation and ANK2 Mutation in a Young Athlete
Gabriele De Masi De Lucaa, b, c, i, Enrico Brancatic, d, Luigi Sciarrac, Arianna Di Danielec, Zefferino Palamac, e, Antonio Gianluca Roblesf, Antonio Scarag, Alessio Borrellig, Martina Nestih, Paola Papadiab, Giuseppe Preteb, Giuseppe De Masi De Lucab, Silvio Romanoc
aCardiology Unit, Card. “G. Panico” Hospital, Tricase, Italy
bCardiomed Medical Center, Maglie, Italy
cDepartment of Life, Health and Environmental Science, University of L’Aquila, L’Aquila, Italy
dHuman Functional Genetics Laboratory, IRCCS San Raffaele Pisana Roma, Rome, Italy
eCardiology Unit, “Villa Verde” Hospital, Taranto, Italy
fCardiology Department, Ospedale “L. Bonomo”, Andria, Italy
gGVM Care and Research, “San Carlo di Nancy” Hospital, Rome, Italy
hCardiology Unit, CNR Fondazione Toscana “Gabriele Monasterio”, Pisa, Italy
iCorresponding Author: Gabriele De Masi De Luca, Cardiology Unit, Card. “G. Panico” Hospital, Tricase, Italy
Manuscript submitted October 27, 2024, accepted December 27, 2024, published online January 6, 2025
Short title: LVNC, AF and ANK2 Mutation in a Young Athlete
doi: https://doi.org/10.14740/jocmr6126
| Abstract | ▴Top |
Left ventricular non-compaction (LVNC) is a rare primary cardiomyopathy with genetic etiology, resulting from an abnormality of myocardial development during embryogenesis. It carries an elevated risk of left ventricular dysfunction, thromboembolic events and malignant arrhythmias. We report the case of LVNC associated with paroxysmal atrial fibrillation and ankyrin 2 (ANK2) mutation at the genetic test. An 18-year-old competitive athlete visited our medical center to undergo the diagnostic investigations protocol preparatory to the release of the suitability for competitive practice. The echocardiographic examination shows LVNC without ventricular remodeling (left ventricular ejection fraction (LVEF) 53%, global longitudinal strain (GLS) -18.3%). The echocardiographic diagnosis was confirmed by cardiac magnetic resonance imaging (cMRI), which revealed dense hypertrabeculation in the left ventricular apex and lateral wall. The cardiogenetic investigation showed a c.9145C>T variant (p.Arg3049Trp) identified in the ANK2 gene. This mutation is associated in the literature with rare cases of LVNC. The patient underwent an extended Holter monitoring which excluded ventricular arrhythmic events but showed two brief episodes of paroxysmal atrial fibrillation. Despite the absence of significant ventricular remodeling, considering the presence of paroxysmal atrial fibrillation and the presence of a mutation in the ANK2 gene, which has several variants related to high-risk phenotypes, it has been decided to suspend the competitive practice, and is defined an adequate clinical-diagnostic follow-up.
Keywords: Left ventricular non-compaction; Atrial fibrillation; ANK2 variant; Cardiac magnetic resonance imaging
| Introduction | ▴Top |
Left ventricular non-compaction (LVNC) is a rare cardiomyopathy due to the failure of myocardial development during embryogenesis, and it is characterized by an elevated risk of left ventricular dysfunction, malignant arrhythmias, and thromboembolic phenomenon [1, 2]. LVNC presents different phenotypic expressions and different pathology evolution. The prevalence of LVNC has been reported as 3-4% among patients with heart failure [3]. During development, most of the heart muscle is a sponge-like meshwork of interwoven myocardial fibers [2, 4]. As normal development progresses, these trabeculated structures undergo significant compaction that transforms them from spongy to solid fibers, which is particularly apparent in the ventricles and particularly in the left ventricle. To date, this type of hereditary cardiomyopathy is not fully understood. The American Heart Association recognized the rapid evolution of molecular genetics in cardiology and classified LVNC as primary, genetic cardiomyopathy [5]. In contrast, a subsequent position statement of the European Society of Cardiology classified LVNC as “unclassified cardiomyopathy”, because it is not clear whether it is a separate cardiomyopathy or merely a morphological trait shared by many phenotypically distinct cardiomyopathies [6]. Currently, LVNC has been reclassified, in recent guidelines on cardiomyopathies, as a phenotypic trait that can occur either in isolation or in association with other cardiac alterations, such as ventricular hypertrophy, dilatation, and/or systolic dysfunction [7]. We present a case report of a young competitive athlete with LVNC, episodes of paroxysmal atrial fibrillation and the presence of a rare ankyrin 2 (ANK2) variant.
| Case Report | ▴Top |
An 18-year-old competitive cyclist visited our diagnostic center of Cardiology and Sport Medicine (Cardiomed Center, Maglie, Italy) to undergo the diagnostic investigations protocol for competitive practice. The patient had a normal body weight based on a body mass index (BMI) of 23.8 kg/m2, adopted healthy lifestyle and has engaged bicycle practice for 7 years. No family history of sudden death or heart disease was reported. The patient did not have major symptoms, except for sporadic palpitations. The physical examination was unremarkable. Electrocardiogram and spirometry did not show abnormalities. No significant abnormalities were detected in laboratory tests. The cycle ergometer test was conducted up to stage 4 of Bruce protocol and showed no signs of myocardial distress, no induced relevant arrhythmias (rare ectopic supraventricular beat during recovery), rapid chronotropic increase, normal pressure increase, and mild dyspnea at the 125-Watt workload. Echocardiographic examination showed multiple trabeculations in apex and lateral wall of the left ventricle with deep endomyocardial recesses, left ventricular ejection fraction (LVEF) 53%, global longitudinal strain (GLS) -18.3%, two-layer myocardial structure with a thin compacted and a thick non-compacted (NC) layer, systolic NC/C ratio > 2 (parasternal short-axis view), color Doppler evidence of perfused intertrabecular recesses, therefore the diagnosis of LVNC is performed (Figs. 1, 2). The echocardiographic diagnosis was confirmed by cardiac magnetic resonance imaging (cMRI), which revealed dense hypertrabeculation in the apex and lateral wall (Fig. 3). The patient did not complain of any skeletal muscle symptoms, and serum muscle enzyme levels were normal. The cardiogenetic investigation showed a c.9145C>T variant (p.Arg3049Trp) identified in the ANK2 gene. The variant identified is already described and related to rare forms of non-compact myocardium but is not described with clear pathogenetic significance. Several variants of the ANK2 gene are associated with different cardiopathy phenotypic expressions, even with an elevated risk of evolving into malignant arrhythmias and sudden death. Therefore, to complete the diagnostic screening, the patient underwent an extended Holter monitoring (1 week), which excluded ventricular arrhythmic events but showed two brief episodes of paroxysmal atrial fibrillation (episode longer than 24 min).
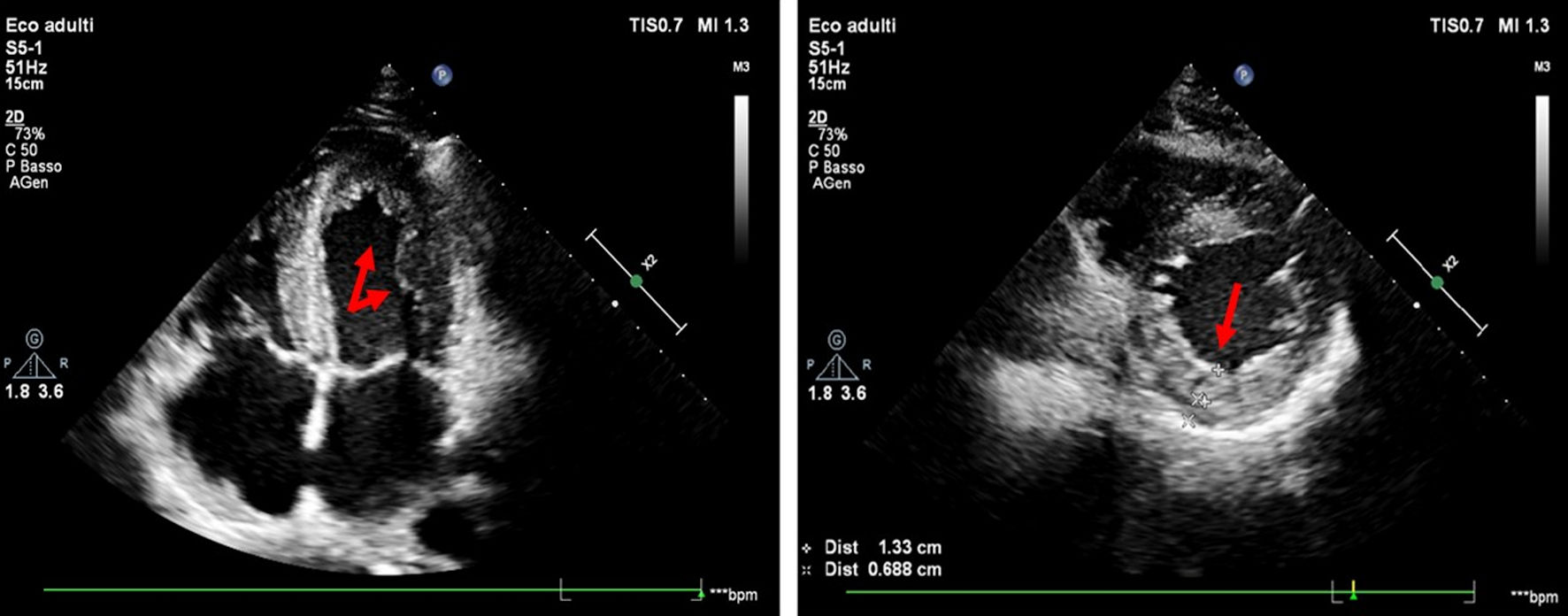 Click for large image | Figure 1. Echocardiographic examination (four-chamber and parasternal short-axis view) shows multiple trabeculations in the apex and lateral wall of the left ventricle, a two-layer myocardial structure with a thin compacted and thick non-compacted (NC) layer, systolic NC/C ratio > 2 (red arrows). |
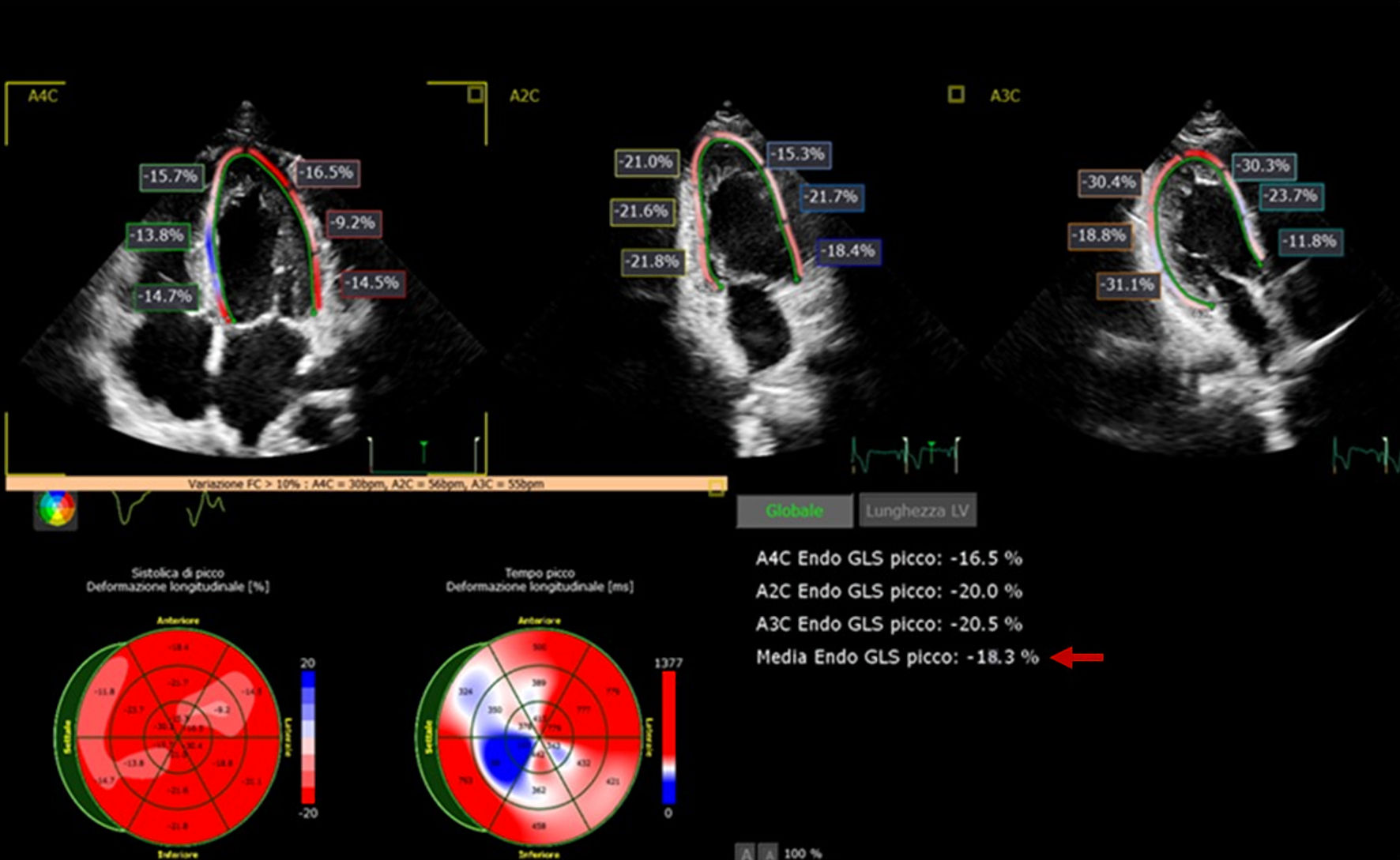 Click for large image | Figure 2. Speckle tracking analysis, global longitudinal strain (-18.3%). |
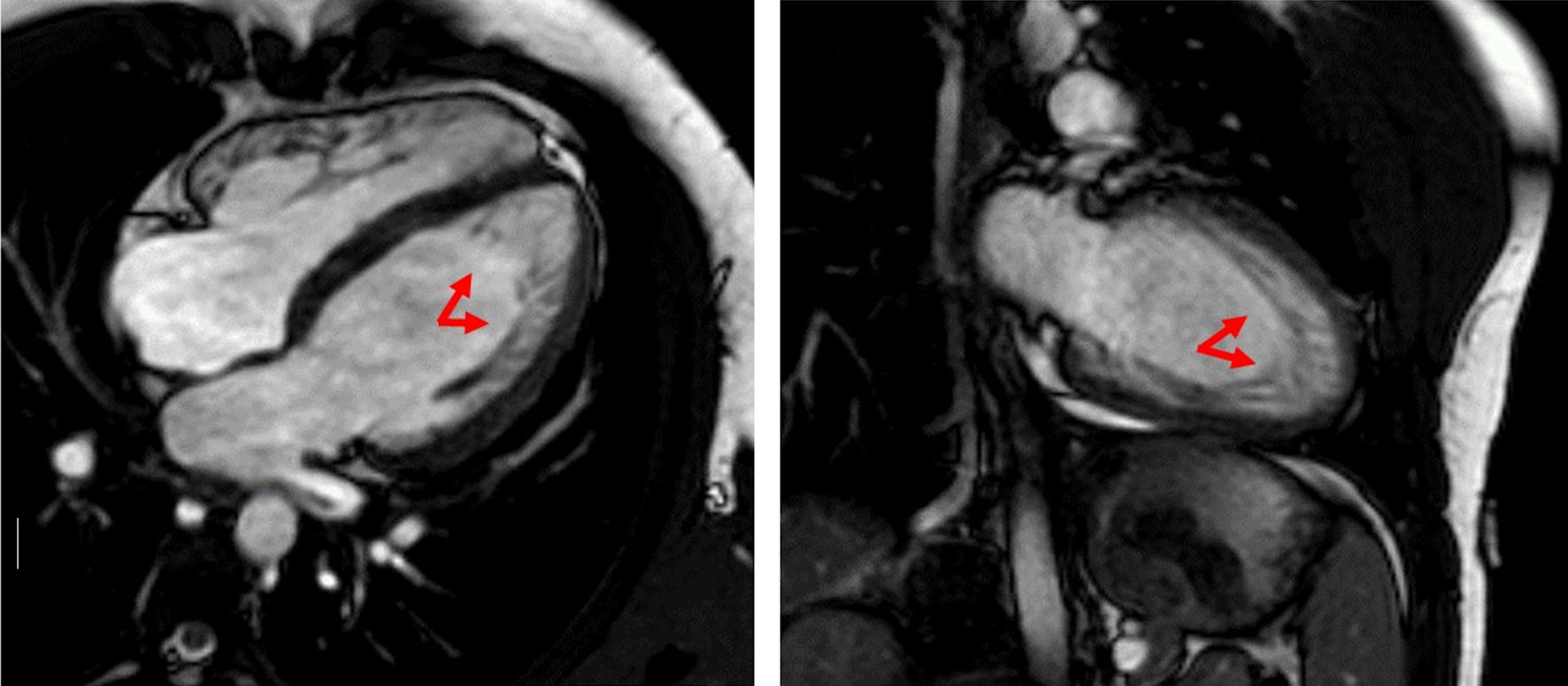 Click for large image | Figure 3. The cMRI showed distinct trabeculations localized to the lateral wall, and part of the posterior wall, as well as the apex (red arrows). cMRI: cardiac magnetic resonance imaging. |
Considering the simultaneous presence of LVNC, atrial fibrillation, and a variant of the ANK2 gene, the suitability for competitive activity is suspended as a precautionary measure, and adequate follow-up is set. Clinical and diagnostic screening of the proband’s family for inherited cardiomyopathy was performed. All family members of the proband did not have any cardiac disease, did not present any cardiac symptoms, and her transthoracic echocardiography (TTE) showed normal cardiac function and geometry. They are all studied with Holter monitoring without detecting any significant anomalies.
Genetic analysis method and result
Genetic counseling was requested, and, after obtaining informed consent from the patient, next-generation sequencing (NGS) analysis of 200 genes associated to cardiovascular diseases was performed on genomic DNA extracted from peripheral leukocytes. The coding regions of the selected genes were isolated and captured using a customized Target Enrichment system (Twist Bioscience, San Francisco, CA, USA); indexed DNA fragments libraries were generated according to the manufacturer’s protocol and sequenced on a ISeq instrument (Illumina, San Diego, CA, USA). Sequence alignment to the National Center for Biotechnology Information (NCBI) reference sequence (GRCh37/hg19) and variant calling were performed using the Borrows-Wheeler Algorithm (BWA) and the Genomic Analysis Toolkit (GATK), respectively. For variant filtering and prioritization, wANNOVAR was used. The heterozygous variant NM_001148:c.C9145T (p.R3049W) in the ANK2 gene, leading to arginine to tryptophan substitution at position 3049 of the beta-ankyrin protein, was identified (Fig. 4). This missense variant was present in publicly available mutation databases, including dbSNP (rs1201870166), ClinVar (classified as variant of uncertain significance (VUS)) and Human Gene Mutation Database (HGMD) (associated to LVNC), with a minor allele frequency of 0.14/1613852 in GnomAD v4.0.0. In silico predictive algorithms support a deleterious effect of the missense mutation on the gene product. According to the American College of Medical Genetics guidelines, the variant was classified of uncertain significance (PM2, PP3, BP1) [8].
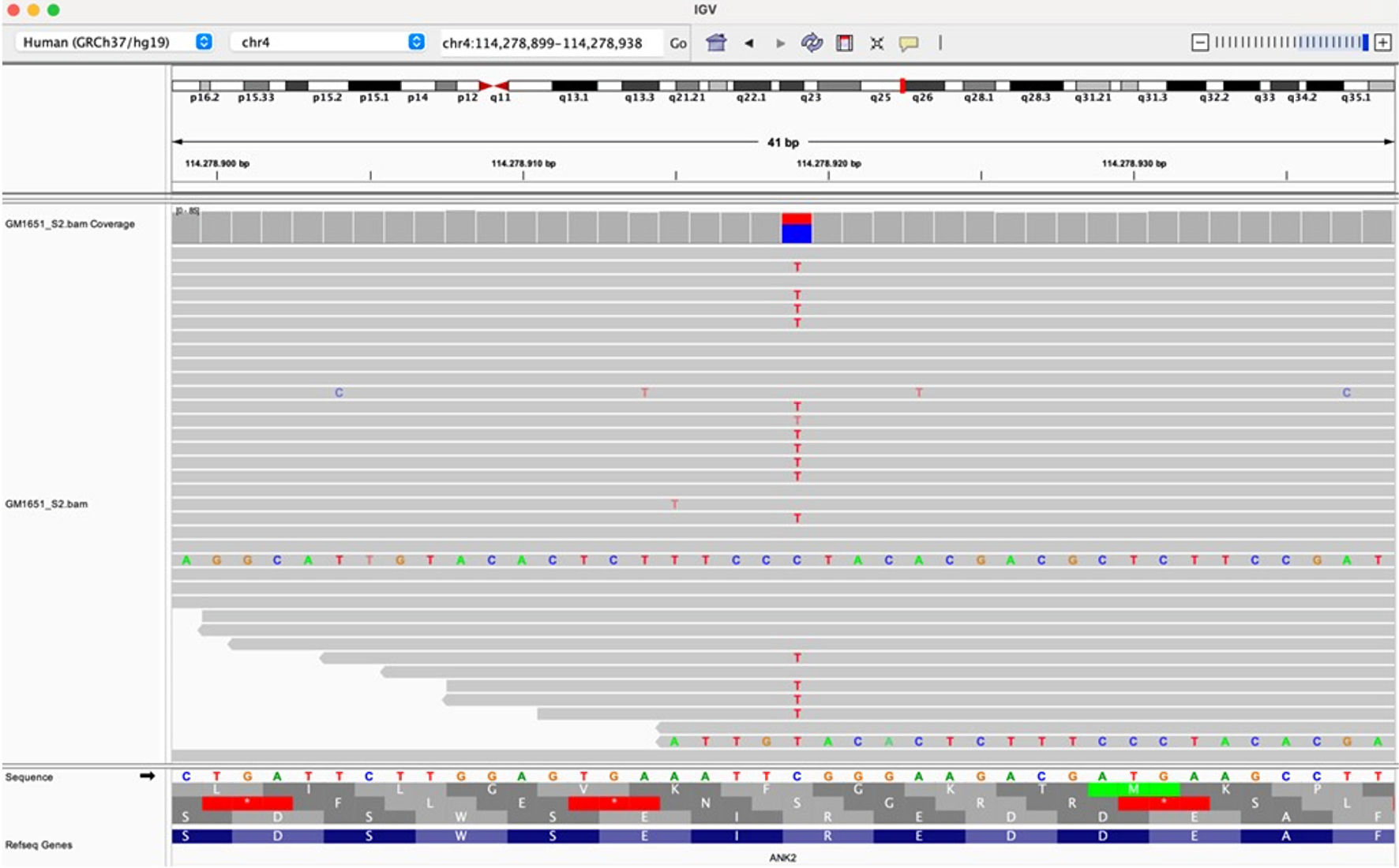 Click for large image | Figure 4. Raw data visualization of the variant c.9145C>T, p.Arg3049Trp in the ANK2 gene (NM_001148) on IGV (Integrative Genomics Viewer). ANK2: ankyrin 2; IGV: Integrative Genomics Viewer. |
| Discussion | ▴Top |
The case of our patient refers to the finding of non-compact myocardium in the context of evaluation for authorization of competitive practice in a young athlete. LVNC is a very rare primary cardiomyopathy characterized by a high risk of left ventricular dysfunction, malignant arrhythmias, and thromboembolic phenomenon [1, 2]. Routinely, the diagnosis of LVNC is based on noninvasive imaging studies, such as TTE and cMRI [9-11]. The disease is caused by heterozygous mutations in different genes, most commonly those coding for the cardiac sarcomere, calcium handling, and other cardiomyopathy-related genes [12-15]. Our patient did not present family history of heart disease or sudden death. The absence of significant symptoms, with evidence of optimal functional capacity at the ergometric test and the absence of important signs of remodeling, led to the hypothesis of an almost benign prognosis. Our cardiogenetic investigation highlighted a variant of the ANK2 gene described in the literature in rare forms of LVNC [16]. Ankyrin-B (encoded by ANK2), originally identified as a key cytoskeletal-associated protein in the brain [17, 18], is highly expressed in the heart and plays a critical role in cardiac physiology. In the heart, ankyrin-B plays key roles in the targeting and localization of key ion channels and transporters [19, 20], structural proteins [21, 22] and signaling molecules [23] The role of ankyrin-B in normal cardiac function is illustrated in animal models lacking ankyrin-B expression, which display significant electrical and structural phenotypes and life-threatening arrhythmias. Further, ankyrin-B dysfunction has been associated with cardiac phenotypes in humans (now referred to as “ankyrin-B syndrome”) including sinus node dysfunction, heart rate variability, atrial fibrillation, conduction block, arrhythmogenic cardiomyopathy, structural remodeling, long QT syndrome, and sudden cardiac death [24-29]. Considering the result of the genetic examination, for a more accurate evaluation of any arrhythmic disorders, the patient underwent prolonged Holter monitoring, which excluded ventricular arrhythmias but showed paroxysms atrial fibrillation. Therefore, considering the simultaneous presence of LVNC, atrial fibrillation, and a variant of the ANK2 gene, the suitability for competitive activity is suspended as a precautionary measure, and adequate follow-up is set.
This case report suggests clinical indications and new evidence in cardiogenetic. Regarding the clinical indications, we can confirm that a multiparametric investigation within a diagnostic procedure preparatory to the release of the suitability for the competitive practice of athletes, which also includes a targeted cardiogenetic investigation, can lead to a better diagnostic and prognostic definition that will guide a more adequate follow-up. About the cardiogenetic indication, it is worth to notice that although this ANK2 mutation was associated with NC myocardium, LVNC has currently been reclassified in recent guidelines on cardiomyopathies, as a phenotypic trait that can occur either in isolation or in association with different other cardiac alterations. Therefore, in view of our patient’s characteristics, our finding might support the evidence of a possible association between mutation in ANK2 gene, atrial fibrillation and LVNC.
Acknowledgments
None to declare.
Financial Disclosure
No funding was received.
Conflict of Interest
The authors declare that there is no conflict of interest relevant to this article.
Informed Consent
Written informed consent was obtained from the patient’s family for publication of this case report.
Author Contributions
De Masi De Luca Gabriele drafted the original manuscript. De Masi, De Luca Gabriele, Prete G. and Papadia P. managed the clinical investigation and performed the instrumental examinations. Brancati and Di Daniele performed the genetic analysis. All authors reviewed and revised the manuscript draft and approved the final version for submission.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
LVNC: left ventricular non-compaction; AF: atrial fibrillation; ANK2: ankyrin 2; LVEF: left ventricular ejection fraction; GLS: global longitudinal strain; cMRI: cardiac magnetic resonance imaging; NGS: next-generation sequencing; BWA: Borrows-Wheeler Algorithm; GATK: Genomic Analysis Toolkit; VUS: variant of uncertain significance; IGV: Integrative Genomics Viewer
| References | ▴Top |
- Pignatelli RH, McMahon CJ, Dreyer WJ, Denfield SW, Price J, Belmont JW, Craigen WJ, et al. Clinical characterization of left ventricular noncompaction in children: a relatively common form of cardiomyopathy. Circulation. 2003;108(21):2672-2678.
doi pubmed - Ichida F. Left ventricular noncompaction - Risk stratification and genetic consideration. J Cardiol. 2020;75(1):1-9.
doi pubmed - Kovacevic-Preradovic T, Jenni R, Oechslin EN, Noll G, Seifert B, Attenhofer Jost CH. Isolated left ventricular noncompaction as a cause for heart failure and heart transplantation: a single center experience. Cardiology. 2009;112(2):158-164.
doi pubmed - Arbustini E, Favalli V, Narula N, Serio A, Grasso M. Left ventricular noncompaction: a distinct genetic cardiomyopathy? J Am Coll Cardiol. 2016;68(9):949-966.
doi pubmed - Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-1816.
doi pubmed - Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29(2):270-276.
doi pubmed - Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C, Bezzina CR, et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur Heart J. 2023;44(37):3503-3626.
doi pubmed - Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424.
doi pubmed - Towbin JA, Lorts A, Jefferies JL. Left ventricular non-compaction cardiomyopathy. Lancet. 2015;386(9995):813-825.
doi pubmed - Petersen SE. CMR and LV noncompaction: does it matter how we measure trabeculations? JACC Cardiovasc Imaging. 2013;6(9):941-943.
doi pubmed - Stacey RB, Caine AJ, Jr., Hundley WG. Evaluation and management of left ventricular noncompaction cardiomyopathy. Curr Heart Fail Rep. 2015;12(1):61-67.
doi pubmed - Hoedemaekers YM, Caliskan K, Majoor-Krakauer D, van de Laar I, Michels M, Witsenburg M, ten Cate FJ, et al. Cardiac beta-myosin heavy chain defects in two families with non-compaction cardiomyopathy: linking non-compaction to hypertrophic, restrictive, and dilated cardiomyopathies. Eur Heart J. 2007;28(22):2732-2737.
doi pubmed - Klaassen S, Probst S, Oechslin E, Gerull B, Krings G, Schuler P, Greutmann M, et al. Mutations in sarcomere protein genes in left ventricular noncompaction. Circulation. 2008;117(22):2893-2901.
doi pubmed - Probst S, Oechslin E, Schuler P, Greutmann M, Boye P, Knirsch W, Berger F, et al. Sarcomere gene mutations in isolated left ventricular noncompaction cardiomyopathy do not predict clinical phenotype. Circ Cardiovasc Genet. 2011;4(4):367-374.
doi pubmed - Richard P, Ader F, Roux M, Donal E, Eicher JC, Aoutil N, Huttin O, et al. Targeted panel sequencing in adult patients with left ventricular non-compaction reveals a large genetic heterogeneity. Clin Genet. 2019;95(3):356-367.
doi pubmed - Paz P, Nguyen T, Rahman R, Elmassry M, El-Nawaa S, Swaminath D, Sethi P. Left ventricular non-compaction associated with long QT syndrome type 4, a case report. JACC Journals. 2022;79(9_Supplement):2402-2402.
- Bennett V, Davis J. Spectrin and ankyrin in brain. Cell Motil. 1983;3(5-6):623-633.
doi pubmed - Davis JQ, Bennett V. Brain ankyrin. Purification of a 72,000 Mr spectrin-binding domain. J Biol Chem. 1984;259(3):1874-1881.
pubmed - Curran J, Mohler PJ. Coordinating electrical activity of the heart: ankyrin polypeptides in human cardiac disease. Expert Opin Ther Targets. 2011;15(7):789-801.
doi pubmed - Camors E, Mohler PJ, Bers DM, Despa S. Ankyrin-B reduction enhances Ca spark-mediated SR Ca release promoting cardiac myocyte arrhythmic activity. J Mol Cell Cardiol. 2012;52(6):1240-1248.
doi pubmed - Cunha SR, Mohler PJ. Ankyrin-based cellular pathways for cardiac ion channel and transporter targeting and regulation. Semin Cell Dev Biol. 2011;22(2):166-170.
doi pubmed - Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81(3):1353-1392.
doi pubmed - Sucharski HC, Dudley EK, Keith CBR, El Refaey M, Koenig SN, Mohler PJ. Mechanisms and alterations of cardiac ion channels leading to disease: role of ankyrin-B in cardiac function. Biomolecules. 2020;10(2):211.
doi pubmed - Robaei D, Ford T, Ooi SY. Ankyrin-B syndrome: a case of sinus node dysfunction, atrial fibrillation and prolonged QT in a young adult. Heart Lung Circ. 2015;24(2):e31-34.
doi pubmed - Mohler PJ, Healy JA, Xue H, Puca AA, Kline CF, Allingham RR, Kranias EG, et al. Ankyrin-B syndrome: enhanced cardiac function balanced by risk of cardiac death and premature senescence. PLoS One. 2007;2(10):e1051.
doi pubmed - Mohler PJ, Splawski I, Napolitano C, Bottelli G, Sharpe L, Timothy K, Priori SG, et al. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc Natl Acad Sci U S A. 2004;101(24):9137-9142.
doi pubmed - Roberts JD, Murphy NP, Hamilton RM, Lubbers ER, James CA, Kline CF, Gollob MH, et al. Ankyrin-B dysfunction predisposes to arrhythmogenic cardiomyopathy and is amenable to therapy. J Clin Invest. 2019;129(8):3171-3184.
doi pubmed - Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421(6923):634-639.
doi pubmed - Adler A, Novelli V, Amin AS, Abiusi E, Care M, Nannenberg EA, Feilotter H, et al. An international, multicentered, evidence-based reappraisal of genes reported to cause congenital long QT syndrome. Circulation. 2020;141(6):418-428.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.