| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 1, January 2025, pages 51-59
Serum Vitamin D Level Correlates Significantly With Leptin and Tumor Necrosis Factor-Alpha in Overweight Postmenopausal Women With Hypertension
Dina M. Qahwajia , Abdulhalim Salim Serafib, j
, Shalan Alaamric
, Zahir Hussainb
, Mohammed A. Bafailb
, Christopher S. Gondid, e, f, g
, Lusine Demirkhanyand, e
, Rizwana Sanaullah Waraichh
, Sumera Sohaili
aDepartment of Clinical Nutrition, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah, Saudi Arabia
bDepartment of Physiology, Faculty of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia
cDepartment of Medicine, Faculty of Medicine, Jeddah University, Jeddah, Saudi Arabia
dDepartment of Medicine, University of Illinois College of Medicine in Peoria, Peoria, IL, USA
eDepartment of Surgery, University of Illinois College of Medicine in Peoria, Peoria, IL, USA
fDepartment of Health Sciences Education and Pathology, University of Illinois College of Medicine in Peoria, Peoria, IL, USA
gThe Grainger College of Engineering, University of Illinois Urbana-Champaign, Urbana, IL, USA
hDepartment of Biomedical & Biological Sciences, Biomedical Research Center, Sohail University, Karachi 78400, Pakistan
iDepartment of Physiology, Faculty of Science, University of Karachi, Karachi, Pakistan
jCorresponding Author: Abdulhalim Salim Serafi, Saleh Hamza Serafi Chair for Research in Coronary Heart Disease (SCRCHD), Scientific Research (DSR), Umm Al-Qura University (UQU), Makkah, Saudi Arabia; Department of Physiology, Faculty of Medicine, Umm Al-Qura University (UQU), Makkah 21955, Saudi Arabia
Manuscript submitted November 20, 2024, accepted January 10, 2025, published online January 14, 2025
Short title: VitD, Leptin and TNF-α in OW-HT PMP Women
doi: https://doi.org/10.14740/jocmr6148
| Abstract | ▴Top |
Background: Association of serum vitamin D (vitD) with leptin (Lep) and tumor necrosis factor-alpha (TNF-α) is not precisely known in overweight hypertensive (OW-HT) postmenopausal (PMP) women. Hence, the present study was carried out to investigate the body mass index (BMI)-based correlation of serum vitD with Lep and TNF-α in OW-HT PMP women.
Methods: Women subjects in their early PMP (n = 346, age: 51 - 60 years) categorized into three groups had main inclusion criteria of specified range of age, BMI and blood pressure (BP). Enzyme-linked immunosorbent assay (ELISA) and other kit methods were employed to investigate the role of various variables in three subject groups (normal weight normotensive (NW-NT, n = 116, BMI (kg/m2): 22 - 24.9), normal weight hypertensive (NW-HT, n = 115, BMI: 22 - 24.9) and OW-HT (n = 115, BMI: 25 - 29.9) PMP women).
Results: A significant negative linear correlation of vitD with serum Lep and TNF-α, and a significant positive linear correlation of BMI with Lep and TNF-α in OW-HT PMP women were obtained. Significantly higher levels of serum Lep, TNF-α and interleukin-6 (IL-6) were found in OW-HT PMP women, as compared to NW-HT PMP women.
Conclusions: The present study suggests that decreased serum vitD levels correlate with the Lep and TNF-α in OW-HT PMP women. However, further studies may help understand the impact of vitD in cardiovascular events and the influencing factors in OW-HT PMP women.
Keywords: Postmenopausal women; Overweight hypertensives; Serum vitamin D; Leptin; TNF-α
| Introduction | ▴Top |
Cardiovascular disease (CVD), type II diabetes mellitus (T2DM) and other cardiometabolic diseases (CMDs) are the main causes of mortality and morbidity [1]. It is well known that lifestyle including body weight (BW) management helps manipulate blood pressure (BP) [1]. Hypertension (HT) is a very common risk factor [1] that occurs generally in overweight (OW) [2] and obese people [3], leading to various forms of CVD [4]. Patients with HT and OW/obesity need proper management, especially the change in lifestyle [5]. Furthermore, decreasing the BW reduces the risk of HT even for those who were never OW or obese [5]. Hence, proper management of lifestyle and medication are essentially required.
The role of a variety of factors/biomarkers has been studied in postmenopausal (PMP) women to understand their association with vitamin D (vitD) in OW/obesity status and HT [1]. Some of these factors are: total cholesterol (TC), triglycerides (TG), homocysteine (Hcy), leptin (Lep), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), other adipocytokines, and proinflammatory and anti-inflammatory markers. However, controversial results were obtained for the involvement of mentioned factors in OW/obesity status or HT [1-3, 5]. No significant association could be obtained for vitD with Lep or TNF-α in healthy normal weight normotensive (NW-NT) subjects [6-8] and low-grade normal weight hypertensive (NW-HT) subjects [9, 10]. The significant association of vitD with Lep or TNF-α in varying body mass index (BMI) or low-grade HT is either controversial or not evident [11, 12].
Low serum levels of vitD (25(OH)D) have been suggested to be associated with tissue inflammation, obesity and a variety of coronary heart disease (CHD) conditions in PMP women [12]. It was explained by the possible mechanisms that vitD receptors are present in smooth muscle and endothelial cells whereby vitD improves the endothelial activity [12].
It was suggested that OW and obese PMP women are linked with CVD but the inflammation via proinflammatory markers, e.g., Lep, TNF-α and IL-6, might have a significant impact on the development of HT [13]. However, since no clearly known impact of the mentioned factors is yet known, and the mechanisms involved in overweight hypertensive (OW-HT) PMP women are not fully understood, further studies are required to clarify the interactive impact of various factors on NW-NT, NW-HT and OW-HT PMP women. The role of vitD in association with Lep and TNF-α in OW-HT is especially not known. Hence, the present study was proposed to find the change and association of vitD with Lep and TNF-α and other related factors in NW-NT, NW-HT and OW-HT PMP women.
| Materials and Methods | ▴Top |
Subjects and data collection
This research work was planned and the proposal was submitted to the College of Medicine, Umm Al-Qura University (UQU), Makkah, for Institutional Review Board approval. The proposal was accepted and finally approved (approval no. HAPO-02-K-012-2021-02-124), for collecting the data and conducting the planned study. The present study was carried out in the hospitals affiliated to UQU from June 2021 till June 2023. This study was conducted in compliance with the ethical standards of the mentioned institution on human subjects as well as with the Helsinki Declaration.
The consent of the subjects was obtained before consultation. The subjects were informed about the nature of the planned work and its implications. It was up to them whether they liked or not to participate in the present study. The subjects who were willing to take part in the current study as volunteers provided the history of their past medical examinations and medication, and they willingly provided the blood samples for this study. The sample size (n) was calculated employing the formula: n = z2 × p × (1 - p)/e2, where z, p and e represent confidence level (α), proportion and margin of error or confidence interval, respectively. To confirm it further, the sample size calculator was used for assessing the number of subjects/samples required for the present study.
The total number of PMP women in the study was 346 (age range: 51 - 60 years). They were categorized into three groups as: group 1: NW-NT (n = 116), group 2: NW-HT (n = 115) and group 3: OW-HT (n = 115). The subjects in all groups in the present study had the specified level of BP. Systolic BP (SBP)/diastolic BP (DBP) having grade 1 HT were in the range of 140 - 159/90 - 99 mm Hg [5]. Women subjects with grade 2 HT (SBP/DBP: 160 - 179/100 - 109) and grade 3 HT (SBP/DBP: ≥ 180/≥ 110) [5] were not taken for the current study. Subjects included in the study were in a specified range of BMI (kg/m2). The BMI range for NW-NT, NW-HT and OW-HT groups was 22 - 24.9, 22 - 24.9 and 25 - 29.9, respectively. Any subject with lower or higher than the mentioned BMI range was not entertained for the present study. Subjects in the present report were studied in their early PMP.
The subjects included in each of the three groups were in specified range of age, BMI and BP. However, the main inclusion criteria related to the confirmation of PMP were used in the selected age range of subjects (51 - 60 years) for all three women groups. Hence, PMP women with age lower than 51 years or higher than 60 years were not included in the present study.
The study design relied upon the criteria of the study. The inclusion criteria were the confirmation of PMP in the selected age range of all subject groups (51 - 60 years), and the BMI and BP range values mentioned in the above paragraph. Only the subjects visiting hospitals from June 2021 till June 2023 were included in the present study. The exclusion criteria were smoking, diabetes mellitus (DM), endocrinological disease, cardiac/CVD (except grade 1 HT), grade 2 and 3 HT, chronic respiratory diseases, stroke, chronic inflammatory disease, renal/hepatic disease, hepatitis B virus (HBV)/hepatitis C virus (HCV) infection, or any other serious discomfort. We selected the NW-NT, NW-HT and OW-HT PMP women that were not under pharmacotherapy.
Methods
The BMI (kg/m2) was determined by dividing the BW (kilograms) by the square of body height (meters) [14, 15]. The BMI levels for NW-NT, NW-HT and OW-HT women were followed from another study [16]. The BPs (SBP and DBP) determined by the routine methods [17] in the NW-NT subjects were in the normal range and NW-HT and OW-HT subjects were in the grade 1 HT range following established reports [18, 19]. The subjects only with grade 1 HT in the range of 140 - 159/90 - 99 [5] were included. A mercury sphygmomanometer (MS-S1500 Mercury Sphygmomanometer, Medical Sources Co., Ltd, Nanjing, China) was employed for measuring the BP. The BP was measured based on two readings taken on two occasions in a comfortable sitting position in the health care setting by the same person recommended for taking the history of the subjects for proper diagnosis. The subjects were recommended to have the check-up of their BP in a suitable occasion without having tension, hurry or any other inconvenience. The subject was instructed to sit comfortably for about 15 min after reaching in the health care setting, and then to proceed for getting check-up of BP. The BP was taken in an airy and a quiet room or hall set at normal temperature. Improper positioning during measurements was not recommended. For an accurate reading, the subject/patient seated in a chair with the feet flat on the floor.
Blood samples of NW-NT, NW-HT and OH-HT groups were obtained in a fasting condition in the morning. The enzyme-linked immunosorbent assay (ELISA) kits were used for analyzing the serum Lep, vitD, TNF-α, IL-6, TC, TG and Hcy. Serum Lep was determined employing a Lep human ELISA kit (ab100581) - the Abcam’s leptin human ELISA (Cambridge, UK), serum vitD was estimated using the ELISA kit following the manufacturer’s instructions (Euroimmun, Lubeck, Germany), serum TNF-α was determined using ELISA assay (catalog no. STA00C, Quantikine® ELISA, R&D Systems Inc., Minneapolis, MN, USA), and serum IL-6 was determined by using ELISA-kit 96T (catalogue no. ELH-IL6, RayBio, USA). The TC was estimated by routine kit methods/enzymatic colorimetric method. Other variables (TG and Hcy) were measured using kit methods.
Statistical analysis
The Statistical Package for Social Sciences (version 24.0) for Windows (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The GraphPad Prism (version 6.0) software (San Diego, CA, USA) was also employed where required. Mean ± standard deviation (SD) values of the analyzed data were obtained as a distribution of the quantitative characteristics corresponding to normal one. The one-way analysis of variance (ANOVA) was obtained for comparing three groups of the PMP women. The results obtained were further confirmed by Tukey-Kramer post hoc test. Regression lines were plotted to determine the significance of associations and the positive or negative correlations/associations. Coefficient of determination (R2) was obtained that indicated the level of significance. Value of significance (P) was obtained considering the previously published biostatistical applications [20] as P ≤ 0.05.
| Results | ▴Top |
The characteristics and parameters of the study in PMP women (n = 346; age range: 51 - 60 years) are given in Table 1. The Tukey-Kramer post hoc test showed that the Lep and TNF-α presented highly significant increases for OW-HT vs. NW-NT, OW-HT vs. NW-HT and NW-HT vs. NW-NT (P < 0.001). Serum vitD showed a close to significant decrease for OW-HT vs. NW-NT and OW-HT vs. NW-HT. Serum IL-6 decreased with P < 0.09 for NW-HT vs. NW-NT. However, other comparisons (OW-HT vs. NW-NT and OW-HT vs. NW-HT) for IL-6 indicated an increase with high significance (P < 0.001).
 Click to view | Table 1. Characteristic Features and Related Parameters Among Groups in Postmenopausal Women (n = 346) |
The ANOVA (Table 1) showed that the mean ± SD of the age (years) of the three groups of women did not vary significantly (P > 0.05). The BMI (kg/m2) range of NW-NT, NW-HT and OW-HT was 22 - 24.9, 22 - 24.9 and 25 - 29.9, respectively. A highly significant difference among groups was obtained for BMI, and serum levels of Lep, TNF-α and IL-6 (P < 0.001) (Table 1).
The BMI of NW-NT showed a significant linear correlation with TC (R2: 0.036, P = 0.04) and IL-6 (R2: 0.037, P = 0.04) (Table 2). A positive linear correction of BMI with TNF-α (R2: 0.028, P = 0.07) for NW-NT was close to significant association. The other parameters of NW-NT did not show any significant correlation (P > 0.05) (Table 2). The women group of NW-HT presented a highly positive linear correlation for BMI against TNF-α (R2: 0.24, P < 0.001). All other parameters in this group showed non-significant association with BMI (P > 0.05) (Table 2). However, the OW-HT group showed a positive linear correlation for BMI against Lep (R2: 0.037, P = 0.04) and TNF-α (R2: 0.288, P < 0.001) (Table 2). Serum vitD correlated negatively and highly significantly with BMI (R2: 0.350, P < 0.001). Other variables in this group were not found having significant correlation with BMI (P > 0.05).
 Click to view | Table 2. Correlation of BMI With Other Variables Based on BMI in Postmenopausal Women |
The analysis of the association of serum Lep with other variables (Table 3) showed a highly significant negative correlation of serum Lep and vitD only for the OW-HT group (R2: 0.106, P < 0.001). Other significant linear correlations of Lep were found with IL-6 (R2: 0.067, P = 0.005) and TNF-α (R2: 0.070, P < 0.004) in NW-NT women subjects (Table 3).
 Click to view | Table 3. Correlation of Serum Lep With Other Variables on the Basis of BMI in Postmenopausal Women |
The data of NW-HT presented a highly significant linear correlation of Lep with TNF-α (R2: 0.147, P < 0.001). Other variables did not show any significant correlation with Lep (P = 0.05) (Table 3). The results for the OW-HT group of women showed a highly significant correlation of Lep with TNF-α (R2: 0.149, P < 0.001). The TC, TG and IL-6 did not show significant correlation. However, Hcy was found to have a close to significant correlation with Lep in this group (R2: 0.028, P = 0.08) (Table 3).
It was quite interesting to note that a significant negative linear correlation of vitD was found with TNF-α and Lep for the OW-HT group of PMP women (R2: 0.241, P < 0.001) (Table 4).
 Click to view | Table 4. Correlation of Serum vitD With Other Variables Based on BMI in Postmenopausal Women |
The plot of serum vitD against Lep levels showed a highly significant negative linear correlation of serum vitD with Lep in OW-HT PMP women (P < 0.0004, Fig. 1). Figure 2 presents a highly significant (P < 0.0001) negative linear correlation for vitD and TNF-α.
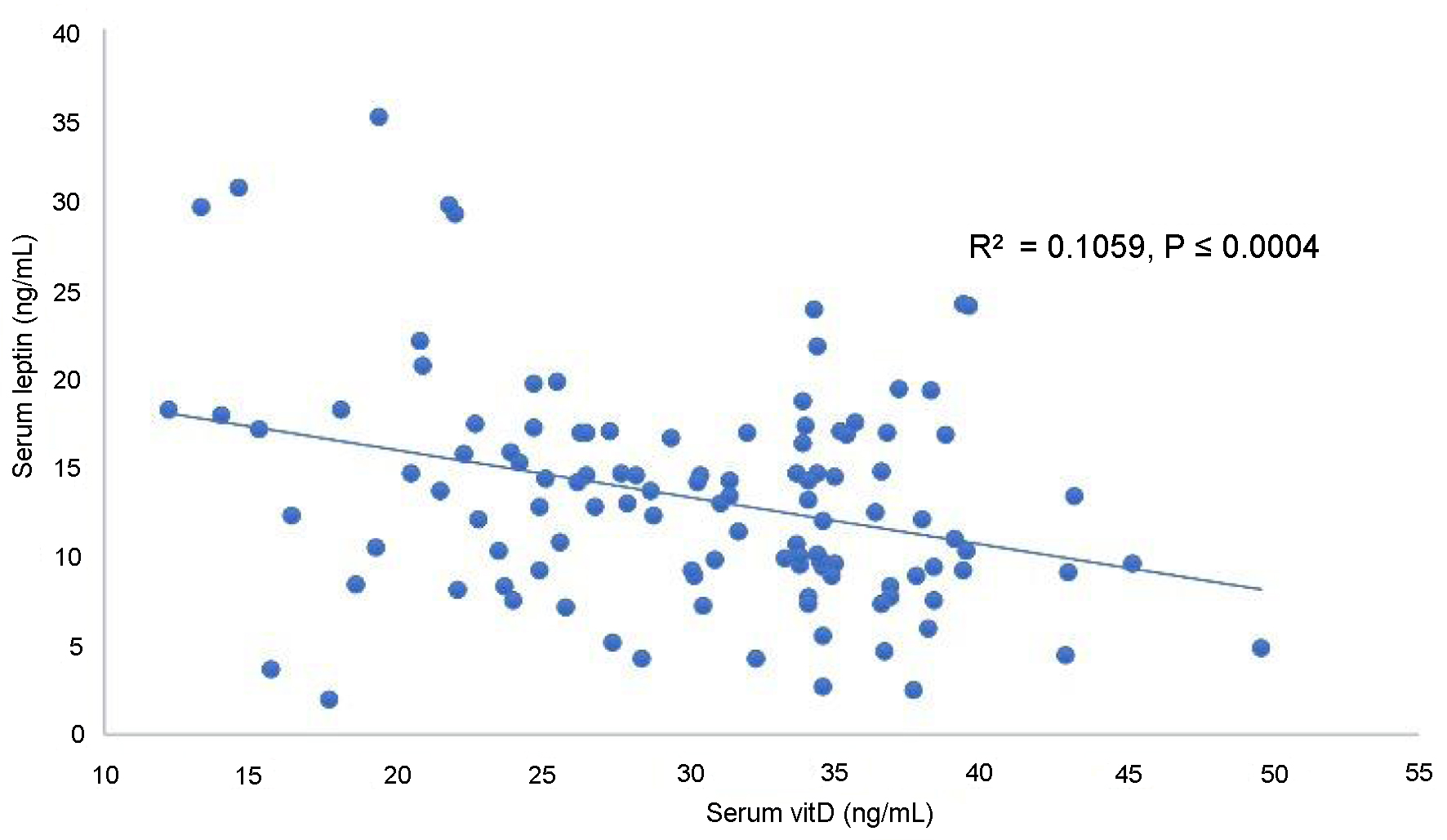 Click for large image | Figure 1. Correlation of serum vitamin D with leptin in overweight hypertensive postmenopausal women. |
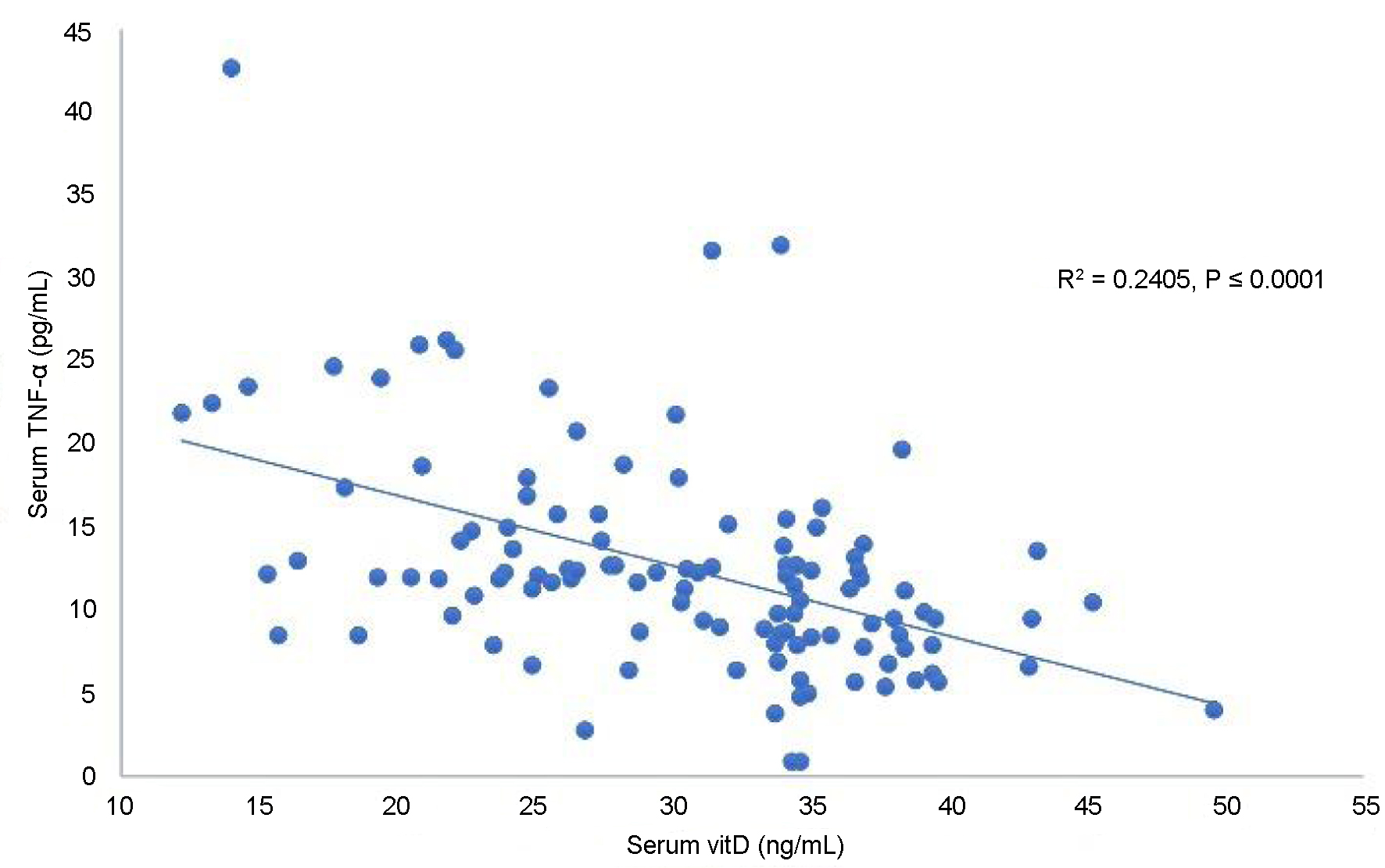 Click for large image | Figure 2. Correlation of serum vitamin D with TNF-α in overweight hypertensive postmenopausal women. TNF-α: tumor necrosis factor-alpha. |
| Discussion | ▴Top |
Consensus could not be established about the interactive impact/involvement of various factors in obesity/OW status, HT and HT with OW or obesity status [21]. Hence, the present study aimed to find the serum levels of various parameters including vitD, Lep, TNF-α, IL-6, and other variables, their comparisons and correlations with each other in NW-NT, NW-HT and OW-HT PMP women.
Various forms of CVD occur worldwide by various factors including substantial increase in BW [3, 4] that may lead to several chronic disorders including high BP/HT [22]. A gradual increase in BP occurs with an increase in BW or BMI over the life course including late in life [23]. A little change in BW was found in response to lifestyle changes [21]. However, the proper management of lifestyle and medication are essentially required regardless of any grade of HT [5].
Women with frequent CVD risks and obesity showed vitD deficiency and the BMI-associated vitD deficiency and polymorphism associated with CVD discomforts in PMP women [24]. These reports indirectly explain our present results obtained for OW-HT PMP women.
An animal study conducted in mice for understanding the role of vitD revealed that decreased levels of vitD caused CVD complications, increased BW and change in proinflammatory biomarkers [25]. This report [25] provides evidence for the results obtained in the present study and helps understand PMP changes occurring in the brain and provides opportunity to conduct studies for the therapeutic impact using vitD therapies [26].
The patients with HT and OW/obesity need proper lifestyle management that decreases the BW, reduces the risk of HT [5] and produces change in the perivascular adipose tissue (PVAT) releasing Lep, and other cytokines/chemokines to the vascular wall and endothelial and inflammatory functions. This indirectly reveals a mechanism whereby significant association was obtained for serum Lep and TNF-α plotted against serum vitD only for OW-HT group of PMP women in the present study. VitD was not found associated with BW and BMI in a meta-analysis study of the randomized controlled trials [11]. Since this study [11] provides findings related to vitD administration in obese PMP women without HT, it differs and does not provide interpretation for our data of OW-HT women.
The second important investigation in the current work in PMP women with OW-HT is the negative linear significant correlation of vitD and TNF-α. The present study reveals the significant role of vitD influencing the OW-HT women via the mechanism partly involving increase in the serum level of TNF-α. It can be interpreted by a previous suggestion that low serum levels of vitD are associated with the tissue injury, obesity and CHD complications [12].
No significant correlation of vitD with Lep or TNF-α was found in the healthy subjects (NW-NT) with similar range of BMI as for healthy NW subject group in the present study. Other reports [8-10] provide confirmation for the results obtained for NW-HT in the present study.
Decreased serum vitD leading to increase in the levels of Lep and TNF-α in the present study relates to the proposed mechanism of inflammatory processes leading to CVD complications wherein the inflammatory status facilitated by the cardiovascular risk factors is regulated by the mechanisms related to immune system activation [27]. This investigation is verified by increased TNF-α and other proinflammatory cytokines causing inflammation [28]. The adipose tissue, fat formation, and OW/obesity are associated with the inflammatory processes through the release of proinflammatory and anti-inflammatory factors [28] and decreased blood supply to adipocytes that causes hypoxia. It then becomes a cascade causing further inflammation by releasing TNF-α for decreasing nitric oxide (NO) in blood vessels and vascular dysfunction.
The significant role of vitD can be interlinked with the immune and inflammatory processes [29]. Quite interesting and valuable information was obtained for cell signaling molecules and immune cells involving genes, genetic loci, inflammation and immune system in the progression and development of HT [29]. This can be explained by the cross-link for the immune system-adiposity-inflammation-BP [30] having a major role in HT with OW/obesity status that seems under the influence of decreased levels of vitD.
The TNF-α is an important and effective proinflammatory adipocytokine that is involved in causing low-grade inflammation and influencing CVD involving atherosclerosis and other disorders. The OW and inflammation in HT influences TNF-α [31] that seems an effect of decreased concentrations of vitD investigated in the present study. There are a variety of immune factors involved in regulating the immune processes in vascular pathologies caused by adipose tissue-derived proinflammatory cytokines and other adipokines [32]. Our present study reveals that probable immune-related inflammatory changes in OW-HT women showed higher levels of Lep and TNF-α, and other factors better characterizing the cardiovascular risk in HT patients. Further studies are needed to be conducted to clarify the role of vitD involvement [27].
Various inflammatory markers elevate in HT [33]. However, it is still not clearly known whether BP changes/HT occur in response to inflammatory changes, or inflammatory changes occur in response to BP changes/HT, as has been revealed for Lep [34], or they both occur at the same time due to other changes/risk factors including age, smoking, use of menopausal hormone therapy, etc. The immune mechanisms related to immune system activation explain our observations in the current study. Inflammatory processes are involved in the pathogenesis and continuation of CVD complications and the inflammatory markers may serve as emergent therapeutic targets [35]. Increased levels of serum Lep and TNF-α in the present study fit nicely in the proposed mechanism of inflammatory processes leading to CVD complications under the influence of decreased concentration of vitD.
Our present report is a well-controlled study. We selected women subjects with a thorough assessment for the PMP stage following the study criteria and a specified age range in all three groups with a range of BMI and HT. Furthermore, our subjects were not smokers and had no serious disorders. However, there are several limitations in the current study. We could not study the higher levels of BMI, HT and comorbidities for comparison purposes. We studied subjects with OW but could not include the PMP women with obesity of various categories. Hence, further studies are required to be conducted in PMP women subjects/patients for various grades of HT and BMI to have a better idea about the intricate association of vitD with the proinflammatory biomarkers (Lep, TNF-α, etc.). Another important demerit in the present investigation was mainly owing to the financial reasons. Some of the proinflammatory markers were assessed but the anti-inflammatory markers could not be studied in PMP women.
Conclusion
In view of a strong negative linear correlation of vitD with serum Lep and TNF-α in OW-HT women, we suggest that the decreased vitD levels consequently increase the serum levels of Lep and TNF-α in OW-HT PMP women. Further relevant studies may help understand the cardiovascular events and the influencing factors in OW-HT PMP women.
Acknowledgments
The authors like to thank the Deanship of Scientific Research at Umm Al-Qura University, Makkah, Saudi Arabia, for the continuous support.
Financial Disclosure
This work was supported financially by the Deanship of Scientific Research at Umm Al-Qura University, Makkah, Saudi Arabia (grant code: 19-MED-1-01-0022).
Conflict of Interest
There is no conflict of interest.
Informed Consent
Informed consent was obtained from the subjects involved in the study.
Author Contributions
Conceptualization and study design: ASS and ZH. Data collection and processing: DMQ, ASS, SA, ZH and MAB. Analysis and interpretation: DMQ, ASS, SA, ZH, MAB, CSG, LD, RSW and SS. Writing - original draft: ASS and ZH. Draft review and editing: DMQ, ASS, SA, ZH, MAB, CSG, LD, RSW and SS. Supervision: ASS and ZH. Resources: ASS.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
ANOVA: analysis of variance; BMI: body mass index; BP: blood pressure; BW: body weight; CHD: coronary heart disease; CMD: cardiometabolic disease; CVD: cardiovascular disease; DBP: diastolic blood pressure; DM: diabetes mellitus; ELISA: enzyme-linked immunosorbent assay; HBV: hepatitis B virus; HCV: hepatitis C virus; Hcy: homocysteine; HT: hypertension/hypertensive; IL-6: interleukin-6; Lep: leptin; n: number of subjects/samples; NO: nitric oxide; NT: normotensive/normotensives; NW-HT: normal weight hypertensive; NW-NT: normal weight normotensive; OW: overweight; OW-HT: overweight hypertensive; PMP: postmenopause/postmenopausal; PVAT: perivascular adipose tissue; R2: coefficient of determination; SBP: systolic blood pressure; SD: standard deviation; SPSS: Statistical Package for Social Sciences; T2DM: type II diabetes mellitus; TC: total cholesterol; TG: triglycerides; TNF-α: tumor necrosis factor-alpha; UQU: Umm Al-Qura University; VitD: vitamin D
| References | ▴Top |
- WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7(10):e1332-e1345.
doi pubmed pmc - Ghomari-Boukhatem H, Bouchouicha A, Mekki K, Chenni K, Belhadj M, Bouchenak M. Blood pressure, dyslipidemia and inflammatory factors are related to body mass index in scholar adolescents. Arch Med Sci. 2017;13(1):46-52.
doi pubmed pmc - Jaacks LM, Vandevijvere S, Pan A, McGowan CJ, Wallace C, Imamura F, Mozaffarian D, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 2019;7(3):231-240.
doi pubmed pmc - Fantin F, Giani A, Zoico E, Rossi AP, Mazzali G, Zamboni M. Weight loss and hypertension in obese subjects. Nutrients. 2019;11(7):1667.
doi pubmed pmc - Whelton PK, Carey RM, Aronow WS, Casey DE, Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248.
doi pubmed - Fatima SS, Farooq S, Tauni MA, Irfan O, Alam F. Effect of raised body fat on vitamin D, leptin and bone mass. J Pak Med Assoc. 2015;65(12):1315-1319.
pubmed - Dinca M, Serban MC, Sahebkar A, Mikhailidis DP, Toth PP, Martin SS, Blaha MJ, et al. Does vitamin D supplementation alter plasma adipokines concentrations? A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2016;107:360-371.
doi pubmed - Madhu SV, Aslam M, Mishra BK, Gupta A, Jhamb R. Association of 25 (OH) vitamin D and leptin in individuals with insulin resistance. Indian J Endocrinol Metab. 2022;26(5):435-438.
doi pubmed pmc - Jorde R, Grimnes G. Lost relation between blood pressure and serum 25-hydroxyvitamin D. Blood Press. 2019;28(1):64-73.
doi pubmed - Baniasad A, Mokhtari Ardekan A, Najafzadeh MJ, Mousavi Mehdiabadi F. The relationship between vitamin D and short-term blood pressure variability. Blood Press Monit. 2023;28(4):193-198.
doi pubmed - Hao L, Lu A, Gao H, Niu J, Prabahar K, Seraj SS, Pan Y. The effects of vitamin D on markers of glucose and obesity in postmenopausal women: a meta-analysis of randomized controlled trials. Clin Ther. 2023;45(9):913-920.
doi pubmed - Norman PE, Powell JT. Vitamin D and cardiovascular disease. Circ Res. 2014;114(2):379-393.
doi pubmed - Gordon JH, LaMonte MJ, Zhao J, Genco RJ, Cimato TR, Hovey KM, Andrews CA, et al. The association between serum inflammatory biomarkers and incident hypertension among postmenopausal women in the Buffalo OsteoPerio Study. J Hum Hypertens. 2021;35(9):791-799.
doi pubmed pmc - Alaamri S, Serafi AS, Hussain Z, Alrooqi MM, Bafail MA, Sohail S. Blood pressure correlates with serum leptin and body mass index in overweight male Saudi students. J Pers Med. 2023;13(5):828.
doi pubmed pmc - Shah NR, Braverman ER. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS One. 2012;7(4):e33308.
doi pubmed pmc - Harakeh S, Kalamegam G, Pushparaj PN, Al-Hejin A, Alfadul SM, Al Amri T, Barnawi S, et al. Chemokines and their association with body mass index among healthy Saudis. Saudi J Biol Sci. 2020;27(1):6-11.
doi pubmed pmc - Oh SY, Ryue J, Hsieh CH, Bell DE. Eggs enriched in omega-3 fatty acids and alterations in lipid concentrations in plasma and lipoproteins and in blood pressure. Am J Clin Nutr. 1991;54(4):689-695.
doi pubmed - Basile JN. Rationale for fixed-dose combination therapy to reach lower blood pressure goals. South Med J. 2008;101(9):918-924.
doi pubmed - de Faria AP, Modolo R, Fontana V, Moreno H. Adipokines: novel players in resistant hypertension. J Clin Hypertens (Greenwich). 2014;16(10):754-759.
doi pubmed pmc - Zahir H, Javaid A, Rehman R, Hussain Z. Statistical concepts in biology and health sciences. J Ayub Med Coll Abbottabad. 2014;26(1):95-97.
pubmed - Wong CY, Byrne NM, O’Moore-Sullivan T, Hills AP, Prins JB, Marwick TH. Effect of weight loss due to lifestyle intervention on subclinical cardiovascular dysfunction in obesity (body mass index >30 kg/m2). Am J Cardiol. 2006;98(12):1593-1598.
doi pubmed - Ertek S, Francesco Cicero A, Erdogan G. The relationship between calcium metabolism, insulin-like growth factor-1 and pulse pressure in normotensive, normolipidaemic and non-diabetic patients. Arch Med Sci. 2011;7(5):776-780.
doi pubmed pmc - Shihab HM, Meoni LA, Chu AY, Wang NY, Ford DE, Liang KY, Gallo JJ, et al. Body mass index and risk of incident hypertension over the life course: the Johns Hopkins Precursors Study. Circulation. 2012;126(25):2983-2989.
doi pubmed pmc - Zhang W, Yi J, Liu D, Wang Y, Jamilian P, Gaman MA, Prabahar K, et al. The effect of vitamin D on the lipid profile as a risk factor for coronary heart disease in postmenopausal women: a meta-analysis and systematic review of randomized controlled trials. Exp Gerontol. 2022;161:111709.
doi pubmed - Borges CC, Bringhenti I, Mandarim-de-Lacerda CA, Aguila MB. Vitamin D deficiency aggravates the liver metabolism and inflammation in ovariectomized mice. Biomed Pharmacother. 2018;107:878-888.
doi pubmed - Siebert C, Berto CG, Ferreira FS, Moreira DS, Dos Santos TM, Wyse ATS. Vitamin D partially reverses the increase in p-NF-kappaB/p65 immunocontent and interleukin-6 levels, but not in acetylcholinesterase activity in hippocampus of adult female ovariectomized rats. Int J Dev Neurosci. 2018;71:122-129.
doi pubmed - Perticone M, Zito R, Miceli S, Pinto A, Suraci E, Greco M, Gigliotti S, et al. Immunity, inflammation and heart failure: their role on cardiac function and iron status. Front Immunol. 2019;10:2315.
doi pubmed pmc - Lafontan M. Fat cells: afferent and efferent messages define new approaches to treat obesity. Annu Rev Pharmacol Toxicol. 2005;45:119-146.
doi pubmed - Rodriguez-Iturbe B, Pons H, Johnson RJ. Role of the immune system in hypertension. Physiol Rev. 2017;97(3):1127-1164.
doi pubmed pmc - Kaur J, Mukheja S, Varma S, Kalra HS, Khosa BS, Vohra K. Serum progranulin/tumor necrosis factor-alpha ratio as independent predictor of systolic blood pressure in overweight hypertensive patients: a cross-sectional study. Egypt Heart J. 2020;72(1):25.
doi pubmed pmc - Goncalves CV, Ribeiro IS, Galantini MPL, Muniz IPR, Lima PHB, Santos GS, da Silva RAA. Inflammaging and body composition: new insights in diabetic and hypertensive elderly men. Exp Gerontol. 2022;170:112005.
doi pubmed - Aladhami AK, Unger CA, Ennis SL, Altomare D, Ji H, Hope MC, 3rd, Velazquez KT, et al. Macrophage tumor necrosis factor-alpha deletion does not protect against obesity-associated metabolic dysfunction. FASEB J. 2021;35(7):e21665.
doi pubmed pmc - Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens. 2005;19(2):149-154.
doi pubmed - Asferg C, Mogelvang R, Flyvbjerg A, Frystyk J, Jensen JS, Marott JL, Appleyard M, et al. Leptin, not adiponectin, predicts hypertension in the Copenhagen City Heart Study. Am J Hypertens. 2010;23(3):327-333.
doi pubmed - Zhang J, Xie F, Yun H, Chen L, Muntner P, Levitan EB, Safford MM, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75(10):1813-1818.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.