| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 000, Number 000, January 2025, pages 000-000
Prevalence of Alloimmunization Events in Thalassemia Patients With Repeated Transfusions in the Rhesus Blood Group System: A Systematic Review and Meta Analysis
Vitasari Indriania, d , Budi Mulyonob
, Teguh Triyonob
, Anastasia Evi Handayaningsiha
, Lukman Ade Chandrac
aDoctoral Program, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada Yogyakarta (UGM), Yogyakarta, Indonesia
bDepartment of Clinical Pathology and Laboratorium Medicine, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada Yogyakarta (UGM), Yogyakarta, Indonesia
cDepartment of Pharmacology and Therapy, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada Yogyakarta (UGM), Yogyakarta, Indonesia
dCorresponding Author: Vitasari Indriani, Doctoral Program, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada Yogyakarta (UGM), Yogyakarta, Indonesia
Manuscript submitted November 16, 2024, accepted January 8, 2025, published online January 17, 2025
Short title: Rh Alloimmunization in Thalassemia
doi: https://doi.org/10.14740/jocmr6142
| Abstract | ▴Top |
Background: Alloimmunization presents a significant challenge for patients with β-thalassemia major who depend on regular transfusion therapy. This systematic review and meta-analysis aimed to evaluate the frequency of alloimmunization within the Rhesus blood group system and identify the most prevalent alloantibodies.
Methods: A comprehensive search across multiple databases was conducted to locate epidemiological studies reporting alloimmunization in thalassemia patients undergoing repeated transfusions, specifically focusing on Rhesus antibodies. Statistical analyses were performed using R software, and heterogeneity was assessed using I2 statistics.
Results: This review included 20 studies with a total of 4,650 patients. The overall prevalence of alloimmunization was 5.4% (95% confidence interval (CI): 3.1-9.3%) across all ages, with a prevalence of 9.1% (95% CI: 5.3-15.2%) in children and 25% (95% CI: 12.7-41.2%) in adults. The pooled overall prevalence was 6.6% (95% CI: 4.2-10.2%). Among the 488 alloimmunized patients, 310 developed Rhesus-specific antibodies, with anti-E (34.58%) and anti-D (13.69%) being the most frequent.
Conclusions: This study underscores the substantial prevalence of Rhesus antibodies among alloimmunized thalassemia patients. Implementing extended phenotype matching for transfusions could significantly reduce the risk of alloantibody formation in this population. Future analyses should explore factors influencing alloimmunization rates, such as ethnic diversity, matching protocols, and age-related variations, to inform clinical practice better.
Keywords: Alloimmunization; Antibodies; Rhesus; Thalassemia; Transfusion
| Introduction | ▴Top |
β-Thalassemia is an inherited blood disorder characterized by insufficient production of β-globin chains, leading to hemolysis and ineffective erythropoiesis [1, 2]. This deficiency in β-globin synthesis, a key component of adult hemoglobin, results in severe anemia, particularly in patients with β-thalassemia major (TM), who become dependent on regular blood transfusions from an early age (a condition known as transfusion-dependent β-thalassemia (TDT)). In contrast, individuals with β-thalassemia intermedia (TI) typically do not require regular transfusions but may need them during specific complications, such as illness or pregnancy [1, 3].
Although transfusion therapy has significantly improved survival and quality of life for thalassemia patients, it is associated with complications, including iron overload, alloimmunization, infections, and adverse transfusion reactions [4, 5]. Frequent transfusions expose patients to foreign antigens, increasing the risk of alloimmunization, where the immune system generates antibodies (alloantibodies) against donor red blood cells (RBCs) [6]. Alloimmunization is influenced by factors such as the immunogenicity of RBC antigens, the patient’s immune status, and the timing and frequency of transfusions [7].
Once developed, alloantibodies can complicate future transfusions, making blood cross-matching more difficult, shortening the lifespan of transfused RBCs, exacerbating iron overload, and delaying access to safe blood products [8]. Reported rates of alloimmunization in thalassemia patients vary widely, ranging from 4% to 50%, with lower rates generally observed in populations, where donors and recipients share similar genetic backgrounds [5]. Alloimmunization against RBC antigens, especially within the Rhesus (Rh) blood group system, poses a significant clinical challenge in β-thalassemia patients [9].
Studies indicate that the most frequently encountered alloantibodies target Rh antigens, particularly anti-E, anti-D, and Kell. The Rh blood group, second only to the ABO system in immunogenicity, is highly polymorphic, which may explain the high alloimmunization rates even when major Rh antigens are matched between donor and recipient due to the presence of Rh variants [10-12].
Given the critical role of Rh antibodies in alloimmunization, understanding their prevalence and distribution among TM patients is essential for improving transfusion strategies. This systematic review and meta-analysis aim to provide valuable insights into the frequency of alloimmunization within the Rh blood group system and identify specific Rh variants common in TM patients undergoing repeated transfusions. While our study focused primarily on Rh blood group alloimmunization, HLA does indeed play a role in alloimmunization events. HLA molecules are highly polymorphic proteins that present peptides to immune cells, particularly T cells. The significant variability in HLA alleles between individuals makes them highly immunogenic. HLA antibodies can develop in response to transfusions and may contribute to transfusion reactions. The recipient is exposed to donor HLA antigens that differ from their own, the immune system can mount an alloimmune response. Although our review did not specifically address HLA-related alloimmunization, it is an important aspect of transfusion medicine.
| Materials and Methods | ▴Top |
Protocol and registration
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines. The review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) on June 6, 2024, under the registration number CRD42024552701.
Eligibility criteria
Studies were included if they met the following criteria: 1) focused exclusively on human subjects; 2) employed cross-sectional or retrospective cohort designs; and 3) involved patients diagnosed with TM who received repeated transfusions. Only articles published in English were considered. Diagnoses of TM were confirmed via patient history, clinical examinations, and hemoglobin electrophoresis. Studies that did not specifically address alloimmunization within the Rh blood group system or lacked full-text availability were excluded.
Information sources and search strategy
We conducted a comprehensive literature search across multiple databases, including EBSCO, Proquest, PubMed, ScienceDirect, Wiley, and Scopus. The search covered all available data from the inception of each database up to February 15, 2024. The search strategy was based on the population, intervention, and outcome (PIO) framework and employed a combination of medical subject headings (MeSH) terms and keywords such as (“Thalassemia” (MeSH terms) OR “beta-Thalassemia” (MeSH terms) OR “thalassemia*”(title/abstract) OR “beta thalassemia*” (title/abstract)) AND (“Blood Transfusion” (MeSH terms) OR “blood transfusion*” (title/abstract) OR “regular transfusion”(title/abstract) OR “repeated transfusion” (title/abstract)) AND (“Rh-Hr blood-group system” (MeSH terms) OR “rh hr blood group system*” (title/abstract) OR “Rh” (title/abstract) OR “Rhesus” (title/abstract)) AND (“isoantibodies” (MeSH terms) OR “isoantibodies*” (title/abstract) OR “alloantibody*” (title/abstract) OR “allo antibody*” (title/abstract) OR “alloimmunization*” (title/abstract) OR “alloimmunisation*” (title/abstract)).
Data collection process and data items
One reviewer (V.I.) was responsible for extracting data from the included studies, which was then verified by a second reviewer (L.A.C.). Any disagreements during this process were resolved through discussion, and if consensus could not be reached, we consulted with verifiers (T.T., B.M., and A.E.H.). The extracted data included study characteristics, patient demographics, and outcomes related to alloimmunization in the Rh blood group system. Additionally, we contacted the authors of conference abstracts to inquire about the availability of full texts for inclusion.
Risk of bias assessment
Two reviewers (V.I. and L.A.C.) independently assessed the risk of bias in the included studies using the Joanna Briggs Institute (JBI) critical appraisal checklist designed for cross-sectional studies [13]. Each potential source of bias was rated as “yes”, “no”, “unclear”, or “not applicable”. Disagreements were resolved through discussion, and in cases where consensus could not be reached, a third or fourth reviewer was consulted.
Synthesis of results
A meta-analysis was conducted using logit-transformed proportions and random-effects models in the R software for our analysis. We categorized studies by age into three groups: 1) all-population; 2) children; and 3) adults. Heterogeneity was evaluated using both I2 and T2 statistics. Additionally, we performed tests for subgroup variances and carried out descriptive analyses to summarize supplementary data, including age at first transfusion, duration between transfusions, and intervals. These analyses were aimed at guiding future research.
Ethics and dissemination
The study is grounded in a synthesis of existing published data, thereby rendering ethical approval unnecessary. We intend to disseminate our findings through publication in a peer-reviewed journal and presentations at pertinent academic conferences
| Results | ▴Top |
Study selection
During the initial database search, we identified 1,011 articles. After the removal of 189 duplicates, 822 articles remained for screening. Using Rayyan automation tools, we reviewed the titles and abstracts, excluding 719 articles based on criteria such as the absence of an abstract, a focus on the thalassemia intermedia population, or a lack of relevance to alloimmunization. Of the remaining 103 articles, three could not be retrieved despite attempts to contact the corresponding authors. After evaluating the remaining 100 articles, 80 were excluded for reasons such as irrelevance to TM, incorrect population, inappropriate study design, or irrelevant outcomes. Ultimately, 20 studies met the inclusion criteria. A detailed PRISMA flow diagram illustrating this selection process can be found in Figure 1 [14].
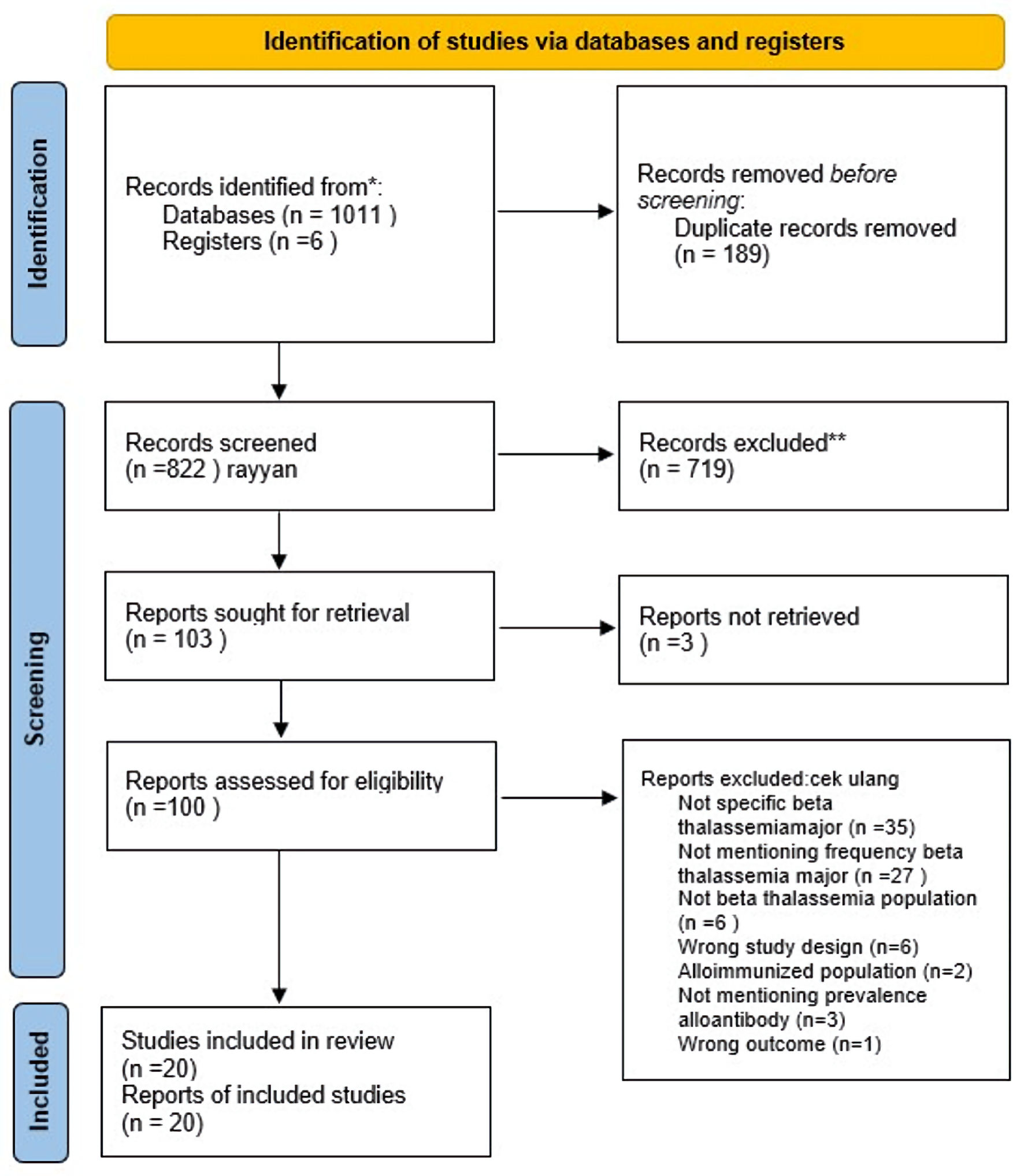 Click for large image | Figure 1. Study selection according to PRISMA flowchart. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses. |
Study characteristics
The 20 studies included in this review were published between 2011 and 2024 [15-34]. Geographically, four studies originated from Pakistan, four from India, four from Iran, and one each from Egypt, Tunisia, Israel, Albania, Canada, Sri Lanka, China, and Yemen. These studies were categorized into three age groups: all-population (14 studies; 70%, age range 0 - 40 years), children (five studies; 25%, age range 1 - 18 years), and adults (1 study; 5%, age range 20 - 47 years). While we recognized the importance of ethnic diversity in blood group antigen distribution, our ability to categorize populations based on ethnicity was limited by the data reported in the original studies. The sample sizes varied widely, ranging from 32 to 1,147 patients across the studies. In terms of methodology, most studies utilized agglutination techniques for RBC antibody screening, with products such as Diamed and Diacell. Alloantibody identification was primarily performed using the Diamed-Diapanel 11 identification panel in 75% of the studies (15 studies). Leukoreduction was reported in six studies, with five applying it universally across all patients. The age at which patients received their first transfusion varied significantly: from 0.3 to 12 years in the all-population group, 0.9 to 8.5 years in children, and 0.25 to 4 years in adults. The total number of transfused blood units also showed substantial variation, ranging from 12 to 191 units in the all-population group, 12 to over 250 units in children, and 493 to 1,363 units in adults. Blood transfusion intervals ranged from 5 to 90 days across half of the studies. A summary of these study characteristics is presented in Table 1 [15-34].
 Click to view | Table 1. General Characteristic and Outcome of the Eligible Studies |
Prevalence of alloimmunization
Of the 4,650 patients included in the review, 488 developed alloimmunization, translating to an overall prevalence rate of 10.49%. Specifically, within the Rh blood group system, alloimmunization was identified in 309 patients, representing 6.64% of the total. While one study did not report the specificity of alloantibodies, the remaining 19 studies provided data for 4,566 patients, with 466 cases of alloimmunization involving Rh antibodies. In total, 44 types of Rh antibodies were identified. Among these patients, 72.35% (212 patients) developed a single antibody, while 27.39% (80 patients) exhibited multiple antibodies. The most frequently identified Rh antibodies were anti-E (34.58%, or 101 cases), followed by anti-D (13.69%, or 40 cases), anti-C (10.27%, or 30 cases), anti-c (7.87%, or 23 cases), and anti-D combined with anti-C (5.47%, or 16 cases). Detailed information on the specificities of Rh blood group antibodies is provided in Table 2 [15-34].
 Click to view | Table 2. Specificity of Red Blood Cell Antibodies in Rhesus Blood Group |
Risk of bias assessment and level of evidence
The risk of bias was assessed using the Joanna Briggs Institute (JBI) critical appraisal tool across eight key domains. Most studies received high ratings in domains such as inclusion criteria, data collection, outcome measurement, statistical analysis, control of confounders, sample size adequacy, and relevance of conclusions. However, three studies (Chaudhari et al [17], Ebrahimisadr et al [21], and Gholami et al [23]) received a “no” rating in domain two, indicating a lack of sufficient detail on study subjects, which could introduce bias due to limited demographic and clinical context.
Despite this, all studies were deemed methodologically sound and included in the meta-analysis. The overall level of evidence (LOE) ranged from level 3 to 4, with most studies classified as level 4, representing moderate-quality evidence. Although some studies had minor weaknesses, the overall strength of the evidence supports the validity of the meta-analysis findings.
Synthesis of results
A meta-analysis was conducted using logit-transformed proportions and random-effects models in the R software. The studies were categorized by age into three groups: 1) all-population; 2) children; and 3) adults. Heterogeneity was evaluated using both I2 and T2 statistics. Additionally, subgroup variance tests and descriptive analyses were performed to summarize supplementary data, including the age at first transfusion, duration between transfusions, and transfusion intervals. These analyses are intended to guide future research.
The forest plot (Fig. 2) illustrates the findings from the meta-analysis of 20 studies investigating the prevalence of alloimmunization in β-thalassemia patients undergoing repeated transfusions. The plot visually represents the individual study estimates, their respective confidence intervals (CIs), and the pooled estimates for various subgroups. The overall pooled prevalence of alloimmunization was 6.9% (95% CI: 4.2% - 11.2%), with significant heterogeneity across studies, indicated by an I2 value of 94.12% (P = 0.000). This high heterogeneity suggests considerable variability in the reported prevalence, likely due to differences in study populations, transfusion practices, and alloimmunization detection methods.
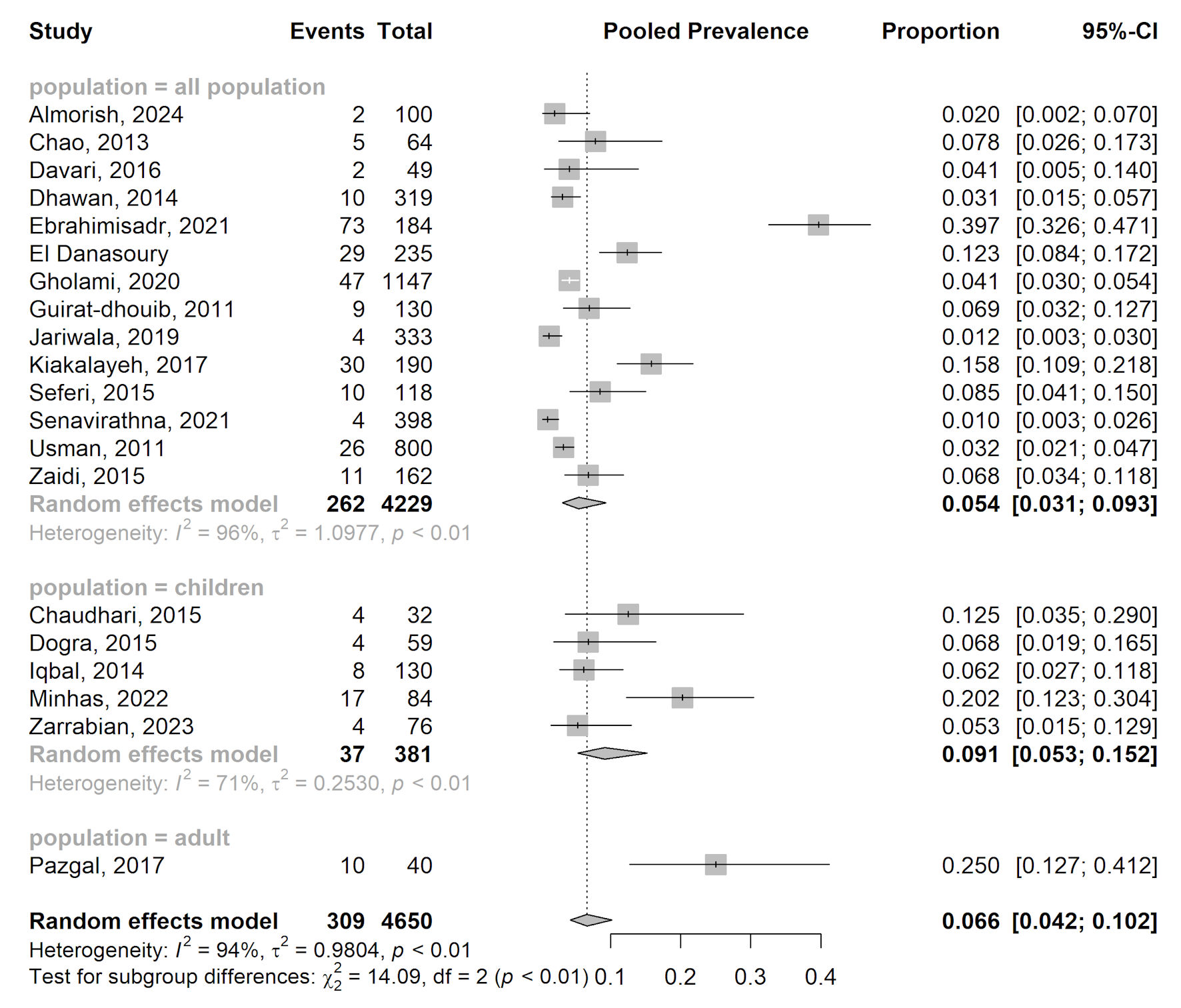 Click for large image | Figure 2. Forest plot of proportion estimate of alloimmunization in rhesus blood group. CI: confidence interval. |
Subgroup analysis
The meta-analysis was divided into three subgroups based on patient age: all-population, children, and adults. In the all-population subgroup, the pooled prevalence of alloimmunization was 5.5% (95% CI: 2.9% - 10.2%), based on data from 16 studies involving 4,229 patients. This subgroup also exhibited high heterogeneity (I2 = 95.54%, P = 0.000), indicating variability in the study estimates. In the children subgroup, the pooled prevalence was higher at 9.4% (95% CI: 5.0% - 17.0%) with moderate heterogeneity (I2 = 70.52%, P = 0.009). This variability suggests that factors such as transfusion protocols and antigen matching may influence alloimmunization rates in children. In the adult subgroup, the pooled prevalence was significantly higher at 30% (95% CI: 17.9% - 45.7%) based on a single study involving 40 patients. Due to the limited number of studies, no heterogeneity measures (I2 or P values) were reported for this subgroup.
| Discussion | ▴Top |
This systematic review and meta-analysis of 20 studies, encompassing 4,650 β-thalassemia patients, examined the prevalence of alloimmunization against Rh blood group antigens in repeatedly transfused individuals. The overall prevalence was 10.49% (95% CI: 8.9% - 12.2%), with 6.9% (95% CI: 4.2% - 11.2%) specifically attributed to Rh antibodies. These findings align with prior research identifying Rh antibodies as the most frequent alloantibodies in thalassemia patients, underscoring the significant immunological challenges posed by lifelong transfusion dependency.
High heterogeneity (I2 = 94.12%) reflects variability driven by regional differences, donor-recipient antigenic disparity, and transfusion practices. Populations with limited antigen matching, such as in Egypt and Southwest Iran, exhibit higher alloimmunization rates, whereas nations employing extended matching, like some European countries, report lower rates. Patient-specific factors, including transfusion frequency, splenectomy, and immune maturity, further contribute to this variability, highlighting the need for tailored transfusion strategies [35-37]
Subgroup analysis
Subgroup analysis revealed notable differences in alloimmunization rates between children and adults. In children, the pooled prevalence was 9.4% (95% CI: 5.0% - 17.0%), while adults showed a much higher prevalence of 30% (95% CI: 17.9% - 45.7%). This disparity may be attributed to the cumulative effects of repeated transfusions over time in adults, increasing their risk of alloimmunization. As patients age and are exposed to foreign antigens from multiple donors, their likelihood of developing alloantibodies increases. These results align with existing literature, which indicates that the risk of alloimmunization rises with the number of transfusions and the duration of exposure [38-40]. Sex was not identified as a statistically significant factor influencing alloimmunization in adult or pediatric transfusion-dependent thalassemia patients. Only in Yemen, alloimmunization was found to be significantly associated with sex, with females more likely to develop alloantibodies than males (P = 0.03). This may suggest immune system differences or transfusion patterns influenced by cultural or clinical factors.
High heterogeneity
The substantial heterogeneity (I2 = 94.12%) observed across studies suggests that factors such as ethnic diversity, transfusion practices, and antigen matching significantly influence alloimmunization rates. Regions with more advanced blood-matching protocols, including extended Rh and minor antigen matching, tend to have lower alloimmunization rates. In contrast, areas with less comprehensive matching experience higher rates [27-29]. Variations in blood group antigen distribution between donors and recipients, particularly in diverse populations, further increase the risk of alloimmunization due to antigen mismatch. The study by Ebrahimisadr et al [21], which reported a 39.7% prevalence of alloimmunization, likely reflects regional or institutional differences in transfusion practices, whereas the 1% prevalence reported by Senavirathna et al [31] may be attributed to stricter matching protocols or differences in genetic backgrounds and transfusion frequencies.
Variability in prevalence rates
The wide variability in alloimmunization rates across different studies can be explained by several factors. Ethnicity plays a critical role, as ethnic differences within populations significantly impact immunogenicity. For example, the low prevalence (1%) reported in a Sri Lankan study may be due to the ethnic homogeneity of the population and effective local transfusion practices [31]. In contrast, higher prevalence rates, such as the 39.7% reported in Iran, may reflect greater genetic diversity and varying immunogenic responses to Rh antigens [21].
Transfusion protocols and age-related factors
Blood transfusion protocols are crucial in mitigating alloimmunization. Studies have shown that matching blood for Rh and Kell antigens significantly reduces the risk of alloimmunization compared to ABO-D matching alone [8]. International guidelines recommend extended phenotyping for C, c, E, e, and Kell antigens before transfusion to minimize alloimmunization risks [39-40]. Variations in the implementation of these guidelines across different centers may account for some of the inconsistencies observed in the reviewed studies.
Age also plays a significant role in the development of alloimmunization. The adult subgroup had the highest prevalence rate of 30%, which is consistent with findings from Eastern India, where 67.86% of alloimmunized cases occurred in individuals aged 21 - 40 years [41]. This suggests that patients requiring multiple transfusions over several years are more likely to develop alloantibodies. On the other hand, children had a lower rate of 9.1%, possibly due to immune tolerance mechanisms that prevent the formation of alloantibodies upon initial exposure to foreign RBC antigens [42]
Specific antibodies and technical considerations
The most frequently identified Rh antibody in this review was anti-E (34.58%), consistent with findings from other regions like Thailand. Anti-D (13.69%) was the second most common, potentially influenced by technical factors such as testing errors or weak D variants. Accurate antibody screening is crucial for managing thalassemia patients and minimizing alloimmunization risks. Variations in detection methods may explain differences in prevalence rates across studies. Standardizing testing protocols can enhance the accuracy of alloantibody identification, leading to improved clinical outcomes for patients undergoing repeated transfusions.
Transfusion practices and alloimmunization
We found that the age at which patients received their first transfusion ranged from 0.25 to 12 years. The age at which patients begin receiving transfusions significantly influences the likelihood of developing alloimmunization. Studies show that younger patients, particularly those starting transfusions before 2 years of age, are less likely to develop alloantibodies compared to those who begin transfusions later in life. For instance, Al-Mousawi et al (2015) observed that the risk of alloimmunization was significantly higher in patients who started transfusions after the age of 2 [43]. Similarly, Yadav et al (2023) reported that patients who received their first transfusion at a median age of 1 year had a higher rate of alloimmunization compared to those who started at 6 months [38]. This phenomenon may be explained by the immune tolerance hypothesis, which suggests that early exposure to foreign antigens during the immune system’s developmental stage might reduce immunological responsiveness. However, not all studies consistently identify a significant correlation between age at first transfusion and alloimmunization risk [44]. However, some studies have found no significant link between age and the risk of alloimmunization [36].
The frequency and volume of transfusions are strongly linked to alloimmunization risk. Studies show that shorter transfusion intervals (< 3 weeks) and higher cumulative transfusion volumes significantly increase alloimmunization rates. El Kababi et al (2019) reported that patients with shorter transfusion intervals had a higher likelihood of developing alloantibodies compared to those with longer intervals [44]. Similarly, Yadav et al (2023) found that alloimmunized patients received more units annually and had shorter transfusion intervals than non-alloimmunized patients, further highlighting the association between frequent transfusions and heightened immune response [38]. These findings emphasize the need for tailored transfusion strategies to minimize exposure to foreign antigens and reduce alloimmunization risks.
Furthermore, evidence from Teawtrakul et al (2022) emphasizes that higher transfusion volumes, particularly exceeding 15 mL/kg, strongly increase the risk of alloimmunization. The study underscored that shorter transfusion intervals and higher total transfusion volumes are among the most predictive factors for the development of alloantibodies in thalassemia patients [45]. This finding is consistent with previous research, which indicates that total transfusion volume is a key factor in predicting the development of alloantibodies in thalassemia patients [35, 46, 47].
Implications for clinical practice
This review underscores the critical need for implementing extended Rh antigen matching protocols in clinical practice. Matching blood for both Rh and K antigens before a patient’s first transfusion can significantly reduce the risk of alloimmunization and improve patient outcomes [48]. Given the diverse ethnic backgrounds of thalassemia patients worldwide, transfusion services should consider implementing extended antigen matching protocols that account for the specific antigen profiles common in their patient populations. This approach could potentially reduce alloimmunization rates by minimizing exposure to foreign antigens. Our findings highlight a significant gap in current practices, particularly in developing countries where access to comprehensive blood typing is often limited.
Limitations
While our review provides valuable insights, it has some limitations, including insufficient data for a detailed meta-analysis on alloimmunization related to specific Rh antigen variants and the absence of heterogeneity analysis in adults. The high heterogeneity observed indicates the need for further research to identify factors influencing alloimmunization in diverse populations, including the potential impact of pharmacotherapy for comorbidities. Future studies should aim to standardize alloimmunization reporting and transfusion practices, conduct longitudinal studies to track alloimmunization risk over time considering variables such as age, sex, and ethnicity, and investigate the influence of various medications, particularly immunosuppressive drugs, on alloimmunization rates in thalassemia patients with comorbidities.
Conclusions
This meta-analysis offers crucial insights into the prevalence of alloimmunization in β-thalassemia patients undergoing repeated transfusions. The findings reveal particularly high rates of alloimmunization among adults, emphasizing the urgent need for improved transfusion strategies. Specifically, the use of extended phenotypic matching, including Rh and minor antigens, could significantly lower the risk of alloimmunization in this population.
The considerable heterogeneity across studies highlights the importance of recognizing regional differences in blood group antigen distribution and transfusion practices when implementing these strategies. Adapting transfusion protocols to accommodate these variations could help clinicians reduce alloimmunization rates, improve patient outcomes, and minimize transfusion-related complications in β-thalassemia care. Future research should aim to standardize alloimmunization reporting and explore the long-term effects of transfusion practices in diverse populations.
Acknowledgments
The authors would like to thank the Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada for their feedback during the initial discussion and planning of this study, and to the Faculty of Medicine, Universitas Jenderal Soedirman, Indonesia for their support of this study.
Financial Disclosure
Financial support provided by the Center for Higher Education Funding (BPPT) and Indonesian Endowment Fund for Education (LPDP) on decree no. 02356/J5.2.3./BPI.06/9/2022 is greatly appreciated.
Conflict of Interest
The authors declared that they had no competing interest.
Informed Consent
Since this manuscript is a systematic review, informed consent is not applicable.
Author Contributions
V.I. initiated the research idea and wrote the initial draft of the manuscript. V.I and L.A.C. performed the literature review, data extraction, and edited the manuscript. T.T., B.M., and A.E.H. verified and accepted the manuscript before submission.
Data Availability
The data supporting the findings of this study are available within the article. Further inquiries can be made to the corresponding authors regarding data requests.
Abbreviations
β-TM: beta-thalassemia major; TDT: transfusion-dependent β-thalassemia; TI: β-thalassemia intermedia; RBC: red blood cell; Rh: Rhesus; CI: confidence interval; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PROSPERO: International Prospective Register of Systematic Reviews; PIO: population, intervention, and outcome; MeSH: Medical Subject Headings; JBI: Joanna Briggs Institute; LOE: level of evidence; HLA: human leukocyte antigen; ABO: A, B, and O blood types
| References | ▴Top |
- Angastiniotis M, Lobitz S. Thalassemias: an overview. International Journal of Neonatal Screening. 2019;5(1):16.
- Shafique F, Ali S, Almansouri T, Van Eeden F, Shafi N, Khalid M, Khawaja S, et al. Thalassemia, a human blood disorder. Braz J Biol. 2021;83:e246062.
doi pubmed - Kattamis A, Forni GL, Aydinok Y, Viprakasit V. Changing patterns in the epidemiology of beta-thalassemia. Eur J Haematol. 2020;105(6):692-703.
doi pubmed - Betts M, Flight PA, Paramore LC, Tian L, Milenkovic D, Sheth S. Systematic literature review of the burden of disease and treatment for transfusion-dependent beta-thalassemia. Clin Ther. 2020;42(2):322-337.e322.
doi pubmed - Jain R, Choudhury N, Chudgar U, Harimoorthy V, Desai P, Perkins J, Johnson ST. Detection and identification of red cell alloantibodies in multiply transfused thalassemia major patients: a prospective study. Indian J Hematol Blood Transfus. 2014;30(4):291-296.
doi pubmed - Rahajeng EP, Samad R, Muhiddin R. Identification of risk factors characteristics of transfusion reaction. Indones J Clin Pathol Med Lab. 2020;26(3):266-271.
- Abdulqader AMR, Mohammed AI, Mohammed NI. Red cell alloimmunization and autoimmunization in multi-transfused thalassemia patients in Sulaymaniyah Province-Iraq. Korean Journal of Clinical Laboratory Science. 2020;52(2):98-104.
- Singer ST, Wu V, Mignacca R, Kuypers FA, Morel P, Vichinsky EP. Alloimmunization and erythrocyte autoimmunization in transfusion-dependent thalassemia patients of predominantly asian descent. Blood. 2000;96(10):3369-3373.
pubmed - Keikhaei B, Yousefi H, Alghasi A, Sharhani A, Khazaei R. Frequency of red cell alloimmunization in patients with thalassemia major: a report from the southwest of Iran. Iranian Journal of Pediatric Hematology & Oncology. 2023.
- Avent ND, Reid ME. The Rh blood group system: a review. Blood. 2000;95(2):375-387.
pubmed - Chou ST, Jackson T, Vege S, Smith-Whitley K, Friedman DF, Westhoff CM. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122(6):1062-1071.
doi pubmed - Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390(10091):311-323.
doi pubmed - Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132-140.
doi pubmed - Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
doi pubmed - Almorish MAW, Al-Absi B, Elkhalifa AME, Alhamidi AH, Abdelrahman M. Red blood cell alloimmunization in blood transfusion-dependent beta thalassemia major patients in Sana'a City-Yemen. Sci Rep. 2024;14(1):1005.
doi pubmed - Chao YH, Wu KH, Lu JJ, Shih MC, Peng CT, Chang CW. Red blood cell alloimmunisation among Chinese patients with beta-thalassaemia major in Taiwan. Blood Transfus. 2013;11(1):71-74.
doi pubmed - Chaudhari CN. Red Cell Alloantibodies in multiple transfused thalassaemia patients. Med J Armed Forces India. 2011;67(1):34-37.
doi pubmed - Davari K, Soltanpour MS. Study of alloimmunization and autoimmunization in Iranian beta-thalassemia major patients. Asian J Transfus Sci. 2016;10(1):88-92.
doi pubmed - Dhawan HK, Kumawat V, Marwaha N, Sharma RR, Sachdev S, Bansal D, Marwaha RK, et al. Alloimmunization and autoimmunization in transfusion dependent thalassemia major patients: Study on 319 patients. Asian J Transfus Sci. 2014;8(2):84-88.
doi pubmed - Dogra A, Sidhu M, Kapoor R, Kumar D. Study of red blood cell alloimmunization in multitransfused thalassemic children of Jammu region. Asian J Transfus Sci. 2015;9(1):78-81.
doi pubmed - Ebrahimisadr P, Bakhshandeh Z, Majidiani H. Red cell alloantibodies in beta-thalassaemia major patients' blood referring to the regional blood transfusion center of Tehran, Iran. Bioimpacts. 2021;11(2):129-133.
doi pubmed - el-Danasoury AS, Eissa DG, Abdo RM, Elalfy MS. Red blood cell alloimmunization in transfusion-dependent Egyptian patients with thalassemia in a limited donor exposure program. Transfusion. 2012;52(1):43-47.
doi pubmed - Gholami MS, Shahidi M, Tabibian S, Naderi M, Dorgalaleh A. Genotyping of blood groups in alloimmunized patients with beta-thalassemia major by T-ARMS-PCR and multiplex-aso-pcr. Transfus Apher Sci. 2021;60(1):102984.
doi pubmed - Guirat-Dhouib N, Mezri M, Hmida H, Mellouli F, Kaabi H, Ouderni M, Zouari B, et al. High frequency of autoimmunization among transfusion-dependent Tunisian thalassaemia patients. Transfus Apher Sci. 2011;45(2):199-202.
doi pubmed - Iqbal I, Ahmed N. Frequency of red cell alloantibodies and autoantibodies in thalassemia major children. Biomedica. 2014;30(1):25-28.
- Jariwala K, Mishra K, Ghosh K. Comparative study of alloimmunization against red cell antigens in sickle cell disease & thalassaemia major patients on regular red cell transfusion. Indian J Med Res. 2019;149(1):34-40.
doi pubmed - Davoudi-Kiakalayeh A, Mohammadi R, Pourfathollah AA, Siery Z, Davoudi-Kiakalayeh S. Alloimmunization in thalassemia patients: new insight for healthcare. Int J Prev Med. 2017;8:101.
doi pubmed - Minhas K, Ejaz MS, Tukruna A, Haider M, Arif A, Saleem Tebha S. Red blood cell alloimmunization in pediatric group with beta thalassemia: a five-year experience. Glob Pediatr Health. 2022;9:2333794X221132679.
doi pubmed - Pazgal I, Yahalom V, Shalev B, Raanani P, Stark P. Alloimmunization and autoimmunization in adult transfusion-dependent thalassemia patients: a report from a comprehensive center in Israel. Ann Hematol. 2020;99(12):2731-2736.
doi pubmed - Seferi I, Xhetani M, Face M, Burazeri G, Nastas E, Vyshka G. Frequency and specificity of red cell antibodies in thalassemia patients in Albania. Int J Lab Hematol. 2015;37(4):569-574.
doi pubmed - Senavirathna SK, Ranasinghe SL, Thilakarathne YK. Estimation of prevalence of red cell alloantibodies in patients with Beta Thalassaemia Major in Sri Lanka. Ceylon Med J. 2021;66(2):96-99.
doi pubmed - Usman M, Saira M, Moinuddun M, Ahmad S, Perveen R, USMAN S. Frequency of red cell alloimmunization among patients with transfusion dependent beta thalassemia in Pakistan. International Journal of Hematology and Oncology. 2011;33(1):166-169.
- Zaidi U, Borhany M, Ansari S, Parveen S, Boota S, Shamim I, Zahid D, et al. Red cell alloimmunisation in regularly transfused beta thalassemia patients in Pakistan. Transfus Med. 2015;25(2):106-110.
doi pubmed - Zarrabian D, Hanna M. Characterization of pediatric transfusion-dependent thalassemia patients in a large academic center. J Clin Lab Anal. 2023;37(17-18):e24962.
doi pubmed - Hussein E, Ahmed Eldesoukey N, Rihan A, Kamal A. Predictors of red cell alloimmunization in multitransfused Egyptian patients with beta-thalassemia. Arch Pathol Lab Med. 2014;138(5):684-688.
doi pubmed - Keikhaei B, Far AH, Abolghasemi H, Mousakhani H, Ghanavat M, Moghadam M. Red blood cell alloimmunization in patients with thalassemia major and intermediate in southwest Iran. Iran J Blood Cancer. 2013;6(1):41-46.
- An GD, Kim K-H. Characteristics of red blood cell alloimmunization in patients with hematologic diseases. The Korean Journal of Blood Transfusion. 2022;33(1):14-23.
- Yadav BK, Chaudhary RK, Elhence P, Phadke SR, Mandal K, Saxena D, Moirangthem A. Red cell alloimmunization and associated risk factors in multiply transfused thalassemia patients: A prospective cohort study conducted at a tertiary care center in Northern India. Asian J Transfus Sci. 2023;17(2):145-150.
doi pubmed - Samarah F, Srour MA, Yaseen D, Dumaidi K. Frequency of red blood cell alloimmunization in patients with sickle cell disease in palestine. Adv Hematol. 2018;2018:5356245.
doi pubmed - Farmakis D, Porter J, Taher A, Domenica Cappellini M, Angastiniotis M, Eleftheriou A. 2021 Thalassaemia international federation guidelines for the management of transfusion-dependent thalassemia. Hemasphere. 2022;6(8):e732.
doi pubmed - Datta SS, Mukherjee S, Talukder B, Bhattacharya P, Mukherjee K. Frequency of red cell alloimmunization and autoimmunization in thalassemia patients: a report from eastern India. Adv Hematol. 2015;2015:610931.
doi pubmed - Poole J, Daniels G. Blood group antibodies and their significance in transfusion medicine. Transfus Med Rev. 2007;21(1):58-71.
doi pubmed - Al-Mousawi MM, Al-Allawi NA, Alnaqshabandi R. Predictors of red cell alloimmunization in Kurdish multi transfused patients with hemoglobinopathies in Iraq. Hemoglobin. 2015;39(6):423-426.
doi pubmed - El Kababi S, Benajiba M, El Khalfi B, Hachim J, Soukri A. Red blood cell alloimmunizations in beta-thalassemia patients in Casablanca/Morocco: Prevalence and risk factors. Transfus Clin Biol. 2019;26(4):240-248.
doi pubmed - Teawtrakul N, Songdej D, Hantaweepant C, Tantiworawit A, Lauhasurayotin S, Torcharus K, Sripornsawan P, et al. Red blood cell alloimmunization and other transfusion-related complications in patients with transfusion-dependent thalassemia: A multi-center study in Thailand. Transfusion. 2022;62(10):2039-2047.
doi pubmed - Mollahoseini Foomani F, Sadeghian MH, Bagheri S, Badiee Z, Bazargani R, Aryanpour Z, Hallajiayan S, et al. Frequency of Kell and Rh alloantibodies in Iranian thalassemia patients in Khorasan Razavi province, Iran. Int J Hematol Oncol Stem Cell Res. 2023;17(1):4-8.
doi pubmed - Al-Riyami AZ, Al-Muqbali A, Al-Sudiri S, Murthi Panchatcharam S, Zacharia M, Al-Mahrooqi S, Al-Hosni S, et al. Risks of red blood cell alloimmunization in transfusion-dependent beta-thalassemia in Oman: a 25-year experience of a university tertiary care reference center and a literature review. Transfusion. 2018;58(4):871-878.
doi pubmed - Compernolle V, Chou ST, Tanael S, Savage W, Howard J, Josephson CD, Odame I, et al. Red blood cell specifications for patients with hemoglobinopathies: a systematic review and guideline. Transfusion. 2018;58(6):1555-1566.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.