| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 1, January 2025, pages 44-50
Temporal Dynamics of Moderate-Intensity Transcranial Static Magnetic Stimulation in Young Adults
Pan Ling Chena, d, Xiang Cuib, d, Qi Zhanga, Hong Da Zhenga, Fan Rong Konga, Hua Yec, Jin Mei Guoa, Min Cuia, e
aDepartment of Rehabilitation Medicine, Affiliated Hospital of Guilin Medical University, Guilin 541001, Guangxi, China
bHealth Management Center, Affiliated Hospital of Guilin Medical University, Guilin 541001, Guangxi, China
cDepartment of Rehabilitation Medicine, Guilin Medical University, Guilin 541004, Guangxi, China
dThese authors contributed equally to this work.
eCorresponding Author: Min Cui, Department of Rehabilitation Medicine, Affiliated Hospital of Guilin Medical University, Guilin 541001, Guangxi, China
Manuscript submitted October 30, 2024, accepted December 2, 2024, published online December 31, 2024
Short title: Temporal Dynamics of tSMS in Young Adults
doi: https://doi.org/10.14740/jocmr6130
| Abstract | ▴Top |
Background: Transcranial static magnetic stimulation (tSMS) as a new noninvasive brain stimulation (NIBS) technique is gradually gaining widespread attention. This study aims to investigate the effects of tSMS on the excitability of the somatosensory cortex in healthy adults.
Methods: Forty healthy volunteers were recruited and randomly assigned to either the intervention group (tSMS) or the control group (sham), with 20 participants in each. The intervention group received 30 min of 180 mT neodymium magnet stimulation at the C3 site, while the control group underwent sham stimulation with a non-magnetic cylinder. Electrodes were placed at the C3 and Fz sites according to the 10-20 system. Somatosensory evoked potentials (SEPs) N20 component amplitudes were measured at baseline, immediately after stimulation (0 - 2 min), 5 - 7 min, and 10 - 12 min post-stimulation to evaluate the effects on cortical excitability.
Results: Following 30 min of static magnetic stimulation, the SEP N20 component amplitude at the C3 site in the tSMS group decreased by an average of 13.2%, with a significant reduction of 13.7% within 0 - 2 min post-stimulation (P < 0.001). This decrease persisted at 5 - 7 min, with a reduction of 16.6% (P < 0.001), and diminished to 9.3% at 10 - 12 min (P = 0.034). Significant differences were observed between time points and groups (P = 0.003). In the control group, no significant changes were observed in SEP N20 component amplitude throughout the experiment (P = 0.382), and there was no significant difference between the two groups (P = 0.195).
Conclusions: These results confirm that a single session of tSMS effectively inhibits cortical excitability in the somatosensory cortex of young adults. This finding underscores the potential of tSMS as a promising, noninvasive brain stimulation technique with broad future applications.
Keywords: Transcranial static magnetic stimulation; Brain stimulation techniques; Somatosensory evoked potentials; Neuromodulation; Neurorehabilitation
| Introduction | ▴Top |
Noninvasive brain stimulation (NIBS) techniques, such as repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), have been extensively utilized in the treatment of various neurological and psychiatric disorders, as well as in rehabilitation. These methods are favored for their noninvasive nature, environmental friendliness, and ease of operation. NIBS has proven effective in managing conditions such as depression [1, 2], anxiety [3], and post-stroke [4, 5] sequelae by modulating neuronal activity and promoting neuroplasticity. However, despite their widespread use and clinical efficacy, traditional NIBS techniques have several limitations.
rTMS, for example, involves the use of electrical currents to generate magnetic pulses that penetrate the skull and stimulate brain tissue [6]. While effective, it still has some limitations. For example, during treatment, scalp discomfort and tingling sensations may occur if the stimulation intensity is too high or if the procedure is not performed correctly. In more severe cases, it could lead to fainting or trigger seizures [7, 8]. Additionally, there are drawbacks such as high equipment costs, complex operation, high maintenance expenses, and poor portability, which limit its accessibility and broader clinical adoption. Similarly, tDCS uses a low electrical current delivered through electrodes placed on the scalp [9], but it also shares the limitations of potential discomfort and the need for sophisticated equipment. Additionally, prolonged use of these devices is often restricted due to overheating issues, further hindering their widespread application.
In response to these challenges, researchers have been actively exploring alternative neuromodulation methods that can overcome the drawbacks of traditional NIBS. One such promising technique is transcranial static magnetic stimulation (tSMS). Unlike rTMS and tDCS, tSMS utilizes a stable static magnetic field to modulate neuronal electrophysiological activity without relying on electrical currents or time-varying magnetic pulses. This method has shown potential in modulating motor [10], cognitive [11], sensory [12], and visual processing [13] functions across various cortical regions. The effects of tSMS are dependent on the intensity and duration of stimulation, and emerging research suggests it could have therapeutic applications in conditions such as Parkinson’s disease [14], epilepsy [15], Huntington’s disease [16], and stroke [17].
tSMS is particularly notable for its portability, safety, cost-effectiveness, and broad potential to influence human brain activity and behavior. The study shows that 2 h of tSMS is safe for cortical stimulation, with no increase in markers of neural damage observed [18]. However, current research on tSMS is limited, with most studies utilizing higher magnetic field strengths around 1T for short durations of 10 - 20 min [19]. These studies have primarily focused on understanding the inhibitory effects of tSMS, which, while effective, are often short-lived. The underlying mechanisms of tSMS are not yet fully understood, and the research is still in its early stages. This study aims to extend this research by investigating whether a moderate-intensity magnetic field of 180 mT, applied over a longer duration (30 min), can similarly modulate cortical excitability. By systematically exploring the efficacy of tSMS in modulating the excitability of the somatosensory cortex, we seek to establish a robust theoretical and practical foundation for its clinical application. This approach not only contributes valuable insights into the potential of tSMS at different intensities and durations but also helps pave the way for its broader use in neuromodulation therapies.
| Materials and Methods | ▴Top |
Subjects
This study recruited 40 healthy subjects, all right-handed, aged 18 to 47 years (16 males and 24 females). None of the subjects had any physical, psychological, or psychiatric disorders, and pregnant or lactating women were excluded. All participants provided informed consent and signed an informed consent form.
Ethical approval
The study was approved by the Ethics Committee of the Affiliated Hospital of Guilin Medical University (2023YJSLL-183), which abides by the ethical principles of the Helsinki Declaration of 1975 (revised in 2000).
tSMS processing
In this study, 40 healthy volunteers were randomly divided into two equal groups using a random number table method: an intervention group (tSMS group) and a control group (sham stimulation group), with 20 healthy subjects in each group. The intervention group received a 30-min application of tSMS using a cylindrical neodymium magnet (central magnetic field strength of 180 mT, diameter 50 mm, height 30 mm, weight 0.52 kg). The study by Dileone et al [20] indicates that 30 min of tSMS produced a more lasting effect on the primary motor cortex area (M1) region. Some studies have shown that the magnetic field strength and magnetic field gradients are relatively high at 23 cm away from the magnet surface when Nd magnets with strength of 120 to 200 mT are applied to human scalps [21]. The magnet’s south pole was centered and placed vertically against the scalp at the C3 location (as determined by the international 10-20 system for electrode placement), as shown in Figure 1. The control group underwent the same setup and procedure, receiving sham stimulation with a non-magnetic stainless steel cylinder of identical dimensions and weight. Electrodes were placed at the C3 site, according to the international 10-20 system, with the reference electrode placed at the Fz site, as shown in Figure 2. Electrophysiological parameters of the N20 component of the somatosensory evoked potential (SEP) at the C3 site were recorded using an electromyography and evoked potential recording system (with electrodes made of Ag-AgCl, and an electrode ring diameter of 10 mm), after stimulating the right median nerve (stimulation intensity: 4 - 10 mA, 80 - 150 repetitions, stimulation frequency: 1.9 Hz, stimulation pulse width: 200 µs). The changes in the amplitude of the N20 component of the SEP before and after the intervention were compared between the two groups. All electrode impedance values were kept below 10 kΩ. SEP recordings were taken at four time points: before stimulation (T0), 0 - 2 min after the end of stimulation (T1), 5 - 7 min after stimulation (T2), and 10 - 12 min after stimulation (T3), as shown in Figures 3 and 4.
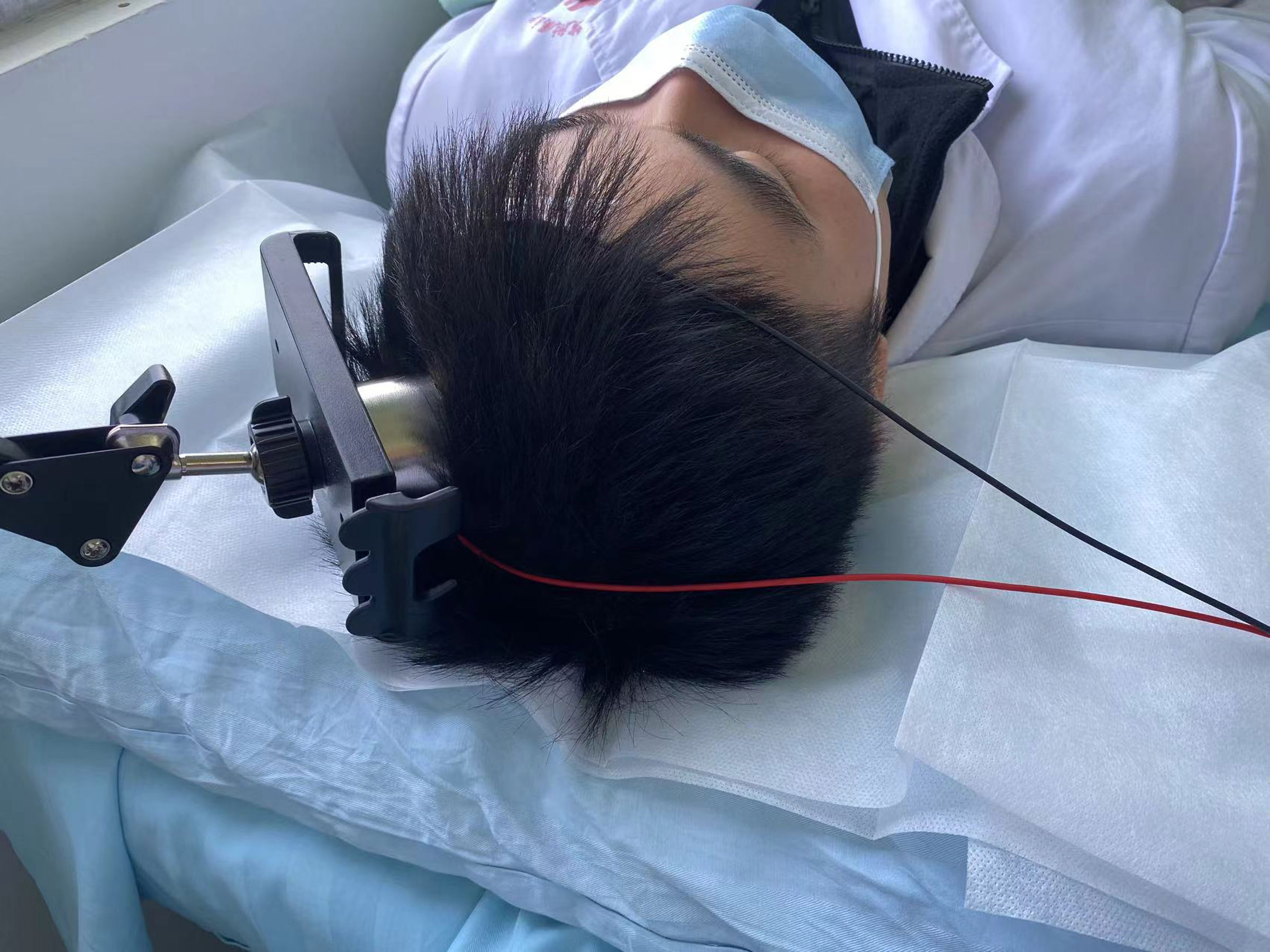 Click for large image | Figure 1. Diagram of the tSMS intervention measures model. Using a floor-to-ceiling mobile stent to attach a neodymium magnet (south pole) and a non-magnetic stainless steel cylinder to the subject’s C3 scalp. tSMS: transcranial static magnetic stimulation. |
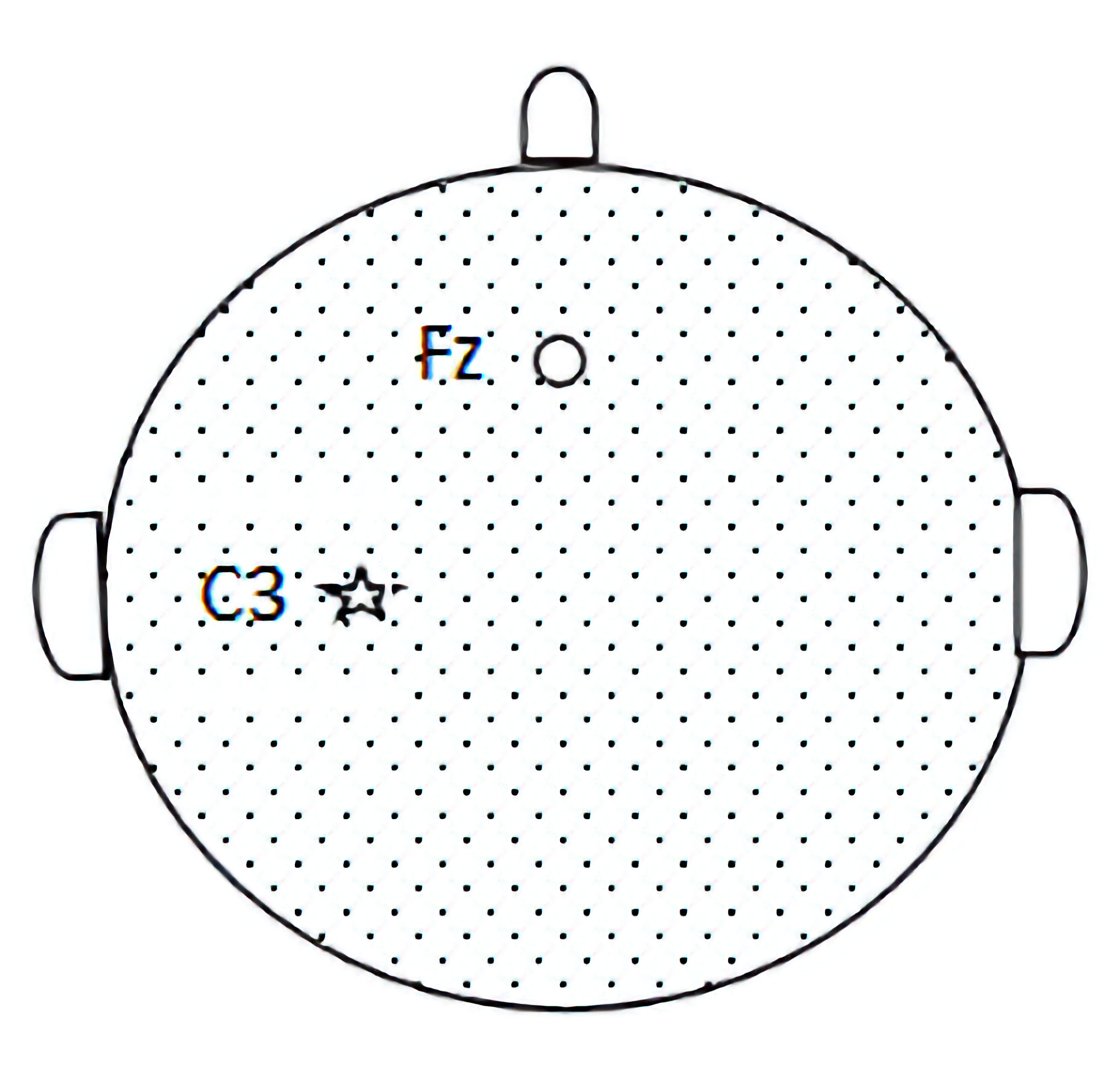 Click for large image | Figure 2. Pattern map of C3 and Fz sites. Based on the international 10-20 system electrode placement method to determine the C3 and Fz sites, SEP is recorded from the C3 (parietal cortex) and Fz (forehead) sites. SEP: somatosensory evoked potential. |
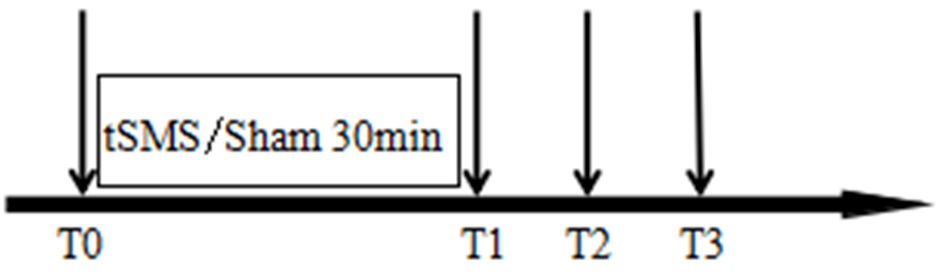 Click for large image | Figure 3. Intervention timeline. T0 represents before stimulation, T1 represents 0 - 2 min after stimulation, T2 represents 5 - 7 min after stimulation, and T3 represents 10 - 12 min after stimulation. A 30-min tSMS or sham stimulation is applied between T0 and T1. tSMS: transcranial static magnetic stimulation. |
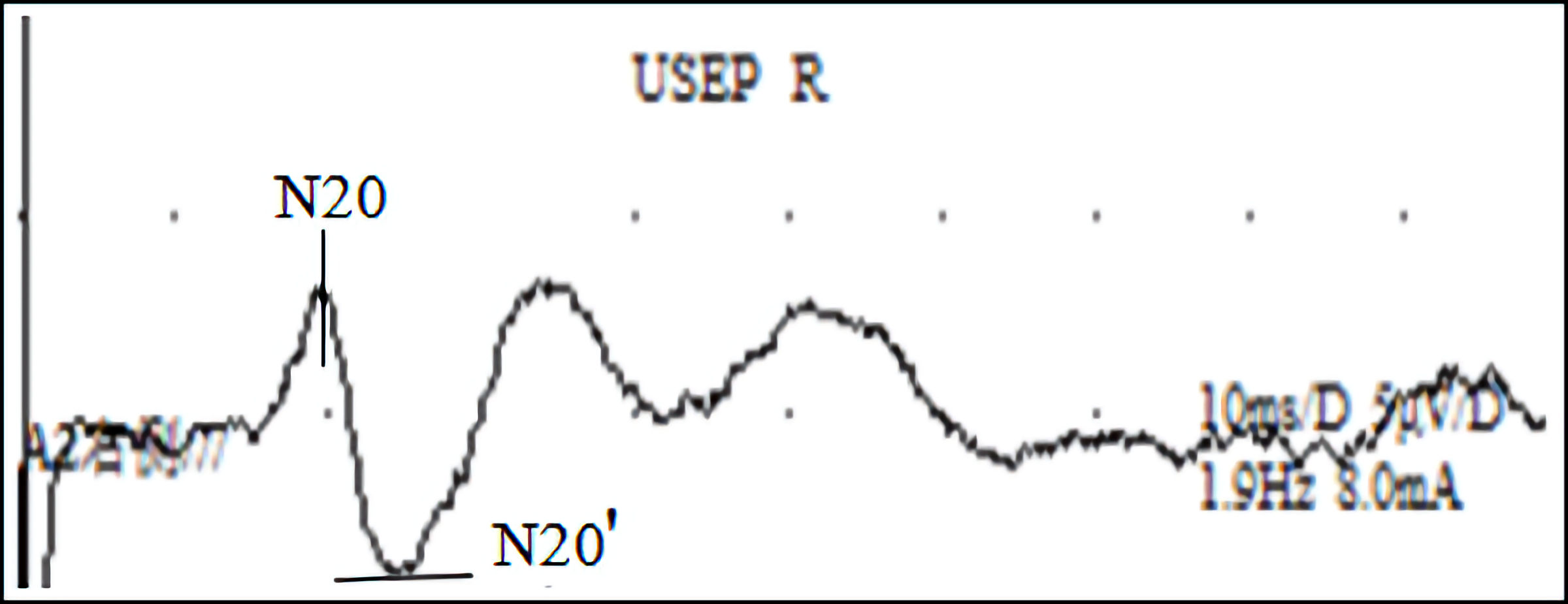 Click for large image | Figure 4. SEP wave map: a SEP waveform recorded using electromyography and evoked potential stimulation of the right median nerve. SEP: somatosensory evoked potential. |
Statistical analysis
Categorical data were compared using the Chi-square test, while quantitative data were compared using the t-test. Normally distributed quantitative data were expressed as mean ± standard deviation (X ± S). For overall comparisons, repeated measures analysis of variance (ANOVA) was employed, and independent sample t-tests were used to examine differences between groups at each time point. If interactions between time points and groups were identified, pairwise comparisons within each group at different time points were conducted separately. A P value < 0.05 was considered statistically significant for all analyses in this study.
| Results | ▴Top |
Demographic characteristics
The demographic characteristics, including gender and age, were well balanced between the intervention and control groups, with no statistically significant differences observed (P > 0.05), as detailed in Table 1.
 Click to view | Table 1. General Information of Two Groups of Subjects Before Intervention |
All participants successfully completed the scheduled interventions without experiencing any adverse reactions, such as dizziness, headache, nausea, palpitations, or chest tightness.
Repeated measures ANOVA before and after intervention
A repeated measures ANOVA was conducted to compare the pre- and post-intervention data between the intervention and control groups. The sphericity of the repeated measures data was evaluated using Mauchly’s test, yielding a P value greater than 0.05 (Table 2, Fig. 5). This result indicates that the assumption of sphericity was met, allowing the study to proceed with the analysis without applying any corrections to the results.
 Click to view | Table 2. Description Statistics of Two Groups of Subjects |
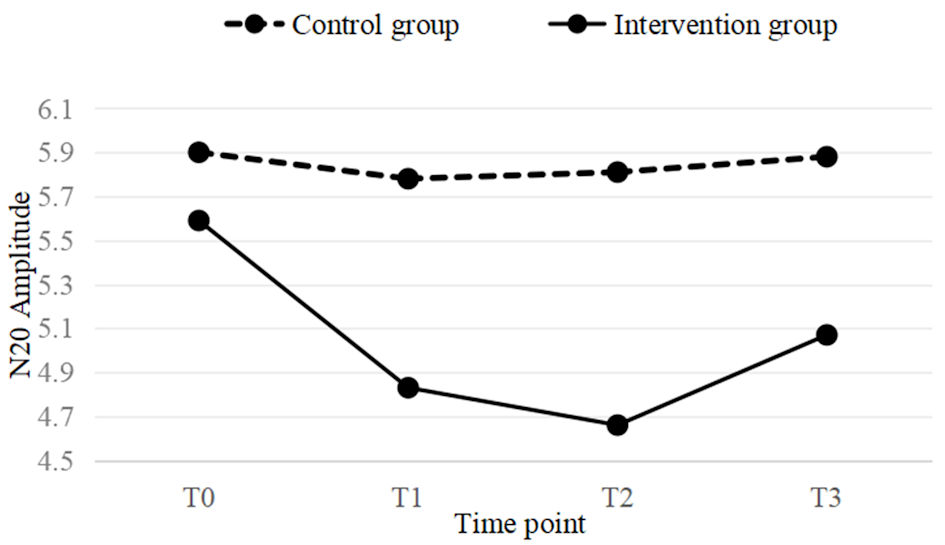 Click for large image | Figure 5. A line graph of the mean values for both the intervention and control groups at four time points (T0, T1, T2, T3). In the intervention group, a significant downward trend is observed at T1 time point, which still remains significant at T2. At T3, the downward trend begins to weaken. |
Comparison of intervention effects between two groups of subjects
When comparing the mean N20 amplitude across four time points between the tSMS group and the sham stimulation group, no statistically significant differences were observed between the groups (P > 0.05). However, within the tSMS group, significant decreases in SEP N20 amplitude were detected between baseline (T0) and the subsequent time points T1, T2, and T3 (P < 0.05), with a specific P value of 0.008. In contrast, within the sham stimulation group, P values were consistently above 0.544, indicating no statistically significant changes across the four pre- and post-intervention time points (P > 0.05). The between-group comparison yielded a P value of 0.195, further confirming that there were no statistically significant differences between the tSMS and sham stimulation groups (P > 0.05). However, the within-group analysis produced a P value of < 0.001, indicating significant effects within each group. Additionally, the interaction between time points and groups was statistically significant, with a P value of 0.003, demonstrating significant differences across time points within and between the groups (P < 0.05). For detailed data, refer to Table 3 and Figures 6, 7.
 Click to view | Table 3. Comparison of the Two Groups of Subjects Before and After Intervention |
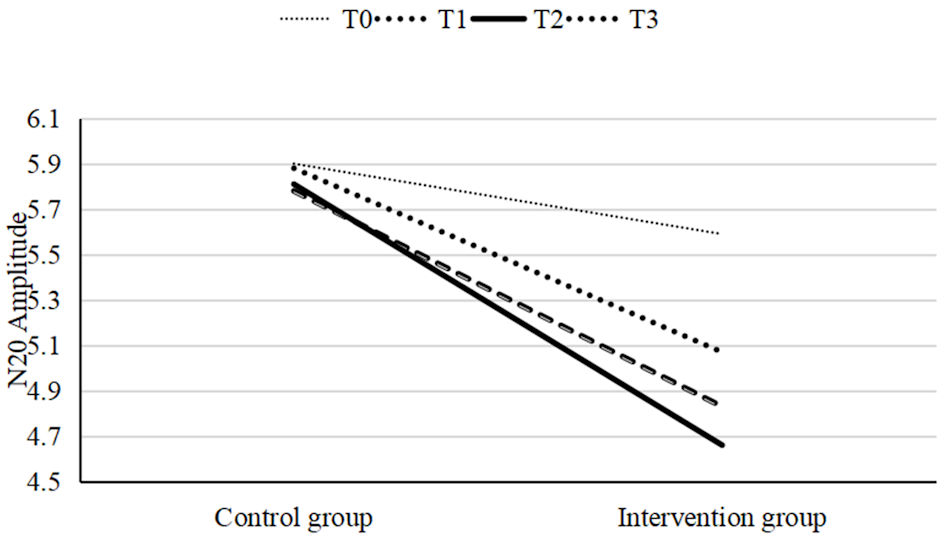 Click for large image | Figure 6. A linear graph of the mean values at four time points for both the intervention and control groups. The mean values at T1, T2, and T3 in the intervention group were significantly different from T0, with the largest difference observed at T2. In contrast, no significant differences were observed in the control group. |
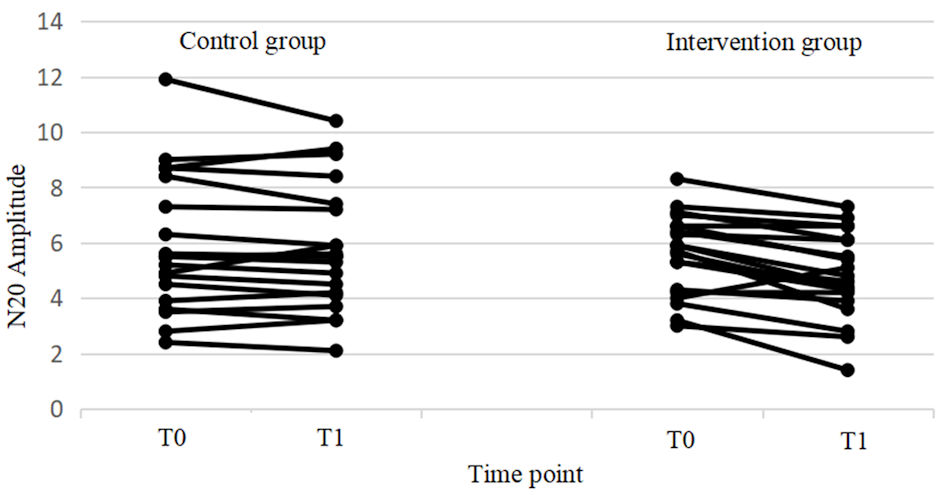 Click for large image | Figure 7. Changes in excitability from T0 to T1 for individual subjects in both the control and intervention groups. In the intervention group, significant differences were found between T0 and T1, while no significant differences were observed in the control group. Additionally, the control group showed large individual differences. |
Subanalysis of four time points within tSMS and sham stimulation groups
The data analysis revealed a significant interaction effect between time points and groups (P < 0.05), necessitating separate analyses for the tSMS and sham stimulation groups.
Within the tSMS group, comparisons between baseline (T0) and subsequent time points T1, T2, and T3 demonstrated statistically significant differences (P < 0.05). However, comparisons between other time points within the tSMS group showed no significant effects (P > 0.05). For detailed statistical outcomes, refer to Table 4.
 Click to view | Table 4. Comparison of Time Before and After Intervention in tSMS Group |
The comparison within the sham stimulation group across four time points did not show statistically significant differences (P > 0.05), as detailed in Table 5.
 Click to view | Table 5. Comparison of Time Before and After Intervention in Control Group |
| Discussion | ▴Top |
Our study demonstrated that a 30-min session of tSMS led to an average decrease of 13.2% in the N20 component amplitude of SEP in the tSMS group, indicating a significant inhibitory effect on the somatosensory cortex. This effect was most pronounced immediately after stimulation, with a peak reduction of 13.7% at 0 - 2 min, remaining significant at 5 - 7 min, and diminishing by 10 - 12 min. These findings confirm that tSMS significantly modulates somatosensory cortex excitability, though the effect is transient. In contrast, the sham stimulation group showed no significant changes, indicating that the observed effects were specific to the tSMS intervention. This aligns with existing literature [19, 22, 23], which suggests that tSMS effects are generally short-term and vary depending on the brain region and stimulation duration. While other studies have explored shorter interventions [24], our findings suggest that the effects of tSMS are context-dependent and may require optimization for different applications.
The exact mechanisms underlying tSMS’s effects are not fully understood, but it is believed to involve changes in membrane phospholipid orientation and ion channel function [25], possibly influenced by the Lorentz force [26]. These changes could affect neuronal excitability and synaptic transmission, contributing to the observed inhibitory effects. The transient nature of these effects suggests that neurons may require adaptive mechanisms to counteract sustained static magnetic fields. Future research should explore the biological mechanisms at play, the potential for cumulative effects with repeated sessions, and the combination of tSMS with other neuromodulation techniques for enhanced outcomes.
While our study provides valuable insights, it is important to acknowledge several limitations. Most literature indicates that tSMS primarily exerts inhibitory effects, a finding corroborated across multiple domains [20, 27, 28]. The small sample size limits the generalizability of the findings, and the lack of research on cumulative effects means that the long-term impact of tSMS remains unclear. Additionally, external factors such as head movement and variations in head anatomy may have influenced the results. The similarity between the neodymium magnets and the sham devices may not have fully eliminated placebo effects. Future research should address these limitations by using larger sample sizes, improving control conditions, and refining study designs to enhance the reliability of the results. Moving forward, we plan to increase the treatment intensity and conduct continuous sessions over 5 - 10 days to observe potential cumulative effects of tSMS.
In the present study, we did not observe that subjects had adverse reactions or discomfort, highlighting the safety of tSMS. Previous research supports its safety even in prolonged sessions, making it a viable option compared to other noninvasive brain stimulation techniques. As a noninvasive brain stimulation technology, tSMS has demonstrated its effectiveness and safety in healthy adults over the past decade [18], and it is now gradually entering the clinical application phase for the treatment and rehabilitation of neurological diseases. With the accumulation of research on its application in the treatment of Parkinson’s disease [14], post-stroke sequelae [17], epilepsy [15], and other conditions, as well as exploration of its effects on brain development in children [29], tSMS shows tremendous potential in the field of neurorehabilitation. Compared to TMS, tSMS operates without electrical currents, minimizes patient discomfort, is easy to administer, suitable for long-term and repeated treatments, and does not require expensive equipment, making it a cost-effective and practical option for both clinical and home-based applications. These advantages make it more favorable in terms of patient compliance, especially in the case of long-term continuous treatments. These features, coupled with its demonstrated efficacy, advocate for the wider adoption of tSMS in clinical research and practice.
This study revealed the inhibitory effect of tSMS on the sensory cortex and provided new insights into its potential mechanisms, particularly in terms of changes in membrane lipid orientation and ion channel function. This mechanistic investigation may help improve the understanding of how static magnetic fields can modulate neural networks and provide foundational theoretical support for future clinical applications. The study also indicates that the effects of tSMS are short-term and related to the stimulation intensity and the selectivity of the targeted brain regions. This suggests that in clinical applications, personalized adjustments could be made based on the specific pathological conditions and needs of individual patients. For instance, some patients may require longer or more frequent stimulation to achieve lasting effects, or they may benefit from combining tSMS with other therapeutic methods to enhance efficacy. This provides clear directions for future preclinical research, including exploring the cumulative effects of repeated treatments, combined applications with other neuromodulation techniques, and individualized treatment plans for patients.
In conclusion, this study confirms that a single 30-min session of tSMS effectively reduces excitability in the somatosensory cortex of young adults, demonstrating its potential as a novel, noninvasive neuroregulatory technique with substantial applications.
Acknowledgments
None to declare.
Financial Disclosure
This research was supported by the Guangxi Natural Science Foundation of China (2021GXNSFBA220005), the Project for Enhancing Young and Middle-Aged Teacher’s Research Basis Ability in Colleges of Guangxi (No. 2024KY0517), and the “13th Five-Year” Science and Technology Research Project of Jilin Provincial Education Department (JJKH20191142KJ), and the Guilin Innovation Platform and Talent Plan in 2022 (No. 20220124-18). Min Cui, Hong Da Zheng, and Qi Zhang were supported by the Guangxi Medical and Health Key Cultivation Discipline Construction Project.
Conflict of Interest
The authors declare no competing interest.
Informed Consent
Informed consent was obtained.
Author Contributions
Pan Ling Chen and Xiang Cui contributed equally to this work and are both considered first authors. They co-designed and performed the experiments, organized the data, and wrote the manuscript. Min Cui, as the corresponding author, provided essential guidance throughout the study and contributed to data analysis and interpretation. Qi Zhang, Hong Da Zheng, Fan Rong Kong, Hua Ye, and Jin Mei Guo assisted with participant recruitment and data collection, making significant contributions to the execution of the study.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
MEP: motor evoked potential; M1: primary motor cortex area; NIBS: non-invasive brain stimulation; rTMS: repetitive transcranial magnetic stimulation; SEP: somatosensory evoked potential; tSMS: transcranial static magnetic stimulation; tDCS: transcranial direct current stimulation; TMS: transcranial magnetic stimulation
| References | ▴Top |
- Gogulski J, Ross JM, Talbot A, Cline CC, Donati FL, Munot S, Kim N, et al. Personalized repetitive transcranial magnetic stimulation for depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2023;8(4):351-360.
doi pubmed - Aust S, Brakemeier EL, Spies J, Herrera-Melendez AL, Kaiser T, Fallgatter A, Plewnia C, et al. Efficacy of augmentation of cognitive behavioral therapy with transcranial direct current stimulation for depression: a randomized clinical trial. JAMA Psychiatry. 2022;79(6):528-537.
doi pubmed - Pigot M, Loo C, Sachdev P. Repetitive transcranial magnetic stimulation as treatment for anxiety disorders. Expert Rev Neurother. 2008;8(10):1449-1455.
doi pubmed - Du J, Yang F, Hu J, Hu J, Xu Q, Cong N, Zhang Q, et al. Effects of high- and low-frequency repetitive transcranial magnetic stimulation on motor recovery in early stroke patients: Evidence from a randomized controlled trial with clinical, neurophysiological and functional imaging assessments. Neuroimage Clin. 2019;21:101620.
doi pubmed - Fridriksson J, Rorden C, Elm J, Sen S, George MS, Bonilha L. Transcranial direct current stimulation vs sham stimulation to treat aphasia after stroke: a randomized clinical trial. JAMA Neurol. 2018;75(12):1470-1476.
doi pubmed - Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55(2):187-199.
doi pubmed - Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMSCG. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008-2039.
doi pubmed - Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201-206.
doi pubmed - Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, et al. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568(Pt 1):291-303.
doi pubmed - Sheffield A, Ahn S, Alagapan S, Frohlich F. Modulating neural oscillations by transcranial static magnetic field stimulation of the dorsolateral prefrontal cortex: A crossover, double-blind, sham-controlled pilot study. Eur J Neurosci. 2019;49(2):250-262.
doi pubmed - Soto-Leon V, Diez-Rodriguez E, Herrera-Perez S, Rosa JM, Aguilar J, Hernando A, Bravo-Sanchez C, et al. Effects of transcranial static magnetic field stimulation over the left dorsolateral prefrontal cortex on random number generation. Clin Neurophysiol. 2023;149:18-24.
doi pubmed - Carrasco-Lopez C, Soto-Leon V, Cespedes V, Profice P, Strange BA, Foffani G, Oliviero A. Static magnetic field stimulation over parietal cortex enhances somatosensory detection in humans. J Neurosci. 2017;37(14):3840-3847.
doi pubmed - Gonzalez-Rosa JJ, Soto-Leon V, Real P, Carrasco-Lopez C, Foffani G, Strange BA, Oliviero A. Static magnetic field stimulation over the visual cortex increases alpha oscillations and slows visual search in humans. J Neurosci. 2015;35(24):9182-9193.
doi pubmed - Dileone M, Carrasco-Lopez MC, Segundo-Rodriguez JC, Mordillo-Mateos L, Lopez-Ariztegui N, Alonso-Frech F, Catalan-Alonso MJ, et al. Dopamine-dependent changes of cortical excitability induced by transcranial static magnetic field stimulation in Parkinson's disease. Sci Rep. 2017;7(1):4329.
doi pubmed - McLean MJ, Engstrom S, Holcomb RR, Sanchez D. A static magnetic field modulates severity of audiogenic seizures and anticonvulsant effects of phenytoin in DBA/2 mice. Epilepsy Res. 2003;55(1-2):105-116.
doi pubmed - Giorgetto C, Silva EC, Kitabatake TT, Bertolino G, de Araujo JE. Behavioural profile of Wistar rats with unilateral striatal lesion by quinolinic acid (animal model of Huntington disease) post-injection of apomorphine and exposure to static magnetic field. Exp Brain Res. 2015;233(5):1455-1462.
doi pubmed - Shimomura R, Shibata S, Koganemaru S, Minakuchi M, Ichimura S, Itoh A, Shimotake K, et al. Transcranial static magnetic field stimulation (tSMS) can induce functional recovery in patients with subacute stroke. Brain Stimul. 2023;16(3):933-935.
doi pubmed - Oliviero A, Carrasco-Lopez MC, Campolo M, Perez-Borrego YA, Soto-Leon V, Gonzalez-Rosa JJ, Higuero AM, et al. Safety study of transcranial static magnetic field stimulation (tSMS) of the human cortex. Brain Stimul. 2015;8(3):481-485.
doi pubmed - Kirimoto H, Tamaki H, Matsumoto T, Sugawara K, Suzuki M, Oyama M, Onishi H. Effect of transcranial static magnetic field stimulation over the sensorimotor cortex on somatosensory evoked potentials in humans. Brain Stimul. 2014;7(6):836-840.
doi pubmed - Dileone M, Mordillo-Mateos L, Oliviero A, Foffani G. Long-lasting effects of transcranial static magnetic field stimulation on motor cortex excitability. Brain Stimul. 2018;11(4):676-688.
doi pubmed - Rivadulla C, Foffani G, Oliviero A. Magnetic field strength and reproducibility of neodymium magnets useful for transcranial static magnetic field stimulation of the human cortex. Neuromodulation. 2014;17(5):438-441; discussion 441-432.
doi pubmed - Kirimoto H, Tamaki H, Otsuru N, Yamashiro K, Onishi H, Nojima I, Oliviero A. Transcranial static magnetic field stimulation over the primary motor cortex induces plastic changes in cortical nociceptive processing. Front Hum Neurosci. 2018;12:63.
doi pubmed - Silbert BI, Pevcic DD, Patterson HI, Windnagel KA, Thickbroom GW. Inverse correlation between resting motor threshold and corticomotor excitability after static magnetic stimulation of human motor cortex. Brain Stimul. 2013;6(5):817-820.
doi pubmed - Oliviero A, Mordillo-Mateos L, Arias P, Panyavin I, Foffani G, Aguilar J. Transcranial static magnetic field stimulation of the human motor cortex. J Physiol. 2011;589(Pt 20):4949-4958.
doi pubmed - Kantak SS, Mummidisetty CK, Stinear JW. Primary motor and premotor cortex in implicit sequence learning—evidence for competition between implicit and explicit human motor memory systems. Eur J Neurosci. 2012;36(5):2710-2715.
doi pubmed - Freire MJ, Bernal-Mendez J, Perez AT. The Lorentz force on ions in membrane channels of neurons as a mechanism for transcranial static magnetic stimulation. Electromagn Biol Med. 2020;39(4):310-315.
doi pubmed - Nojima I, Koganemaru S, Fukuyama H, Mima T. Static magnetic field can transiently alter the human intracortical inhibitory system. Clin Neurophysiol. 2015;126(12):2314-2319.
doi pubmed - Nojima I, Watanabe T, Gyoda T, Sugata H, Ikeda T, Mima T. Transcranial static magnetic stimulation over the primary motor cortex alters sequential implicit motor learning. Neurosci Lett. 2019;696:33-37.
doi pubmed - Hollis A, Zewdie E, Nettel-Aguirre A, Hilderley A, Kuo HC, Carlson HL, Kirton A. Transcranial static magnetic field stimulation of the motor cortex in children. Front Neurosci. 2020;14:464.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.