| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 2, February 2025, pages 76-88
Impact of Prior Metformin Use on Stroke Outcomes: A Systematic Review and Updated Meta-Analysis
Anas Elgenidya, k, Ahmed Yasser Shabanb, k, Khaled Saadc, l, Yasser Hamedd, Ahmed Elhadi Rhabb, Mohamed Khalafalla Darwishe, Alaa Essam Kamalf, Mohamed Salem Abdelkaderg, Hamza Anas Marzoukg, Mohamed Mahmoud Gomaah, Hassan Ahmed Hashemd, Amira Elhoufeyi, Hamad Ghaleb Dailahi, Rami A. Metwallyj, Noran ElBazzarj, Nevin Shalabya
aDepartment of Neurology, Faculty of Medicine, Cairo University, Cairo, Egypt
bFaculty of Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt
cDepartment of Pediatrics, Faculty of Medicine, University of Assiut, Assiut, Egypt
dDepartment of Neurology, Faculty of Medicine, Al-Azhar University, Assiut, Egypt
eFaculty of Medicine, Minia University, Minia, Egypt
fFaculty of Medicine, Tanta University, Tanta, Egypt
gFaculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan
hFaculty of Medicine, Al-Azhar University, Cairo, Egypt
iDepartment of Community Health Nursing, Faculty of Nursing, Assiut University, Assiut, Egypt
jDepartment of Internal Medicine, Benha University, Benha, Egypt
kThe authors contributed equally to the paper.
lCorresponding Author: Khaled Saad, Department of Pediatrics, Faculty of Medicine, University of Assiut, Assiut 71516, Egypt
Manuscript submitted December 1, 2024, accepted January 27, 2025, published online February 4, 2025
Short title: Prior Metformin Use on Stroke Outcomes
doi: https://doi.org/10.14740/jocmr6159
| Abstract | ▴Top |
Background: Metformin is a commonly prescribed oral hypoglycemic agent for diabetic patients. Its effect in reducing the incidence of stroke has already been proven. We aimed to explore the impact of prior metformin use on stroke outcomes.
Methods: The Web of Science, PubMed, Embase, and Cochrane Library were searched to identify relevant studies involving stroke patients with a history of metformin use and comparing them to non-metformin users. We analyzed the following outcomes: modified Rankin Scale (mRS), National Institutes of Health Stroke Scale (NIHSS), mortality, or length of hospitalization.
Results: Eleven studies, with 13,825 participants, were included. The metformin group showed higher favorable mRS 0 - 2 than the non-metformin group (risk ratio (RR) = 1.14, 95% confidence interval (CI): 1.09 - 1.19, P value < 0.01). Also, significantly lower mortality rates were seen in the metformin group (RR = 0.54, 95% CI: 0.46 - 0.63, P value ≤ 0.01). NIHSS at discharge was lower in the metformin group than the non-metformin group (mean difference (MD) = -0.46, 95% CI: -0.82 - -0.11, P value < 0.01). The mRS 3 - 6 indicates less favorable outcomes were higher in the non-metformin group (RR = 0.85, 95% CI: 0.77 - 0.93). At the same time, NIHSS at admission showed no statistically significant difference between the two groups. These results indicate that metformin has a beneficial impact on the severity of stroke.
Conclusions: Pre-stroke metformin therapy is associated with better post-stroke clinical outcomes and lower mortality rates. These results highlight the potential neuroprotective role of metformin and emphasize its role as an adjunctive treatment in stroke management. Further research is required to understand its mechanism better.
Keywords: Metformin; Stroke; mRS; NIHSS; Meta-analysis
| Introduction | ▴Top |
Metformin is the first-line treatment for type 2 diabetes mellitus (T2DM) according to the American Diabetic Association, due to its blood glucose-lowering effect. It belongs to biguanides, a class of antidiabetic drugs [1, 2]. Metformin increases peripheral tissue sensitivity to insulin, promotes peripheral glucose uptake, and inhibits hepatic gluconeogenesis. This effect is mediated through the activation of adenosine 5’-monophosphate-activated protein kinase (AMPK). It regulates energy homeostasis and contains two regulatory subunits (β and γ) and α catalytic subunit [2].
Stroke incidence has recently increased. It has emerged as a leading cause of disability and the second most frequent cause of death globally [3]. Diabetic patients experience a higher rate of stroke compared to the general population, with a 10-year cumulative recurrence rate of ischemic stroke being 40.9% for type 1 diabetes mellitus (T1DM) (14 events), 29.7% for T2DM (15 events), and 12.0% for non-diabetic individuals. This means that diabetes is a significant risk factor for stroke [4].
Metformin has demonstrated significant effectiveness in lowering stroke occurrences among 14,856 diabetic patients (10,857 on metformin and 3,999 on other oral hypoglycemic agents). A total of 1,695 stroke events were recorded (994 in the metformin group and 701 in the non-metformin group), with notably fewer stroke events observed in the metformin-treated patients (9.2% vs. 17.5%, P < 0.001) over a 4-year follow-up period [5].
Metformin’s clinical benefits in reducing stroke incidence are already proven, but what happens if a stroke has occurred? Then, AMPK activation in metformin users shows lower odds of poor functional outcomes [6].
Metformin activates AMPK, which has protective effects against cerebral ischemia by either inhibiting the NF-κB cascade to reduce post-ischemic neuroinflammation or by activating the nuclear factor-erythroid 2-related factor 2 (Nrf2) antioxidant pathway. Administering metformin to diabetic patients before the onset of stroke may be associated with decreased neurological severity and enhanced outcomes during acute-phase therapy [7].
Previous reviews assessed metformin’s effects on stroke incidence. Therefore, this review aimed to evaluate whether prior metformin use was associated with better stroke outcomes and improved prognosis.
| Materials and Methods | ▴Top |
We reported this study following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [8]. Furthermore, the review protocol was registered with PROSPERO (CRD42024530349), the international prospective registry for systematic reviews. The Institutional Review Board approval and ethical compliance with human studies are not applicable to this study.
Search strategy
We searched medical electronic databases, PubMed, Web of Science, Cochrane Library, and Embase, for relevant studies until April 2024. For a sensitive search strategy, we used the MESH database and the following search queries: (“Metformin” OR “Glucophage”) AND (“Stroke*” OR “Cerebrovascular Accident”). The full search strategy is provided here (Supplementary Material 1, jocmr.elmerjournals.com). After the literature search, the retrieved studies were downloaded and imported into Endnote X20 for duplicate removal and then exported into an Excel sheet. To enhance the validation of our search approach, we developed an additional search strategy that focused on stroke type. These search terms related to stroke type are presented here for reference (Supplementary Material 1, jocmr.elmerjournals.com).
Selection of the studies
Two independent authors reviewed each study. The disagreement was resolved by a discussion between the two authors and a senior author’s decision. The study was retrieved for a full-text check to see the eligibility criteria. The full text of all related articles was then obtained and checked by at least two independent authors.
Inclusion criteria
We included studies that met these inclusion criteria: 1) primary studies that used metformin therapy before stroke onset and had a control group of non-metformin users; 2) assessed the post-stroke clinical outcomes and reported clear outcomes related to modified Rankin Scale (mRS), National Institutes of Health Stroke Scale (NIHSS), and mortality; 3) published in international peer-reviewed journals. We excluded the previous reviews, preclinical studies, animal studies, pharmacokinetics, and pharmacodynamics studies, which had no clear clinical outcomes.
Data extraction and risk of bias assessment
Two authors independently extracted the data, and a third reviewer resolved any conflict for the studies included. Extracted data were divided into four domains: 1) study characteristics (study ID, study design, duration, inclusion criteria, exclusion criteria, results); 2) characteristics of the included study population (age, sex, body mass index (BMI), systolic blood pressure (SBP), and diastolic blood pressure (DBP)) and their risk factors (hypertension, smoking, and hyperlipidemia); 3) risk of bias domains; 4) study outcomes (mRS, NIHSS, mortality rate, and length of hospitalization).
At least two independent authors reviewed each study protocol, and full text and supplementary material are available for risk of bias assessment. Randomized controlled trials (RCTs) were evaluated using the Cochrane Risk-of-Bias assessment tool for randomized trials (ROB-2), as recommended by the Cochrane Handbook for Systematic Reviews [9]. This tool involves eight questions covering areas such as randomization methods, allocation concealment, blinding of participants, personnel, and outcome assessors, management of incomplete outcome data, selective reporting of outcomes, and identification of other potential biases. Each aspect was rated as having a high, unclear, or low risk of bias (ROB). For observational cohort and cross-sectional studies, we employed the National Institutes of Health (NIH) Quality Assessment Tool [10] to determine their quality.
Two authors independently evaluated the risk of bias in the included studies, and a third reviewer resolved any disagreements.
Statistical analysis
All statistical analyses were performed with the R software (version 4.1.3). Continuous variables were presented as the mean difference (MD) and the corresponding 95% confidence intervals (CIs), which describe the difference between metformin and non-metformin groups for an outcome, having taken into account the weighting of the individual studies.
The categorical variables were presented as risk ratios (RRs) and the corresponding 95% CIs, which describe the ratio of the risk of an outcome event in the metformin group to the risk of the outcome event in the non-metformin group.
Dealing with missing data
When the standard deviation (SD) of the change in outcome was unavailable, we calculated it using the standard error (SE) or the 95% CI, as recommended by Altman [11].
Assessment of heterogeneity
After visually inspecting the forest plot, we used the Chi-square (χ2) test and the I2 statistic to evaluate heterogeneity among the studies. The χ2 test assessed whether significant heterogeneity existed, while the I2 statistic quantified the extent of heterogeneity when present. We interpreted the I2 values following the guidelines from the Cochrane Handbook for Systematic Reviews [9], considering the following ranges: 0-40% (might not be important), 30-60% (may indicate moderate heterogeneity), 50-90% (may indicate substantial heterogeneity), and 75-100% (considerable heterogeneity). As per the Cochrane Handbook (Part 2, Chapter 9), a significance level (α) less than 0.1 in the χ2 test was considered evidence of significant heterogeneity.
Publication bias
We did not assess publication bias because, according to Cochrane guidelines [9], tests for funnel plot asymmetry should only be performed when each outcome includes at least 10 studies.
| Results | ▴Top |
Study results and characteristics
The electronic databases search identified 2,063 studies. After duplicate removal by Endnote, 1,143 articles were imported into an Excel sheet for title and abstract screening. Twenty-six articles were read in full text for eligibility. A further 15 articles were excluded. The reason for exclusion and details of included studies are presented in the PRISMA flow diagram (Fig. 1). Finally, 11 studies with 13,825 patients were included after full-text screening based on the inclusion criteria. Except for one randomized control trial, all included studies were observational cohorts. Two studies [12, 13] were published in 2024, five in 2022 [14-18], two in 2020 [19, 20], one in 2018 [21], and one in 2015 [7]. Studies were conducted in different countries: two studies in China [15, 16], two studies in Japan [7, 13], and the others in Qatar, Netherlands, Switzerland, Korea, Turkey, and Iran [12, 14, 17, 19-21], respectively. More details about the study’s characteristics are presented in Table 1 [7, 12-15, 17-22].
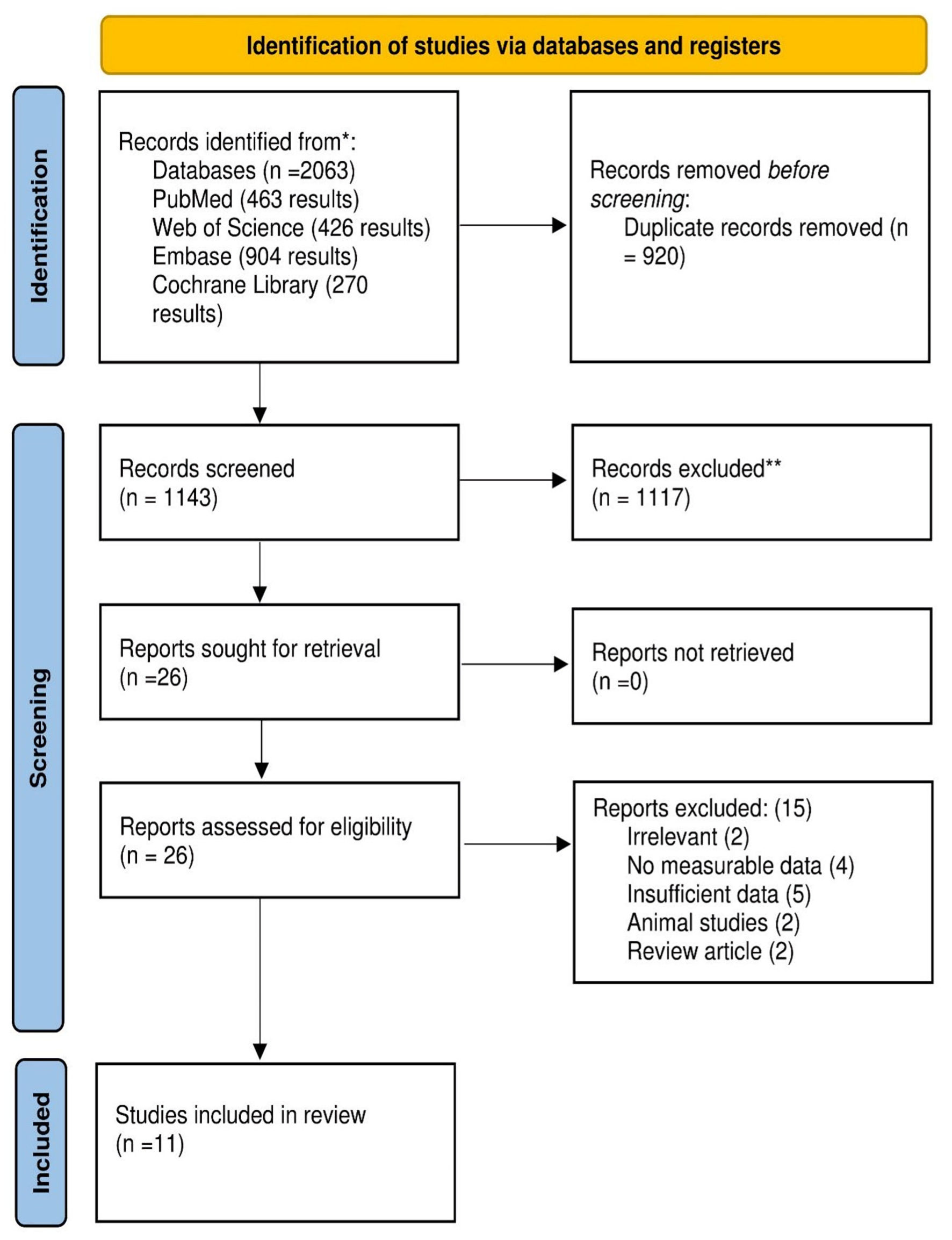 Click for large image | Figure 1. PRISMA flow diagram. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses. |
 Click to view | Table 1. Summary Characteristics of the Included Studies |
Baseline characteristics
Patients’ mean ages in the included studies ranged between 54 and 75 years, and 60% were male. Three studies reported the mean BMI (kg/m2) ranging between 23 and 28. Seventy-two percent and 34% of the population were hypertensive and hyperlipidemic, respectively. Baseline creatinine was within normal limits. Nine studies assessed the post-stroke outcomes in diabetic patients on metformin, but two studies [13, 21] reported it in metformin users with no diabetes. Blood glucose level was reported in eight studies; it was below 200 mg/dL except in the study by Jian et al [15] (2023), which showed high blood glucose level (higher than 200 mg/dL). Six studies reported previous stroke events, which estimated 18% of their total population. More details including the baseline characteristics of the included population are shown in Table 2 [7, 12-15, 17, 18-22].
 Click to view | Table 2. Baseline Characteristics of the Included Population |
Quality assessment
We utilized the NIH Quality Assessment Tool for observational cohort studies. Two studies [16, 17] showed a low risk of bias as they scored 10 or more. Seven studies [7, 12-15, 18, 19] were of moderate risk; their sample size justification, power description, or variance and effect estimates were not provided, and their exposures were not assessed more than once over time, as they ranged between 5 to 9 in the scoring system. The last observational study [20] was of high risk, as most domains of NIH were not provided or assessed in this study (Supplementary Material 2, jocmr.elmerjournals.com). We also used RoB 2 (a revised Cochrane risk-of-bias tool for randomized trials) for the RCT study [21]. The study was stratified as having a high risk of bias because of some missing outcome data, and there were some concerns with the selection of the reported data [9] (Supplementary Material 3, jocmr.elmerjournals.com).
Meta-analyses
mRS outcomes
The mRS 0 - 2 between the metformin and non-metformin groups was reported in six studies. The overall RR is statistically significant in favor of the metformin group (RR = 1.14, 95% CI: 1.09 - 1.19), and there is no significant heterogeneity (I2 = 40%, P value = 0.14) (Fig. 2). But the overall RR for mRS 3 - 6 between the two groups was reported in three studies and statistically significantly higher in the non-metformin group (RR = 0.85, 95% CI: 0.77 - 0.93), and the pooled studies were homogenous (I2 = 0%, P value = 0.40). These results are displayed in Figure 3.
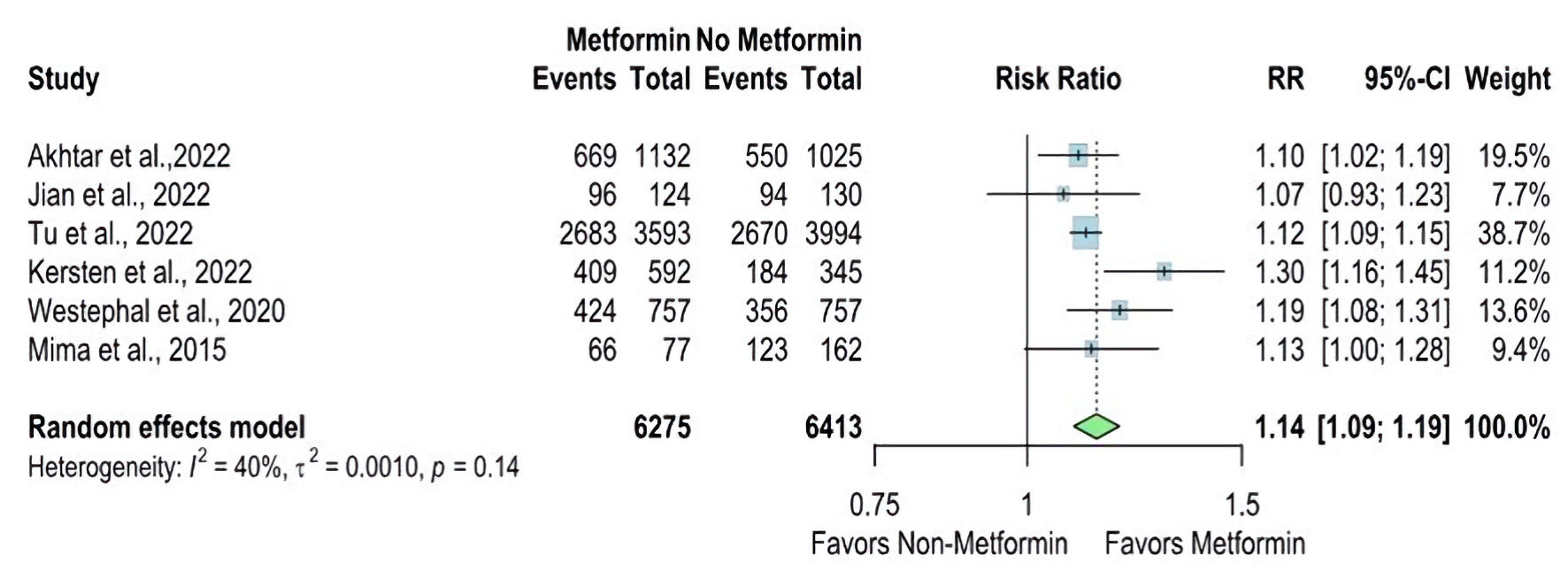 Click for large image | Figure 2. Forest plot of modified Rankin Scale (mRS 0 - 2) outcome. RR: risk ratio; CI: confidence interval; mRS: modified Rankin Scale. |
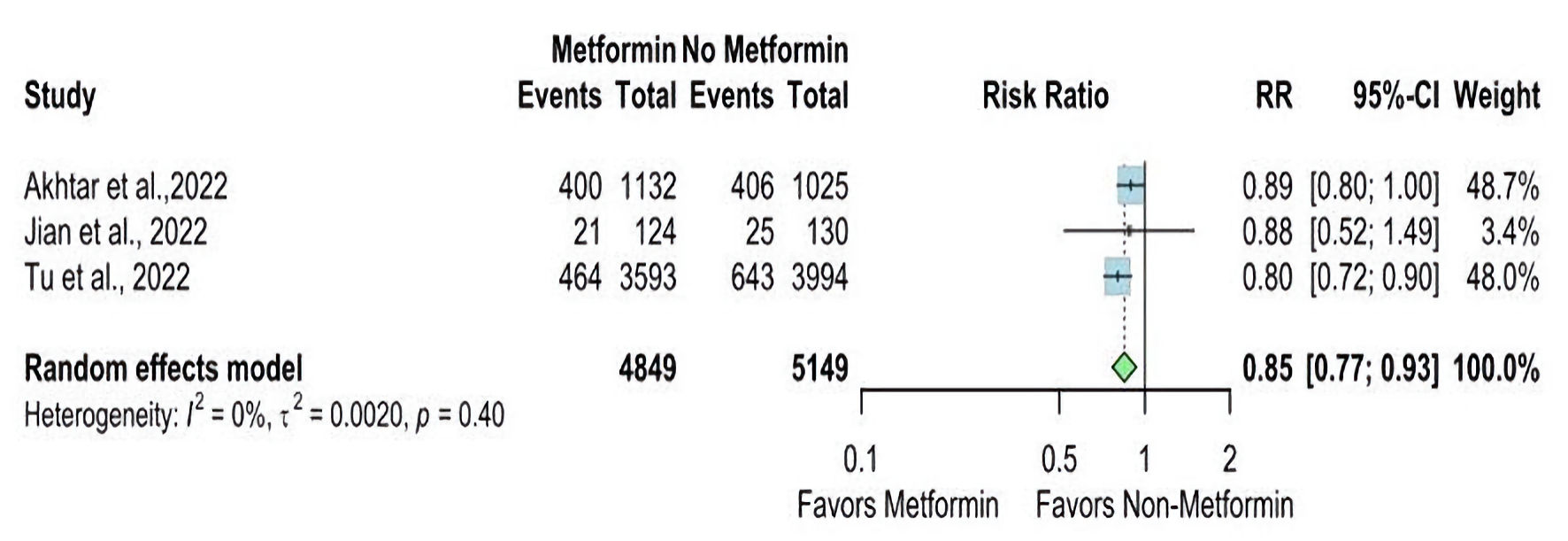 Click for large image | Figure 3. Forest plot of modified Rankin Scale (mRS 3 - 6) outcome. RR: risk ratio; CI: confidence interval; mRS: modified Rankin Scale. |
Akhtar et al [14] reported mRS 0 - 2 at discharge as a proportion and revealed that the metformin group had a higher proportion than the non-metformin group (55.4% vs. 51.9%, respectively). Kersten et al [17] reported the mRS 0 - 22 as the odds ratio (OR) for the metformin users compared with non-metformin users and reported a higher OR in the metformin group (OR = 1.96, CI 1.49 - 2.57).
NIHSS outcomes
The NIHSS at discharge was reported in two studies. The overall MD was statistically significantly lower in the metformin group than the non-metformin group (MD = -0.46, 95% CI: -0.82 - -0.11; P value ≤ 0.01), and pooled studies were homogenous (I2 = 0%, P = 0.62) (Fig. 4). In contrast, The NIHSS at admission was reported in eight studies. The overall MD showed a non-statistically significant difference between the two groups (MD = 0.15, 95% CI: -1.46 - 1.75; P value ≥ 0.05), and there is significant heterogeneity found (I2 = 92%, P < 0.01) (Fig. 5).
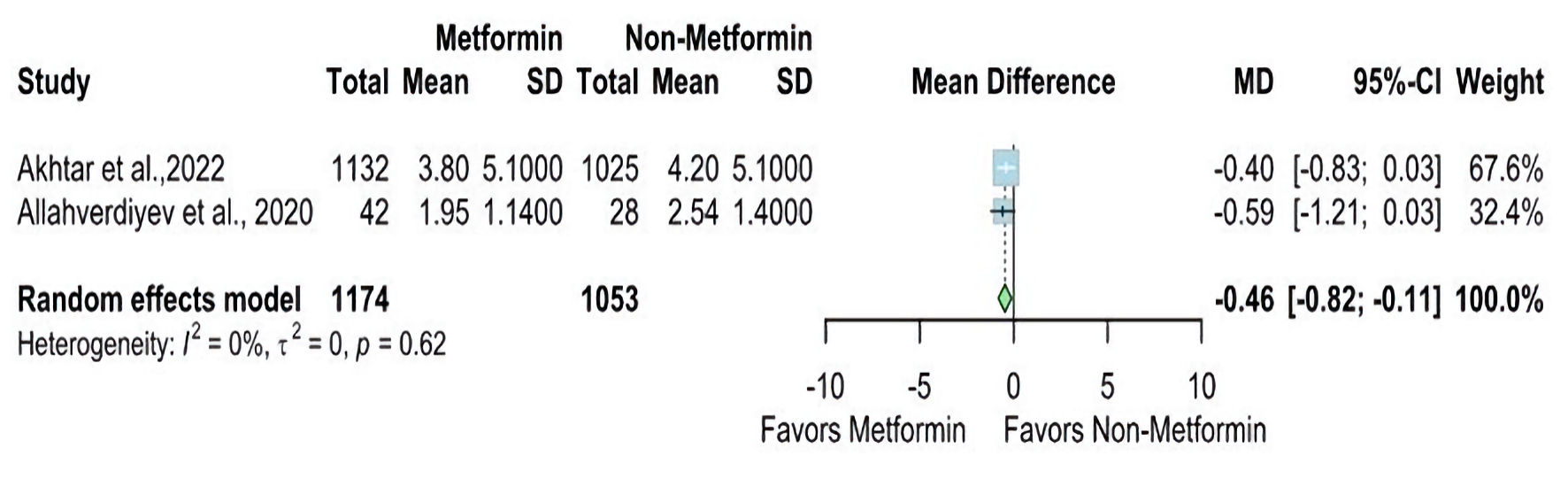 Click for large image | Figure 4. Forest plot of NIHSS at discharge outcome. SD: standard deviation; MD: mean difference; CI: confidence interval; NIHSS: National Institutes of Health Stroke Scale. |
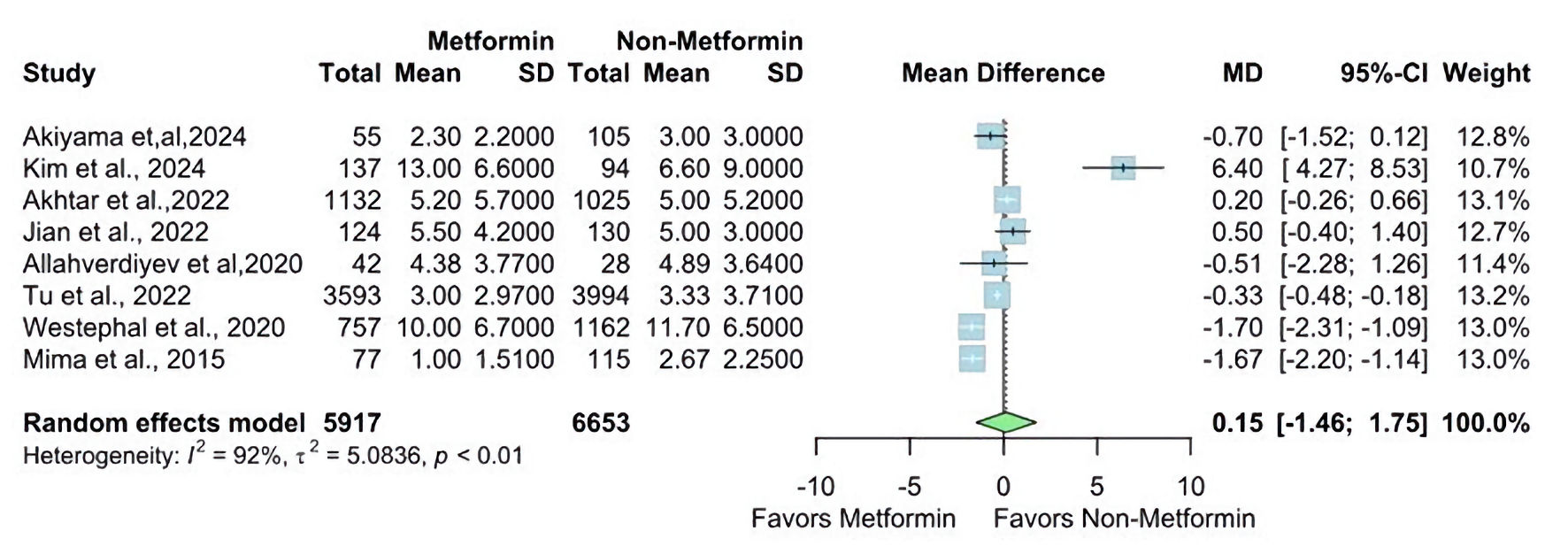 Click for large image | Figure 5. Forest plot of NIHSS at admission outcome. SD: standard deviation; MD: mean difference; CI: confidence interval; NIHSS: National Institutes of Health Stroke Scale. |
Kersten et al [17] reported the OR for mild stroke (NIHSS score below 4) in the metformin group compared to the non-metformin and reported a higher OR in the metformin group (OR = 1.53 (1.16 - 2.01)).
Mortality and length of stay outcomes
The overall RR for mortality was statistically significantly lower in the metformin group compared with the non-metformin group (RR = 0.54, 95% CI: 0.46 - 0.63; P value < 0.01), and there is no significant heterogeneity found (I2 = 46%, P = 0.13) (Fig. 6).
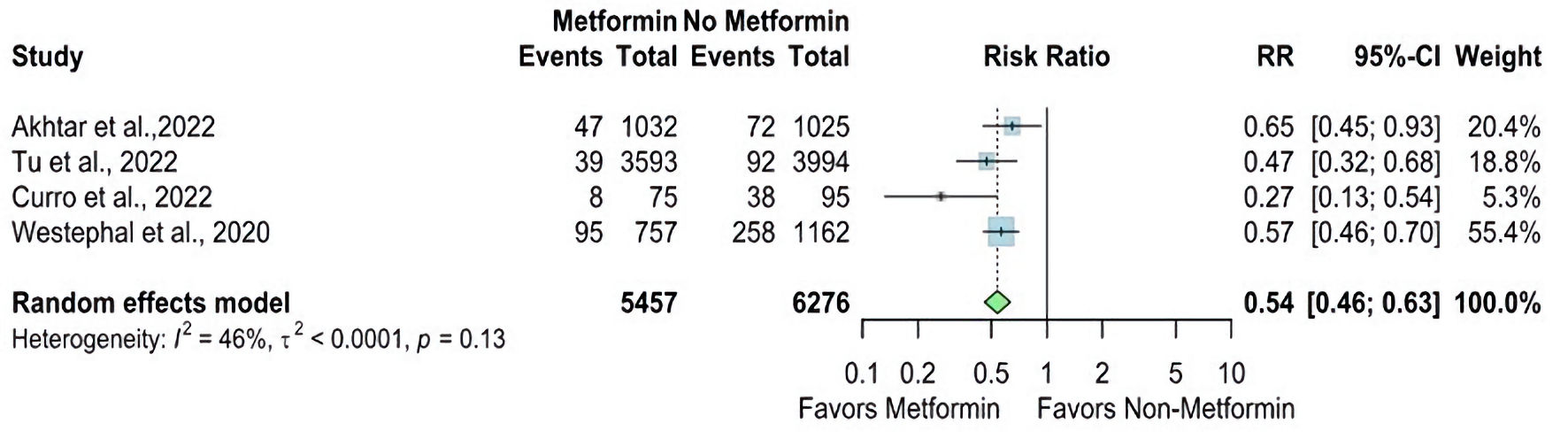 Click for large image | Figure 6. Forest plot of mortality outcome. RR: risk ratio; CI: confidence interval. |
Two studies reported the length of hospital stay. There is no statistically significant difference (MD = -0.02, 95% CI: -0.21 - 0.18) between both groups, which may be due to the limited number of studies that reported these outcomes. Pooled studies were homogenous (I2 = 0%, P = 0.50) (Fig. 7).
 Click for large image | Figure 7. Forest plot of length of stay outcome. SD: standard deviation; MD: mean difference; CI: confidence interval. |
Some outcomes showed heterogeneity, so we used random models to report them. We also conducted a leave-out meta-analysis to explain the cause of the heterogeneity.
Sensitivity analysis and leave-one-out meta-analysis
We performed a sensitivity analysis in multiple scenarios for mRS 0 - 2, mRS 3 - 6, NIHSS at admission, and mortality, by excluding one study at each time and conducting the forest plot for other studies. It did not significantly change the pooled results or the heterogeneity levels (Figs. 8-11).
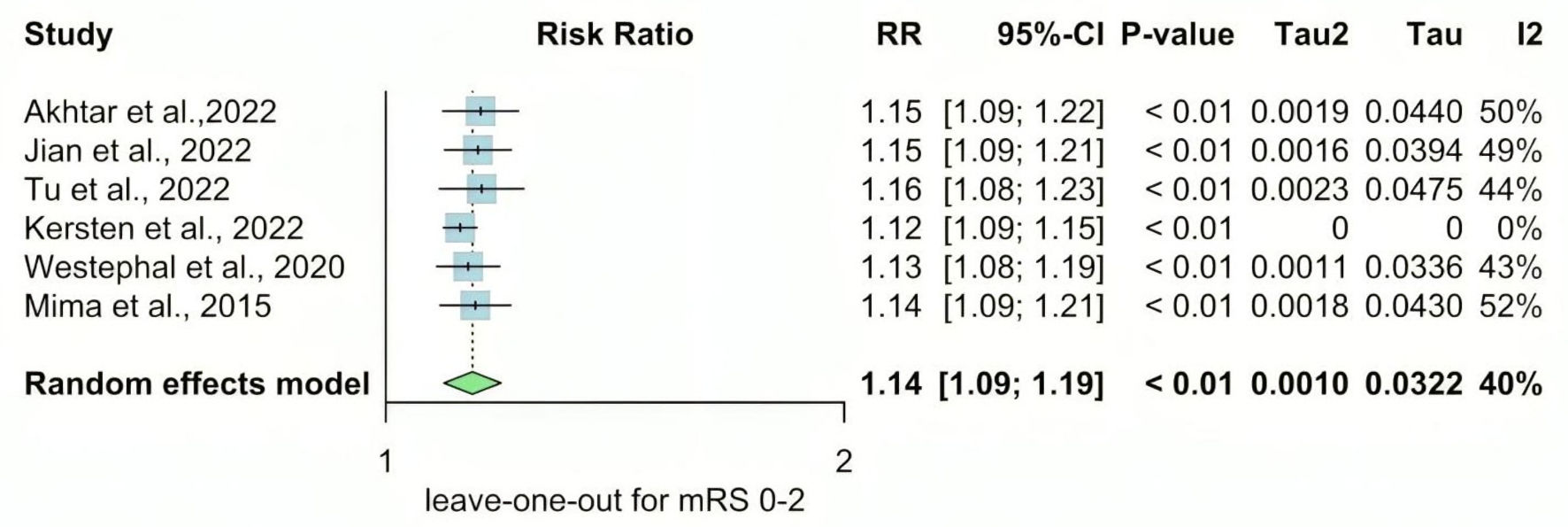 Click for large image | Figure 8. Leave-one-out meta-analysis for mRS 0 - 2. RR: risk ratio; CI: confidence interval; mRS: modified Rankin Scale. |
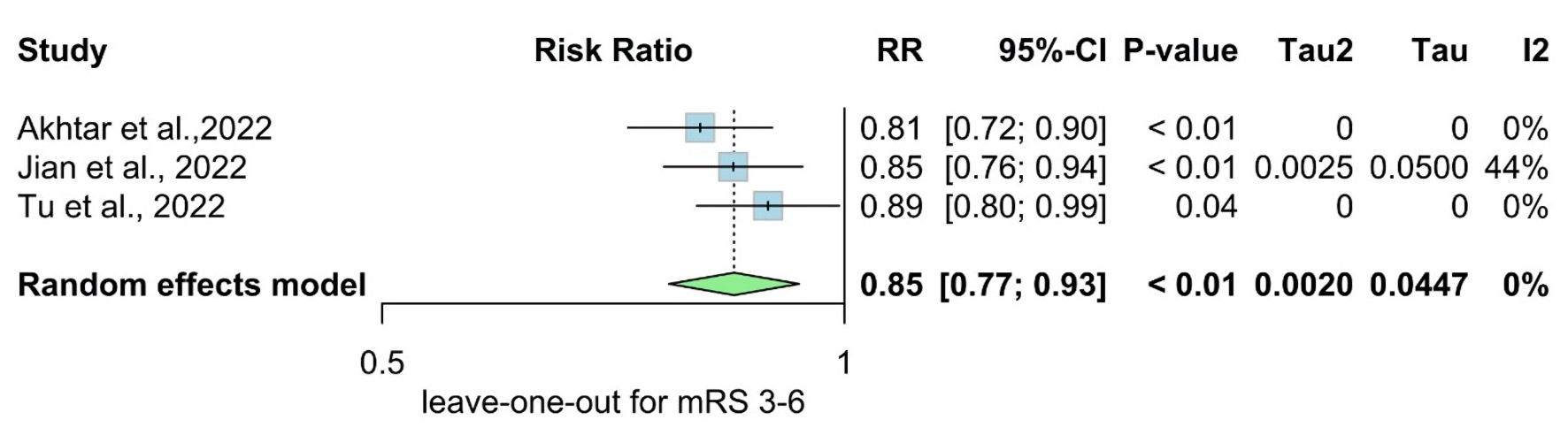 Click for large image | Figure 9. Leave-one-out meta-analysis for mRS 3 - 6. RR: risk ratio; CI: confidence interval; mRS: modified Rankin Scale. |
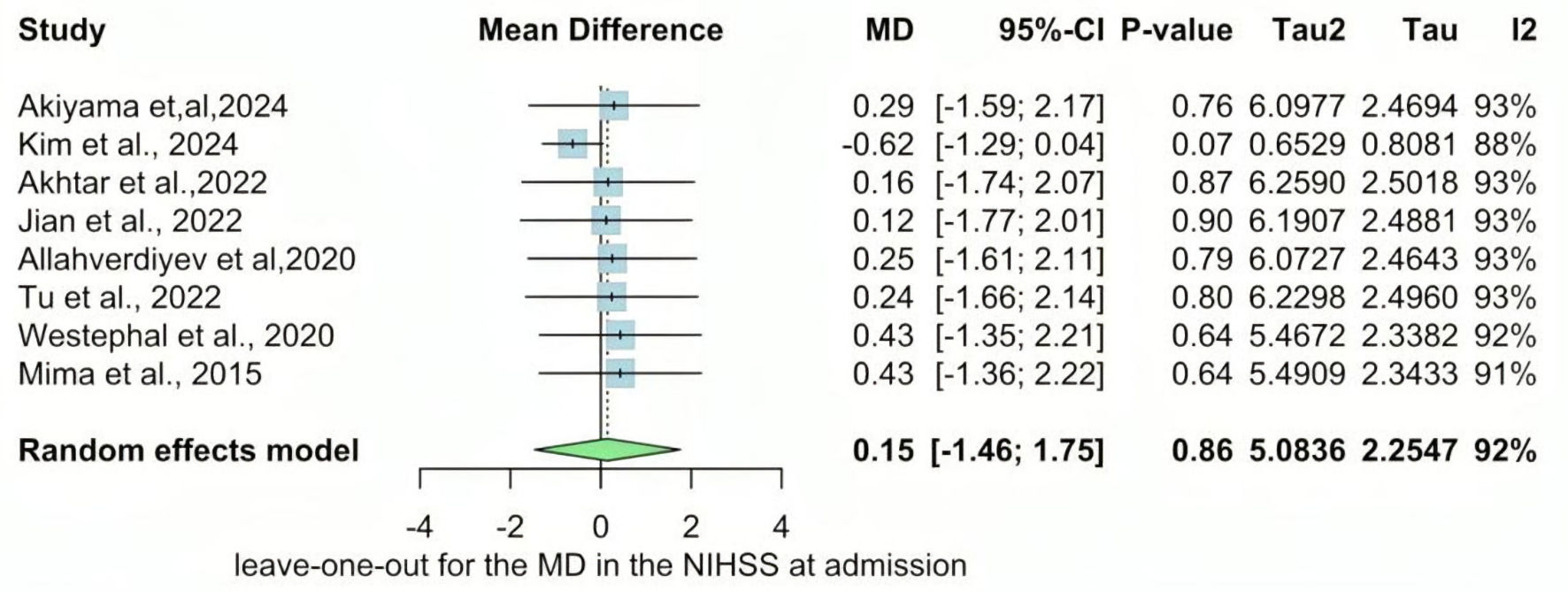 Click for large image | Figure 10. Leave-one-out of the meta-analysis for NIHSS at admission. MD: mean difference; CI: confidence interval; NIHSS: National Institutes of Health Stroke Scale. |
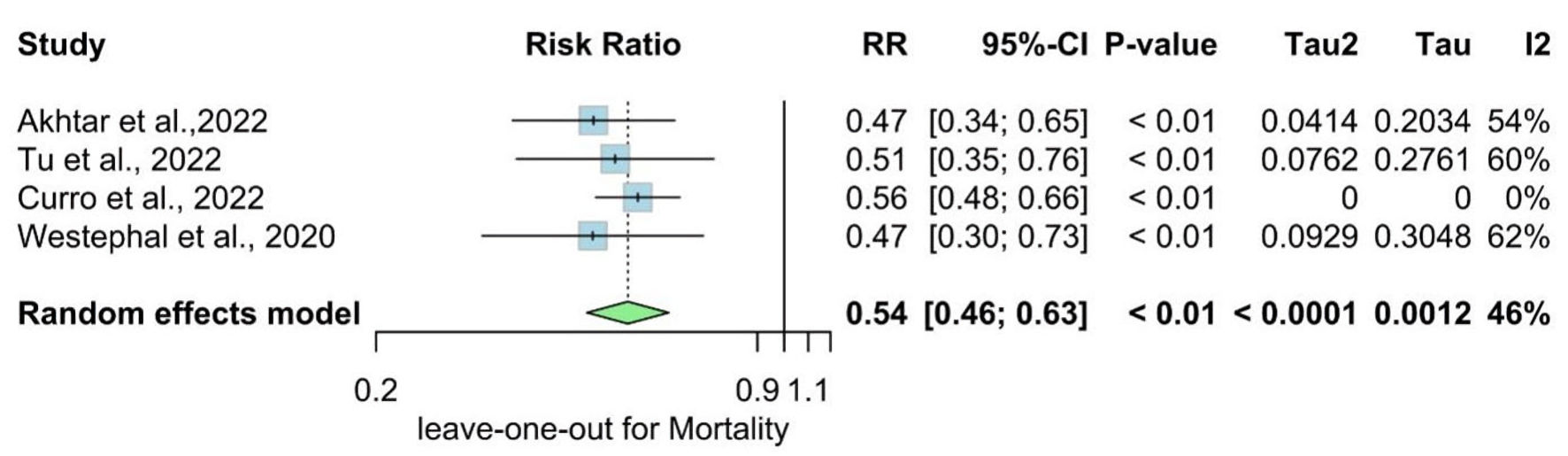 Click for large image | Figure 11. Leave-one-out of the meta-analysis for mortality. RR: risk ratio; CI: confidence interval. |
| Discussion | ▴Top |
This meta-analysis revealed that the metformin group has higher favorable mRS 0 - 2 than the non-metformin group (RR = 1.14, 95% CI: 1.09 - 1.19, P value ≤ 0.01), and the mRS 3 - 6 shows that the non-metformin group had a higher rate of fewer favorable results (RR = 85, 95% CI: 0.77 - 0.93, P value ≤ 0.01). While NIHSS at discharge was lower in the metformin group than the non-metformin group (MD = -0.46, 95% CI: -0.82 - -0.11, P value ≤ 0.01), the RR for mortality was statistically significantly lower in the metformin group compared with the non-metformin group (RR = 0.54, 95% CI: 0.46 - 0.63, P value ≤ 0.01). Conversely, the NIHSS at admission revealed no statistically significant difference between the two groups. Based on these findings, Metformin reduces the severity of stroke.
Metformin may improve stroke prognosis through several mechanisms. Firstly, it exerts antioxidant and anti-inflammatory effects by activating AMPK [22]. Through increasing angiogenesis, metformin promotes post-stroke recovery; AMPK signaling mediates these benefits [23]. Furthermore, by inducing AMPK, metformin may protect cells by regulating Nrf2 antioxidant and inflammatory pathways [24]. Patients with diabetes who were taking metformin at the time of their stroke were more likely to have a better prognosis than those who were not [14]. Secondly, the polarization of microglia and macrophages mediated by AMPK and angio-neurogenesis could be essential in metformin-promoted recovery [16]. Thirdly, metformin demonstrated neuroprotective effects against ischemic brain injury following cardiopulmonary resuscitation and sudden cardiac arrest via enhancing autophagy, which is dependent on AMPK initiation [25].
Several studies included in our meta-analysis reported a statistically significant reduction in the risk of recurrent stroke among patients treated with metformin. These findings align with our analysis, suggesting a potential protective effect of metformin in the post-stroke period, and it is not limited to diabetic patients. Abbasi et al conducted an RCT, which reported that metformin reduced the severity and stroke symptoms and accelerated recovery and clinical outcome in patients with cortical stroke on metformin [21]. However, it is important to note that one study [20] reported conflicting results or non-significant findings. These conflicting findings may be because of the bias predicted in the methodology of this study. According to the NIH, the quality of the assessment tool was of high risk due to bias predicted in multiple domains (its score: 4 out 14). Regarding the benefits of metformin in specific age groups, we found no significant differences in the effect of metformin across certain age groups in our included studies. We can attribute that to the high prevalence of stroke in elderly patients. However, metformin has already showed a beneficial effect in obese patients, as it has less weight gain and fewer hypoglycemic attacks compared to other antidiabetic medications [26]. The dose of metformin did not show a dose-dependent effect on prognosis or clinical outcomes. Correlation analysis conducted by Westphal et al showed no significant association between metformin dose and NIHSS at admission, mRS after 3 months, mortality, and intracerebral hemorrhage (ICH) [19]. Same findings were reported by Kim et al [12].
Pakkam et al [27] conducted the most recent meta-analysis, which showed a significantly higher rate of mRS 0 - 2 score at discharge and a lower rate of 90-day mortality, which is consistent with our study. Our meta-analysis is more comprehensive and included 11 studies with larger populations. We also reported NIHSS scores at admission and discharge, which showed that the NIHSS at discharge was significantly lower in the metformin group [27].
The strength of our study is that it is the most updated meta-analysis to discuss the impact of prior metformin on clinical outcomes in stroke patients with 11 included studies. Another strength is the inclusion of a substantial number of studies in our meta-analysis. We incorporated 11 studies to allow strong quantitative analysis. The large sample also enhanced the statistical power of our analysis and increased confidence in the result. Moreover, our meta-analysis included studies with diverse study designs, encompassing randomized clinical trials and observational studies; including various study designs adds strength to our findings and increases applicability.
Despite these strengths, our study also has some limitations that should be taken into consideration. Firstly, most studies included in our meta-analysis were of moderate quality, although we conducted a thorough risk of bias assessment using appropriate tools. The limitations of individual studies could influence the overall quality of evidence. Secondly, the included studies exhibited some heterogeneity in outcome measures, which affected the interpretation of the results. To address this, we performed a sensitivity analysis to explore the impact of these factors on the overall findings. Lastly, our meta-analysis focused on the impact of metformin on stroke outcomes and did not explore potential adverse effects or safety concerns associated with metformin use in stroke patients. Therefore, future research should address the safety aspect to provide a more comprehensive understanding of the risk-benefit profile of metformin in this population. We cannot reach a solid conclusion regarding publication bias due to the limited study numbers according to Cochrane guidelines.
Conclusions
Pre-stroke metformin therapy is associated with better post-stroke clinical outcomes and lower mortality rates. These findings demonstrate the possible neuroprotective effects of metformin and also highlight its practical value as an adjuvant treatment for stroke patients. Further research is required to understand its mechanism.
| Supplementary Material | ▴Top |
Suppl 1. The detailed search strategy for each electronic database.
Suppl 2. NIH Quality Assessment scores.
Suppl 3. Cochrane risk of bias tool for randomized controlled trials (ROB).
Acknowledgments
None to declare.
Financial Disclosure
No funding was provided to complete this work.
Conflict of Interest
We declare no conflict of interest.
Informed Consent
Not applicable.
Authors Contributions
AE, KS, and NS conceived the idea and supervised the project. AE, AYS, AER, MKD, AEK, HAM, MMG, and MSA collected and analyzed the data. AYS, AEh, YH, HGD, and AER drafted the manuscript. All authors have revised and agreed to the final version of the manuscript.
Data Availability
The data supporting this study’s findings are available from the corresponding author KS upon reasonable request.
Abbreviations
AMPK: adenosine 5’-monophosphate-activated protein kinase; DBP: diastolic blood pressure; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; Nrf2: nuclear factor-erythroid 2-related factor; SBP: systolic blood pressure; T2DM: type 2 diabetes mellitus
| References | ▴Top |
- Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20(6):953-966.
doi pubmed - Jia J, Cheng J, Ni J, Zhen X. Neuropharmacological actions of metformin in stroke. Curr Neuropharmacol. 2015;13(3):389-394.
doi pubmed - Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38(2):208-211.
doi pubmed - Putaala J, Liebkind R, Gordin D, Thorn LM, Haapaniemi E, Forsblom C, Groop PH, et al. Diabetes mellitus and ischemic stroke in the young: clinical features and long-term prognosis. Neurology. 2011;76(21):1831-1837.
doi pubmed - Cheng YY, Leu HB, Chen TJ, Chen CL, Kuo CH, Lee SD, Kao CL. Metformin-inclusive therapy reduces the risk of stroke in patients with diabetes: a 4-year follow-up study. J Stroke Cerebrovasc Dis. 2014;23(2):e99-105.
doi pubmed - Wang M, Zhang Z, Georgakis MK, Karhunen V, Liu D. The impact of genetically proxied AMPK activation, the target of metformin, on functional outcome following ischemic stroke. J Stroke. 2023;25(2):266-271.
doi pubmed - Mima Y, Kuwashiro T, Yasaka M, Tsurusaki Y, Nakamura A, Wakugawa Y, Okada Y. Impact of metformin on the severity and outcomes of acute ischemic stroke in patients with type 2 diabetes mellitus. J Stroke Cerebrovasc Dis. 2016;25(2):436-446.
doi pubmed - Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583.
doi pubmed - Cochrane. Cochrane handbook for systematic reviews of interventions. 2022. Available from: https://training.cochrane.org/handbook/current. Accessed January 5, 2023.
- National Institutes of Health. Study quality assessment tools. 2021. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed January 5, 2023.
- Altman DG, Bland JM. Standard deviations and standard errors. BMJ. 2005;331(7521):903.
doi pubmed - Kim C, Kim Y, Sohn JH, Sung JH, Han SW, Lee M, Kim Y, et al. Effects of prior metformin use on stroke outcomes in diabetes patients with acute ischemic stroke receiving endovascular treatment. Biomedicines. 2024;12(4):745.
doi pubmed - Akiyama N, Yamashiro T, Ninomiya I, Uemura M, Hattori Y, Ihara M, Onodera O, et al. Neuroprotective effects of oral metformin before stroke on cerebral small-vessel disease. J Neurol Sci. 2024;456:122812.
doi pubmed - Akhtar N, Singh R, Kamran S, Babu B, Sivasankaran S, Joseph S, Morgan D, et al. Diabetes: chronic metformin treatment and outcome following acute stroke. Front Neurol. 2022;13:849607.
doi pubmed - Jian Y, Wang H, Zhao L, Li T, Zhang L, Wang X, Zhang Y, et al. Metformin treatment and acute ischemic stroke outcomes in patients with type 2 diabetes: a retrospective cohort study. Neurol Sci. 2023;44(3):989-997.
doi pubmed - Tu WJ, Liu Z, Chao BH, Yan F, Ma L, Cao L, Ji XM, et al. Metformin use is associated with low risk of case fatality and disability rates in first-ever stroke patients with type 2 diabetes. Ther Adv Chronic Dis. 2022;13:20406223221076894.
doi pubmed - Kersten C, Knottnerus ILH, Heijmans E, Haalboom M, Zandbergen AAM, den Hertog HM. Effect of metformin on outcome after acute ischemic stroke in patients with type 2 diabetes mellitus. J Stroke Cerebrovasc Dis. 2022;31(9):106648.
doi pubmed - Curro CT, Fiume G, Cotroneo M, Russo G, Casella C, Dell'Aera C, Fazio MC, et al. Ischemic stroke and reperfusion therapies in diabetic patients. Neurol Sci. 2022;43(7):4335-4348.
doi pubmed - Westphal LP, Widmer R, Held U, Steigmiller K, Hametner C, Ringleb P, Curtze S, et al. Association of prestroke metformin use, stroke severity, and thrombolysis outcome. Neurology. 2020;95(4):e362-e373.
doi pubmed - Allahverdiyev T, Sorgun MH, Togay Isikay C, Yucesan C. The effect of metformin therapy on severity and prognosis in acute ischemic stroke. Turk J Cerebrovasc Dis. 2020;26(3):256-261.
- Beduk Esen CS, Gedik ME, Canpinar H, Yedekci FY, Yildiz F, Gunaydin G, Gultekin M. Radiosensitising effects of metformin added to concomitant chemoradiotherapy with cisplatin in cervical cancer. Clin Oncol (R Coll Radiol). 2023;35(11):744-755.
doi pubmed - Mudgal J, Nampoothiri M, Basu Mallik S, Kinra M, Hall S, Grant G, Anoopkumar-Dukie S, et al. Possible involvement of metformin in downregulation of neuroinflammation and associated behavioural changes in mice. Inflammopharmacology. 2019;27(5):941-948.
doi pubmed - Venna VR, Li J, Hammond MD, Mancini NS, McCullough LD. Chronic metformin treatment improves post-stroke angiogenesis and recovery after experimental stroke. Eur J Neurosci. 2014;39(12):2129-2138.
doi pubmed - Ashabi G, Khalaj L, Khodagholi F, Goudarzvand M, Sarkaki A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab Brain Dis. 2015;30(3):747-754.
doi pubmed - Zhu J, Liu K, Huang K, Gu Y, Hu Y, Pan S, Ji Z. Metformin improves neurologic outcome via AMP-activated protein kinase-mediated autophagy activation in a rat model of cardiac arrest and resuscitation. J Am Heart Assoc. 2018;7(12):e008389.
doi pubmed - Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854-865.
pubmed - Pakkam M, Orscelik A, Musmar B, Tolba H, Ghozy S, Senol YC, Bilgin C, et al. The impact of pre-stroke metformin use on clinical outcomes after acute ischemic stroke: A systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2024;33(6):107716.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.