| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Review
Volume 17, Number 1, January 2025, pages 1-13
Strategies in Management of Pulmonary Embolism With Acute Ischemic Stroke: A Systematic Review
Sheilabi Seeburuna, c , Carlos Valladaresa
, Jose Iglesiasb
aDepartment of Internal Medicine, Rutgers Health - Community Medical Center, Toms River, NJ 08755, USA
bDepartment of Nephrology, Rutgers Health - Community Medical Center, Toms River, NJ 08755, USA
cCorresponding Author: Sheilabi Seeburun, Department of Internal Medicine, Rutgers Health - Community Medical Center, Toms River, NJ 08755, USA
Manuscript submitted November 23, 2024, accepted January 9, 2025, published online January 14, 2025
Short title: Management of PE With AIS
doi: https://doi.org/10.14740/jocmr6153
| Abstract | ▴Top |
Pulmonary embolism (PE) and acute ischemic stroke (AIS) are serious conditions with high morbidity and mortality. In the USA, PE causes around 100,000 deaths annually, with higher incidence in males. AIS following PE occurs in 1-10% of cases and is a leading cause of death within 2 - 4 weeks post-stroke. Managing concurrent PE and AIS is complex due to the need for anticoagulation, which is contraindicated after thrombolysis for AIS. This review evaluates the impact of various PE treatments - anticoagulation, thrombolysis, and embolectomy - on mortality in patients with both conditions. Following PRISMA 2020 guidelines, a systematic review was conducted across six databases from January 2010 to December 2023. The primary outcome measured was mortality, comparing treated vs. untreated patients for PE. Secondary outcomes included marked symptom improvement, slight improvement or deterioration of symptoms, and the complications. Data were analyzed descriptively, summarizing patient demographics, clinical characteristics, and treatment outcomes. Treatment modalities, such as anticoagulation, thrombolysis, catheter-directed thrombectomy, surgical thrombectomy, and conservative management, were evaluated based on their impact on symptom improvement, survival, and mortality. Initial querying of six databases yielded 1,679 articles, with only 21 remaining after a thorough review. Thrombolysis led to 100% symptom improvement and survival, with 0% mortality. Anticoagulation resulted in symptom improvement and survival in 62.5% of cases, with a 12.5% mortality rate. Catheter-directed and surgical thrombectomy had symptom improvement and survival in 66.7% and 75% of cases, respectively, with no mortality. Conservative management, defined here as management without anticoagulation or thrombolytic therapy, was associated with symptom worsening or no improvement and 50% mortality. This systematic review, based on observational data from case reports, highlights the diverse strategies used by physicians. Proactive and aggressive treatments, especially thrombolysis, show better outcomes and lower mortality rates. However, specific recommendations cannot be made from these results alone, emphasizing the need for well-designed prospective, randomized controlled trials to design structured guidelines for healthcare providers.
Keywords: Pulmonary embolism; Ischemic stroke; Case reports; Anticoagulation; Thrombolysis; Mechanical thrombectomy; Catheter-directed thrombolysis; Patent foramen ovale
| Introduction | ▴Top |
Pulmonary embolism (PE) and acute ischemic stroke (AIS) are significant medical conditions associated with significant morbidity and mortality. In the USA, PE is a major cause of death, with around 100,000 fatalities each year [1]. The incidence of PE is higher in males, with 56 cases per 100,000 compared to 48 per 100,000 in females [2]. AIS following PE occurs in 1-10% of cases, and PE is the leading cause of death within the first 2 - 4 weeks post-stroke [3-5]. When PE and AIS occur concurrently, management becomes particularly complex due to the need for anticoagulation in PE, which is contraindicated after pharmacological thrombolysis for ischemic stroke due to the increased risk of cerebral bleeding [6]. The lack of a standardized management protocol for this scenario highlights the urgent need for effective strategies.
The objective of this study is to evaluate the impact of various treatments for PE, including anticoagulation therapy, thrombolysis, and embolectomy, compared to no treatment, on mortality among patients concurrently diagnosed with AIS. Secondary outcomes include assessing improvement and deterioration in symptoms and incidence of complications associated with these interventions.
| Methods | ▴Top |
The systematic review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guidelines [7] as shown in Figure 1.
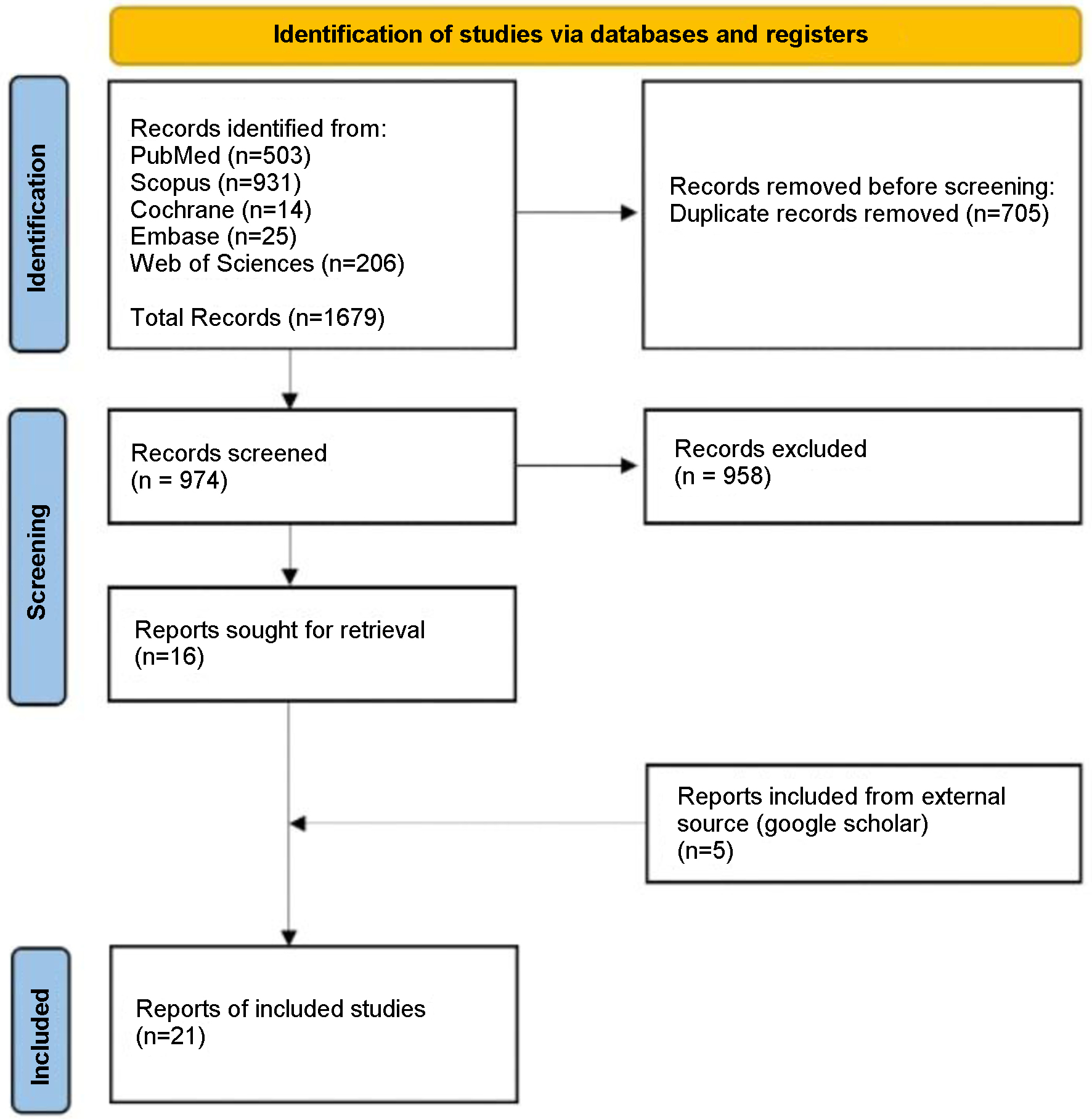 Click for large image | Figure 1. Article selection flow sheet per PRISMA 2020 guidelines [7]. |
Inclusion and exclusion criteria
Studies included in this review consisted of case reports, case series, and abstracts documenting the concurrent presentation of AIS and PE. Both diagnoses had to be either simultaneous or within 96 h prior or subsequent to each other. Confirmation of PE and ischemic stroke was required through computed tomography pulmonary angiography (CTPA) and brain imaging (computed tomography (CT) and/or magnetic resonance imaging (MRI)). Only studies published between January 2010 and December 2023 were considered. All age groups were included, and only cases documented in English were selected for analysis.
Articles were excluded if they were cohort studies, meta-analyses, review articles, or clinical trials. Additionally, cases where the presentation of PE and AIS occurred more than 96 h apart were not included. Publications from before January 2010, abstracts with limited information, and studies not available in English were also excluded from this review.
Information sources and search strategy
We performed a comprehensive search across six databases - PubMed, Scopus, Cochrane, Embase, Web of Science, and Google Scholar - for case reports, case series, and abstracts documenting the concurrent presentation of PE and AIS from January 2010 to December 2023. The search strategy employed the following search string: (“Pulmonary embolism” OR “lung embolism”) AND (“acute ischemic stroke” OR “ischemic stroke” OR “stroke” OR “embolic stroke” OR “cerebral infarct”) AND (“case reports” OR “case series”). The initial article search was conducted by SS on May 15, 2024.
Duplicate studies were initially identified and addressed using Rayyan.ai. After duplicates were removed, two reviewers (SS and CV) manually checked the remaining 1,976 articles to ensure all duplicates were eliminated.
Study selection
After duplicates were identified and sorted through, titles and abstracts were screened, leading to a full-text appraisal by SS and CV for inclusion. In the event of any disagreements regarding study inclusion, these were resolved by a third reviewer (JI). Data were extracted from studies deemed eligible for data analysis.
Data collection and analysis
Full-text appraisal involved an initial critical evaluation, followed by data extraction. The extracted data were assessed for relevance, significance, and generalizability. The primary measure extracted by the reviewers was the outcome of patients diagnosed with PE and AIS. Subsequently, the articles were critiqued for their study design.
The analysis in this systematic review of case reports was primarily descriptive, given the nature of the data. Patient demographics (age, sex) and clinical characteristics (risk factors, co-morbidities) were summarized using descriptive statistics. Continuous variables, such as age, were reported as means with standard deviations (SDs) and medians while categorical variables (e.g., sex, presence of risk factors, treatments administered) were presented as frequencies and percentages.
For treatment outcomes, we evaluated the frequency of symptom improvement, survival, and mortality rates based on different treatment strategies, including anticoagulation, thrombolysis, catheter-directed thrombectomy, surgical thrombectomy, and conservative management. Treatment outcomes were reported as percentages of cases showing symptom improvement, survival, or mortality, and a comparative summary of outcomes was made across treatment groups. The data were presented as a narrative synthesis, summarizing the main findings and identifying potential trends or gaps in the literature related to the concurrent presentation and treatment of AIS and PE.
Certainty of evidence and risk of bias assessment
The included articles were evaluated for the certainty of evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria by two reviewers (SS and CV) and assessed for bias based on study design.
The methodological quality was evaluated using a modified Newcastle-Ottawa Scale (NOS) for case series, case reports, and case-control studies, following Murad et al’s approach [8]. Reviewers used a standardized procedure to reach consensus on risk of bias. The summarized results are in Tables 1 and 2 [9-29].
 Click to view | Table 1. Risk of Bias |
 Click to view | Table 2. GRADE Analysis for Included Studies |
| Results | ▴Top |
Querying the six databases yielded 1,679 articles. After using Rayyan.ai to remove duplicates, 974 original articles remained. Following abstract and title appraisal, 20 case reports and one case series (two cases) met the inclusion criteria, resulting in 22 cases for analysis. Patient data from the included articles (Table 3 [9-29]) were then extracted and analyzed (Table 4).
 Click to view | Table 3. Patient Information, Risk Factors and Presenting Symptoms |
 Click to view | Table 4. Descriptive Analysis of the Extracted Data From Included Studies |
Table 3 summarizes symptoms of patients with concurrent PE and AIS. Table 4 shows patient demographics: 15 (68.18%) females and seven (31.82%) males. The mean age was 56.68 years (SD: 16.8), with a median age of 59.5 years (Table 4).
In our cohort (Table 5), the prevalent risk factors for PE include oral contraceptive pill (OCP) use and post-major surgical interventions (18.18% each). Other common factors are immobility and varicose veins (9.09% each), and prior lower extremity deep vein thrombosis (DVT) (4.55%). Hypertension is the primary risk factor for ischemic stroke (22.73%), followed by dyslipidemia (13.64%), type 2 diabetes, and prior thromboembolic strokes (9.09% each). Tobacco abuse is a shared risk factor for both conditions (4.55%).
 Click to view | Table 5. Prevalence of Main Predisposing Factors |
Table 6 details the locations of PE and acute stroke, the various treatment approaches for PE and stroke and the associated outcomes in each study. Table 7 details various treatment approaches for PE and their frequencies. Heparin was used in six cases [9-14]. Thrombolysis was used in five cases [15-19]. Low molecular weight heparin (LMWH) was used in two cases [20, 21]. One case was managed with medical therapy alone [19] and another with an inferior vena cava (IVC) filter alone [22]. Catheter-directed thrombectomy was performed in three cases [23-25], including one mechanical thrombectomy (MT) [24] and two percutaneous thrombectomies with heparin [23, 25]. Surgical approaches were utilized in four cases [26-29].
 Click to view | Table 6. Case Reports Describing Multiple Permutations of Treatment Combinations and Their Outcomes |
 Click to view | Table 7. Frequencies of Treatment Approaches for PE |
Of our patients, 72.72% had a patent foramen ovale (PFO), with 37.5% of those having a biatrial thrombus straddling the PFO (Table 8). Among cases mentioning DVT, 31.81% of patients had DVT.
 Click to view | Table 8. Prevalence of PFO, PFO With Thrombus, and DVT in Patients With Concurrent PE and AIS |
A detailed breakdown of outcomes by different treatment modalities is presented in Table 9. Anticoagulation encompasses treatments like heparin infusion and LMWH, while thrombolysis involves therapies such as recombinant tissue plasminogen activator (rt-PA), urokinase, and streptokinase. Cases categorized as no treatment involve PE being managed conservatively. Among the 22 cases analyzed (Table 9), anticoagulation led to symptom improvement and survival in 62.5% of cases, with a 12.5% mortality rate. Thrombolysis achieved symptom improvement and survival in 100% of cases. Catheter-directed thrombectomy had a 66.7% symptom improvement and survival while surgical thrombectomy had a 75%. Conservative management without anticoagulation or thrombolytic therapy resulted in 50% mortality.
 Click to view | Table 9. Outcomes by Treatment Modality in Patients With PE and AIS |
| Discussion | ▴Top |
In our analysis, females predominated (68.18%) over males (31.82%), with other demographic details such as race or occupation infrequently reported, except for Saleh Velez and Ortiz Garcia [19], which included two African American patients, and Pelletier et al [9], identifying a patient as a general surgeon. Most PEs are located in lobar, segmental, or subsegmental branches of the pulmonary artery with saddle PE, occurring in 3-6% of cases [30, 31]. In our study, 72.7% of cases involved bilateral PE.
Research indicates that 50% of individuals with proximal vein DVT also have concurrent PE. PE is linked to an elevated risk of ischemic stroke, potentially through paradoxical embolism (PDE) via a PFO. The prevalence of PFO in the general population is around 25%, and those with a PFO have a stroke risk four times higher than those without [3, 32]. In our study, 72.7% of patients with ischemic stroke had a PFO, and 27.3% had both DVT and PFO. PDE may occur when a clot from DVT passes through a PFO, causing cerebral ischemia. Additionally, in cases of acute PE, elevated right atrial pressure can lead to a right-to-left shunt through the PFO, resulting in systemic embolization [33, 34].
| Management Strategies and Outcomes | ▴Top |
Anticoagulation
PE left untreated has a mortality rate of up to 30% [35]. Anticoagulation with unfractionated heparin significantly reduces mortality in confirmed PE cases [1], but managing PE concurrent with AIS is challenging. It is recommended to defer initiating anticoagulation for PE until 24 h after administering rt-PA after the stroke, due to the risk of hemorrhagic conversion of stroke [6]. However, delaying anticoagulation initiation for more than 24 h in PE leads to a threefold increase in mortality [36], creating a dilemma in deciding when to start anticoagulation in PE patients with concurrent AIS. In our reviewed cases, anticoagulation led to symptom improvement and survival in 62.5% of cases, while 25% had worsening or no improvement in symptoms, and 12.5% resulted in mortality. Two cases demonstrated marked improvements when heparin was administered within 24 h of rt-PA for ischemic stroke [9, 10]. Jayalakshmi et al [14] reported a patient who underwent decompression craniotomy followed by full-dose heparin, achieving a positive outcome under close monitoring. Similarly, Pan et al [20] preferred LMWH due to its lower bleeding risk postoperatively, also resulting in a positive outcome.
Conversely, anticoagulation led to deterioration in functional status in some cases. Bagate et al [12] reported a case where heparin caused hemorrhagic transformation of the ischemic stroke, resulting in the patient’s brain death. In Chakir et al’s COVID-19 case [21], the patient was treated with aspirin for ischemic stroke and LMWH for PE, leading to only slight neurological improvement. Lio et al [13] described a patient who, treated with unfractionated heparin for bilateral lobar PE and later undergoing emergent thrombectomy for basilar occlusion, deteriorated and died from progressive obstructive shock and renal failure. In contrast, Gunta and Kamath [11] reported a pediatric case where unfractionated heparin for PE followed by mechanical clot retrieval and aspirin for stroke led to long-term favorable functional improvement.
Thrombolysis
Thrombolytic therapy, available as systemic or catheter-directed, is an alternative treatment for PE. Systemic thrombolysis is typically reserved for hemodynamically unstable patients, with an absolute contraindication being a non-hemorrhagic stroke within the previous 3 months. Despite this, we identified five cases [15-19] using thrombolysis, all of which resulted in symptom improvement and survival. For instance, Naidoo and Hift [15] observed rapid neurological improvement, likely due to reperfusion of critical cerebral ischemia. Christiansen et al [17] treated a post-surgical patient with intravenous (IV) alteplase for ischemic stroke and PE, along with MT. Although thrombolysis is contraindicated within 14 days of major surgery, they argued that the intracerebral hemorrhage risk with anticoagulation was too high, and thrombolysis would address both PE and stroke. With optimal surgical control of the resulting abdominal hematoma, complete neurological and respiratory recovery was observed.
For PE complicated by right heart thrombus, thrombolysis and surgical thrombectomy are treatment options. Konala et al [18], referencing the pulmonary embolism-3 trial results [37], chose thrombolysis with alteplase followed by IV heparin, resulting in a post-thrombolysis ischemic stroke. This underscores the risk of PDE in PE with PFO post-thrombolysis. Management of such cases may involve catheter-directed thrombolysis (CDT) with percutaneous PFO closure or surgical embolectomy with PFO closure.
Evidence from the ULTIMA and SEATTLE-II trials provides insights into the effectiveness of CDT in PE management. The ULTIMA trial, though limited by a small sample size and design constraints, suggests potential benefits of CDT in reducing right ventricular dilation, pulmonary hypertension, and thrombus burden compared to anticoagulation alone. SEATTLE-II, a single-arm study without a heparin-only comparison, further supports CDT’s potential advantages, though the lack of a control group means that results should be cautiously interpreted given the study’s inherent limitations [38-40].
Embolectomy
Embolectomy is a viable alternative when thrombolysis is contraindicated, with options for surgical or catheter-directed procedures. In our study, percutaneous thrombectomy using unfractionated heparin yielded symptomatic improvement in two cases [23, 25], though Barros-Gomes et al [24] did not report outcomes. Surgical embolectomy is recommended when an embolus is trapped within a PFO, right atrium, or right ventricle [41]. In cases where a thrombus is identified within a PFO, closure of the PFO is sometimes considered to prevent further embolic events. However, closing the PFO during the acute phase might increase the risk of right-sided heart failure, as the PFO can function as a pressure-release mechanism to relieve elevated right atrial pressure. Consequently, some clinicians may delay closure until the patient is stable, typically several months after the acute event [3]. However, mortality rates for pulmonary embolectomy range from 27% to 41% [42, 43]. In our cohort, surgical thrombectomy resulted in symptom improvement and survival in 75.0% cases, with no reported mortality and 25.0% having functional status deterioration.
Conservative management
When management without anticoagulation or thrombolytic therapy was utilized, outcomes were poor: 50% of cases resulted in functional status deterioration (bed-bound and non-verbal), and 50% ended in mortality, highlighting the crucial role of treatment strategies. In Omar et al’s case [22], where anticoagulation and thrombolysis were withheld due to hemorrhagic transformation on follow-up brain imaging, an endovascular approach was employed for the stroke, and an IVC filter was placed. Despite this approach aiming to mitigate bleeding risks, the patient remained bed-ridden and non-verbal. Similarly, the first case reported by Saleh Velez and Ortiz Garcia [19] relied solely on medical therapy for both PE and stroke, which tragically resulted in the patient’s death. The decision to avoid aggressive treatments like thrombolysis and thrombectomy, in this case, appears to have contributed to impairment in functional status.
Strengths
This systematic review boasts a comprehensive search strategy, adherence to PRISMA guidelines, and stringent inclusion criteria, ensuring high-quality studies. Detailed data extraction, GRADE scoring for quality assessment, and Rayyan.ai for duplicate identification enhance accuracy. The review provides valuable insights into clinical characteristics, management strategies, and outcomes, highlighting the lack of established protocols and the need for further research. By documenting diverse treatment approaches and offering a balanced discussion of outcomes, the review contributes to the robustness and clinical relevance of potential management strategies.
Limitations
The review’s limitations include a small sample size, with only 22 patients, which restricts the generalizability of the findings to a broader population. The lack of complete demographic data, such as information on race and occupation, further limits the ability to fully characterize the patient population. The heterogeneity in the quality and completeness of reporting across case reports may affect the reliability and consistency of the results. Several case reports did not account for multiple comorbidities, which could confound the relationships between treatment modalities and outcomes. Furthermore, the absence of control groups in this study of case reports and case series limits the ability to make causal inferences. The inherent biases of the study, particularly selection bias, also affect the validity of the conclusions drawn from these findings. The influence of age and sex on survival prognosis in this complex presentation remains unclear.
Gaps in the existing literature
Several gaps require further research and clarification.
Optimal treatment strategies for combined PE and AIS
The effectiveness and safety of thrombolytic therapy, MT, and anticoagulation in managing both PE and AIS need further study [19]. The efficacy of combining treatments, such as rt-PA with MT, remains unclear and requires more clinical evidence. The role of CDT alone or with mechanical methods in high-risk PE patients unable to receive thrombolysis or those who are unstable post-thrombolysis also needs investigation [44].
Safety and timing of anticoagulation post-rt-PA
There is no consensus on the optimal timing for initiating anticoagulation after rt-PA administration for AIS. Literature shows delay up to 24 h post-rt-PA, highlighting the need to balance bleeding risks with early anticoagulation in patients with both AIS and PE [19].
Over-treatment and management of subsegmental PE (SSPE)
Current approaches often lead to over-treatment of SSPE, in asymptomatic or low-risk patients. Research is needed to identify which patients can safely forgo anticoagulation without increasing recurrent event risks [45].
| Conclusion | ▴Top |
The findings from this systematic review, primarily based on observational data from case reports, illustrate the diverse strategies employed by physicians in the management of combined PE and AIS. While proactive treatments, such as thrombolysis and thrombectomy, tend to yield better outcomes, the lack of robust clinical evidence and the reliance on case reports limit the ability to draw definitive conclusions and specific treatment recommendations. Therefore, this comprehensive overview underscores the importance of well-designed prospective, randomized controlled trials to confirm the efficacy and safety of various treatments. Such trials would investigate standardized treatment protocols, to guide structured clinical guidelines to manage this complex presentation.
Acknowledgments
None to declare.
Financial Disclosure
No funding was received for this paper.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author Contributions
SS designed the research, performed the research, analyzed data, and wrote the paper. CV performed the research, analyzed data, and contributed to writing the paper. JI reviewed the paper.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
AC: anticoagulation; AIS: acute ischemic stroke; DVT: deep vein thrombosis; L: left; LMWH: low molecular weight heparin; MCA: middle cerebral artery; MT: mechanical thrombectomy; PE: pulmonary embolism; R: right
| References | ▴Top |
- Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163(14):1711-1717.
doi pubmed - Zghouzi M, Mwansa H, Shore S, Hyder SN, Kamdar N, Moles VM, Barnes GD, et al. Sex, racial, and geographic disparities in pulmonary embolism-related mortality nationwide. Ann Am Thorac Soc. 2023;20(11):1571-1577.
doi pubmed - Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59(1):17-20.
doi pubmed - Clergeau MR, Hamon M, Morello R, Saloux E, Viader F, Hamon M. Silent cerebral infarcts in patients with pulmonary embolism and a patent foramen ovale: a prospective diffusion-weighted MRI study. Stroke. 2009;40(12):3758-3762.
doi pubmed - Pongmoragot J, Rabinstein AA, Nilanont Y, Swartz RH, Zhou L, Saposnik G, Investigators of Registry of Canadian Stroke N, et al. Pulmonary embolism in ischemic stroke: clinical presentation, risk factors, and outcome. J Am Heart Assoc. 2013;2(6):e000372.
doi pubmed - Adams HP, Jr., Brott TG, Furlan AJ, Gomez CR, Grotta J, Helgason CM, Kwiatkowski T, et al. Guidelines for thrombolytic therapy for acute stroke: a supplement to the guidelines for the management of patients with acute ischemic stroke. A statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Circulation. 1996;94(5):1167-1174.
doi pubmed - Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
doi pubmed - Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60-63.
doi pubmed - Pelletier M, Bugeaud R, Ibrahim R, Morency G, Kouz S. Successful thrombolysis of a stroke with a pulmonary embolism in a young woman. J Emerg Med. 2010;39(4):443-448.
doi pubmed - Delgado MG, Mauri G, Vega J. Massive pulmonary thromboembolism after intravenous stroke thrombolysis. BMJ Case Rep. 2012;2012:bcr1020115008.
doi pubmed - Gunta S, Kamath S. A case of pulmonary embolism and stroke in a 16-year-old girl. WMJ. 2012;111(2):58-60.
pubmed - Bagate F, Bedet A, Mekontso Dessap A, Carteaux G. Paradoxical brain embolism shadowing massive pulmonary embolism. Am J Emerg Med. 2018;36(8):1527.e1521-1527.e1522.
doi pubmed - Lio KU, Kumaran M, Rali P. Patent foramen ovale: Connecting dots from massive pulmonary embolism to acute ischemic stroke. Lung India. 2019;36(6):564-566.
doi pubmed - Jayalakshmi S, Khandalia H, Vooturi S, Jiwani PA, Kaul S. Simultaneous acute pulmonary thromboembolism and stroke - a management dilemma. Neurol India. 2021;69(5):1371-1373.
doi pubmed - Naidoo P, Hift R. Massive pulmonary thromboembolism and stroke. Case Rep Med. 2011;2011:398571.
doi pubmed - Xie XJ, Jiang JB, Jiang J, Wang JA. Concurrent pulmonary thrombosis with systemic embolism: a case report. J Zhejiang Univ Sci B. 2014;15(9):838-844.
doi pubmed - Christiansen ME, Kumar G, Mahabir RC, Helmers RA, Bendok BR, O'Carroll CB. Intravenous alteplase for acute stroke and pulmonary embolism in a patient with recent abdominoplasty. Neurologist. 2017;22(4):150-152.
doi pubmed - Konala VM, Naramala S, Adapa S, Aeddula NR, Bose S. An elderly man with syncope, hypoxia, and confusion: a case report and review of literature. Cureus. 2019;11(9):e5567.
doi pubmed - Saleh Velez FG, Ortiz Garcia JG. Management dilemmas in acute ischemic stroke and concomitant acute pulmonary embolism: Case series and literature review. eNeurologicalSci. 2021;23:100341.
doi pubmed - Pan S, Wang Z, Wei X, Tang H, Li Z, Zhang Y, Zhou S, et al. Simultaneous pulmonary embolism and ischemic stroke in a patient with Cor Biloculare after glenn anastomosis. Heart Surg Forum. 2019;22(3):E180-E182.
doi pubmed - Chakir M, El Jamili M, Boudhar Z, El Hattaoui M. Simultaneous acute myocardial infarction, bilateral pulmonary embolism, and acute ischaemic cerebral stroke, a delayed complication in a patient with COVID-19 infection: case report. Eur Heart J Case Rep. 2021;5(6):ytab218.
doi pubmed - Omar HR, Huang C, Miller JH, Mangar D, Kabemba A, Camporesi EM. Simultaneous pulmonary embolism and cerebrovascular stroke. Herz. 2013;38(8):884-886.
doi pubmed - Ozsancak Ugurlu A, Cinar O, Caymaz I, Cevik H, Gumus B. Combined catheter thrombus fragmentation and percutaneous thrombectomy in a patient with massive pulmonary emboli and acute cerebral infarct. Anatol J Cardiol. 2015;15(1):72-74.
doi pubmed - Barros-Gomes S, El Sabbagh A, Eleid MF, Mankrad SV. Concomitant acute stroke, pulmonary and myocardial infarction due to in-transient thrombus across a patent foramen ovale. Echo Res Pract. 2018;5(4):I9-I10.
doi pubmed - Duy TM, Dang LV, Viet PD, Van CN, Nguyen QA, Minh TP, Dat AN, et al. Multiple recurrent acute ischemic strokes treated by thrombectomy in a patient with acute pulmonary embolism. Open Access Maced J Med Sci. 2019;7(5):801-804.
doi pubmed - Nam SB, Kim CM, Cho SA, Chung S, Shim YH. Thrombus entrapped by patent foramen ovale in a patient with pulmonary embolism: a case report. Korean J Anesthesiol. 2015;68(1):70-73.
doi pubmed - de Oliveira MAB, Sabbag AT, Brandi AC, dos Santos CA, Botelho PH, Patriarcha FA, Braile DM. Surgical treatment for thrombus straddling a patent foramen ovale. Braz J Cardiovasc Surg. 2016;31(5):406-408.
doi pubmed - Dada R, Dada J, Abdelsalam M, Agrawal Y. Thrombus straddling a patent foramen ovale, pulmonary embolism and paradoxical embolism: a rare trifecta. BMJ Case Rep. 2018;2018:bcr2018227505.
doi pubmed - Hattori K, Daitoku K, Taniguchi S, Fukuda I. Surgical embolectomy for paradoxical cerebral embolism with massive pulmonary embolism. Gen Thorac Cardiovasc Surg. 2020;68(4):385-388.
doi pubmed - Ryu JH, Pellikka PA, Froehling DA, Peters SG, Aughenbaugh GL. Saddle pulmonary embolism diagnosed by CT angiography: frequency, clinical features and outcome. Respir Med. 2007;101(7):1537-1542.
doi pubmed - Sardi A, Gluskin J, Guttentag A, Kotler MN, Braitman LE, Lippmann M. Saddle pulmonary embolism: is it as bad as it looks? A community hospital experience. Crit Care Med. 2011;39(11):2413-2418.
doi pubmed - Le Moigne E, Timsit S, Ben Salem D, Didier R, Jobic Y, Paleiron N, Le Mao R, et al. Patent foramen ovale and ischemic stroke in patients with pulmonary embolism: a prospective cohort study. Ann Intern Med. 2019;170(11):756-763.
doi pubmed - Kasper W, Geibel A, Tiede N, Just H. Patent foramen ovale in patients with haemodynamically significant pulmonary embolism. Lancet. 1992;340(8819):561-564.
doi pubmed - Konstantinides S, Geibel A, Kasper W, Olschewski M, Blumel L, Just H. Patent foramen ovale is an important predictor of adverse outcome in patients with major pulmonary embolism. Circulation. 1998;97(19):1946-1951.
doi pubmed - Weinberg AS, Rali P. Treatment, prognosis, and follow-up of acute pulmonary embolism in adults, UpToDate. Edited by J. Madel, K. Zachrison, and G. Finlay. Available at: https://www.uptodate.com/contents/treatment-prognosis-and-follow-up-of-acute-pulmonary-embolism-in-adults?search=pulmonary+embolism+&topicRef=8253&source=see_link (Accessed: May 12, 2024).
- Smith SB, Geske JB, Maguire JM, Zane NA, Carter RE, Morgenthaler TI. Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest. 2010;137(6):1382-1390.
doi pubmed - Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W, Management S, Prognosis of Pulmonary Embolism-3 Trial I. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347(15):1143-1150.
doi pubmed - Piazza G, Hohlfelder B, Jaff MR, Ouriel K, Engelhardt TC, Sterling KM, Jones NJ, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv. 2015;8(10):1382-1392.
doi pubmed - Engelberger RP, Kucher N. Reperfusion Treatment for Acute Pulmonary Embolism. Hamostaseologie. 2018;38(2):98-105.
doi pubmed - Kucher N, Boekstegers P, Muller OJ, Kupatt C, Beyer-Westendorf J, Heitzer T, Tebbe U, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129(4):479-486.
doi pubmed - Bloomfield P, Boon NA, de Bono DP. Indications for pulmonary embolectomy. Lancet. 1988;2(8606):329.
doi pubmed - Fauveau E, Cohen A, Bonnet N, Gacem K, Lardoux H. Surgical or medical treatment for thrombus straddling the patent foramen ovale: impending paradoxical embolism? Report of four clinical cases and literature review. Arch Cardiovasc Dis. 2008;101(10):637-644.
doi pubmed - Kilic A, Shah AS, Conte JV, Yuh DD. Nationwide outcomes of surgical embolectomy for acute pulmonary embolism. J Thorac Cardiovasc Surg. 2013;145(2):373-377.
doi pubmed - Todoran TM, Petkovich B. Aggressive therapy for acute pulmonary embolism: systemic thrombolysis and catheter-directed approaches. Semin Respir Crit Care Med. 2021;42(2):250-262.
doi pubmed - Yoo HH, Nunes-Nogueira VS, Fortes Villas Boas PJ. Anticoagulant treatment for subsegmental pulmonary embolism. Cochrane Database Syst Rev. 2020;2(2):CD010222.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.