| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 3, March 2025, pages 164-173
Predictive Factors for Diagnosing Diabetic Ketoacidosis or Simple Hyperglycemia in Adults With High Blood Glucose: The “1-DKA Alert” Study
Wanwisa Peamyaoa, Thanin Lokeskraweea, e, Suppachai Lawanaskolb, Jayanton Patumanondc, Suwapim Chanlaord, Wanwisa Bumrungpagdeed, Chawalit Lakdeed
aDepartment of Emergency Medicine, Lampang Hospital, Lampang, Thailand
bChaiprakarn Hospital, Chiang Mai, Thailand
cClinical Epidemiology and Clinical Statistics Unit, Faculty of Medicine, Naresuan University, Phitsanulok, Thailand
dDepartment of Radiology, Buddhachinaraj Phitsanulok Hospital, Phitsanulok, Thailand
eCorresponding Author: Thanin Lokeskrawee, Department of Emergency Medicine, Lampang Hospital, Lampang 52000, Thailand
Manuscript submitted January 6, 2025, accepted March 3, 2025, published online March 11, 2025
Short title: Predictive Factors for DKA in Adults
doi: https://doi.org/10.14740/jocmr6180
| Abstract | ▴Top |
Background: Hyperglycemia is commonly encountered in the Emergency Departments, necessitating the differential diagnosis between diabetic ketoacidosis (DKA) and simple hyperglycemia, as the treatment and prognosis differ significantly. In clinical practice, it is essential to investigate DKA in all patients; however, the final diagnosis of actual DKA is found in only 1-5% of these cases, resulting in unnecessary costs. This study aimed to develop an application for predicting the probability of DKA in patients with capillary blood glucose levels exceeding 250 mg/dL in the Emergency Department.
Methods: This study was conducted as diagnostic prediction research, employing a retrospective observational delayed-type cross-sectional design. Data were collected from patients with capillary blood glucose levels exceeding 250 mg/dL between January and April 2023. The predictive variables were available at the time of prediction. Analysis was performed using multivariable risk ratio regression analysis, with results reported as multivariable risk ratios. The area under the receiver operating characteristic (AuROC) curve was calculated. Internal validation was performed using bootstrapping and calibration plots. An application named “1-DKA Alert” was developed to predict the probability of DKA for use in real-world clinical settings.
Results: The study included 274 adult patients, of whom 52.9% were female, with an average age of 59 years. Predictive factors for DKA included initial capillary blood glucose, type of diabetes mellitus, insulin usage, poor compliance, respiratory rate, and suspected infection. These variables were readily available in clinical practice and yielded an AuROC of 0.8777 (95% confidence interval (CI): 0.8294 - 0.9259). Bootstrapping internal validation demonstrated an AuROC of 0.8770 and a shrinkage factor of 0.991.
Conclusions: The “1-DKA Alert” demonstrates excellent discriminative ability, and the model is valid, suggesting its potential for use in clinical practice. However, further studies for external validation are necessary.
Keywords: Diabetic ketoacidosis; Hyperglycemia; Clinical prediction rules; Diagnosis, Mobile applications; Diabetes mellitus
| Introduction | ▴Top |
In the Emergency Department (ED) of Lampang Hospital, a substantial number of hyperglycemic patients are encountered, approximately 1,080 to 1,440 per year (data from Lampang Hospital). It is essential to differentiate between diabetic ketoacidosis (DKA) and simple hyperglycemia, as treatment and prognosis differ [1]. Patients with blood glucose levels exceeding 250 mg/dL, hyperglycemic state, undergo testing with a DKA laboratory panel (including blood glucose, serum ketone, venous pH, and bicarbonate) in all cases. However, actual DKA is confirmed in only 1-5% of these patients [2].
Clinical prediction models for DKA have been developed, but they primarily focus on predicting mortality, hospitalization, and early resolution of DKA [3, 4]. Moreover, existing clinical diagnostic models have been primarily studied in pediatric populations [5, 6].
The absence of predictive models for DKA in adults may partly be due to the clarity of the diagnostic criteria for DKA in adults [1]. However, in the context of Thailand, where access to healthcare services is relatively convenient, there is a financial burden associated with testing the DKA laboratory panel, amounting to approximately 750 Baht per case and totaling 1.1 million Baht per year (approximately $32,000). Therefore, this study aims to develop a mobile application to differentiate between DKA and simple hyperglycemia to avoid excessive investigation, particularly in developing countries.
| Materials and Methods | ▴Top |
Study design
This study was conducted as diagnostic prediction research, designed as a retrospective observational delayed-type cross-sectional study in the ED of Lampang Hospital (a tertiary regional hospital in northern Thailand with 743 general beds and 82 intensive care unit beds) from January to April 2023. Patients with hyperglycemia underwent diagnostic evaluation according to routine clinical practice. Data on baseline characteristics, clinical symptoms, and laboratory results were collected to develop a clinical prediction model for differentiating between DKA and simple hyperglycemia.
Participant and data collection
Participants
Patients aged 18 years and older with blood glucose levels exceeding 250 mg/dL underwent testing with a DKA laboratory panel, which includes blood glucose, serum ketone, venous pH, and bicarbonate. Data were collected on clinical characteristics, presenting symptoms, clinical signs, vital signs, comorbidities, medication history, and laboratory results from the electronic medical record. However, pregnant women were excluded from the study due to the very low incidence of diabetic crises.
Endpoints
This study references the diagnostic criteria for DKA as outlined by the American Diabetes Association (ADA) in 2009 [1], which require the presence of all three of the following criteria: 1) blood glucose > 250 mg/dL; 2) β-hydroxybutyrate concentration (serum ketone) ≥ 3.0 mmol/L, or urine ketone strip 2+ or greater; 3) pH ≤ 7.3 and/or bicarbonate concentration ≤ 18 mmol/L. Data collection for this research was completed prior to the revision of the ADA’s diagnostic criteria for DKA in August 2024 [7].
Candidate predictors
Age
Age is directly measured as a quantitative variable.
Capillary blood glucose (CBG) (mg/dL)
All patients underwent CBG testing (mg/dL) in the ED. For patients referred from outpatient departments with hyperglycemia, a repeat test was conducted, using the ED value as the primary measure. All testing devices were standardized to the same brand and model. If the CBG reading was reported as “Hi” (unmeasurable), indicating a level exceeding 600 mg/dL [8], confirmation with venous blood glucose testing was required. Since laboratory results were not available at that moment, the “Hi” reading was substituted with a maximum value of 600 mg/dL. CBG greater than 250 mg/dL, not yet confirmed by the DKA laboratory panel, is considered “hyperglycemia” prior to the moment of prediction.
Respiratory rate (breaths/min) [9]
We use respiratory rate instead of dyspnea because it is an objective measure obtained from the vital signs medical record.
Type of diabetes mellitus (DM) (undiagnosed, type 1, or type 2) [10]
If the patient had a prior history of diabetes, the type of DM was documented in the medical records. However, if the patient experienced hyperglycemia for the first time, their diabetes status was recorded as undiagnosed.
Previous DKA (yes or no) [11] and type of hypoglycemic agent (oral (yes/no) or insulin usage (yes/no)] [12] can also be retrieved from the medical record, taking only a few minutes.
Compliance (poor or good) [12]
Compliance was primarily assessed through medical records, particularly in diabetes clinics, where it is typically documented as “good” or “poor”. If such records were unavailable, compliance was evaluated by taking a history from patients and reliable close relatives, along with an assessment of dietary habits, lifestyle, and medication adherence. When available, a hemoglobin A1c (HbA1c) level of ≥ 9.0% was also used as an indicator of poor compliance [13-15].
Suspected infection (yes or no) [12]
Patients often present with fever and/or localized symptoms. In some cases, they may lack a fever or present with hypothermia, but infection is suspected based on the physician’s clinical examination.
All candidate variables were available at the moment of prediction, and there was no missing data. The individuals who collected the predictor variables were different from those who collected the endpoints in order to reduce information bias.
Specific time point of prediction
This study utilized a specific time point of prediction following the patient’s initial assessment, with a CBG > 250 mg/dL, just before the physician decided to order the DKA laboratory panel.
Study size estimation
The study size estimation for a multivariable prediction model with a binary endpoint, as proposed by Riley et al [16], set the C-statistic and shrinkage factor at 0.8 and 0.9, respectively. With a total of six predictor variables and a prevalence of DKA (based on a pilot study at the Lampang Hospital) of 20%, a minimum of 266 participants was required, including at least 54 cases of DKA.
Statistical analysis
Model derivation
The final prediction model was developed using the stepwise backward elimination method from the full multivariable risk ratio regression, with a significance level set at P < 0.05. Additionally, predefined candidate factors were manually entered to ensure the equation was as clinically relevant as possible. The researchers have already tested the linear relationship between CBG, respiratory rate, and the risk of DKA.
Clustered robust variance correction was used to address the dependency in the data resulting from multiple visits for hyperglycemia.
Model performance and internal validation
The model’s discriminative ability was assessed using the C-statistic, presented as the area under the receiver operating characteristic (AuROC) curve [17]. Calibration performance was evaluated through calibration plots, calibration slopes, expected-to-observed outcomes (E:O) ratio, and calibration-in-the-large (CITL). Internal validation was conducted using the bootstrapping method with 200 cycles to assess model optimism.
To evaluate the clinical utility of the prediction model, we used decision curve analysis (DCA) [18, 19]. This method assesses the net benefit (NB) of prediction models by subtracting false positives from true positives. DCA revealed how the NB changed across threshold probabilities for patients with DKA.
Identifying cut points for clinical implications
Diagnostic indices were calculated, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), according to the cut points derived from various predicted probabilities. This aimed to achieve a cut point that resulted in a false negative of less than 5% from total and a false positive of less than 10% from total, as calculated from the confusion matrix.
The study protocol was registered in the Thai Clinical Trials Registry (TCTR) (TCTR20241212001). The Institutional Review Board of Lampang Hospital approved the study protocol (CERT No.: 047/66). Informed consent was waived due to the observational nature of the study. This study reported according to the standards of the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline [20].
| Results | ▴Top |
During January to April 2023, a total of 274 eligible patients were included, consisting of 218 cases of simple hyperglycemia and 56 cases of DKA, respectively. The prevalence of DKA was 20.4% (Fig. 1).
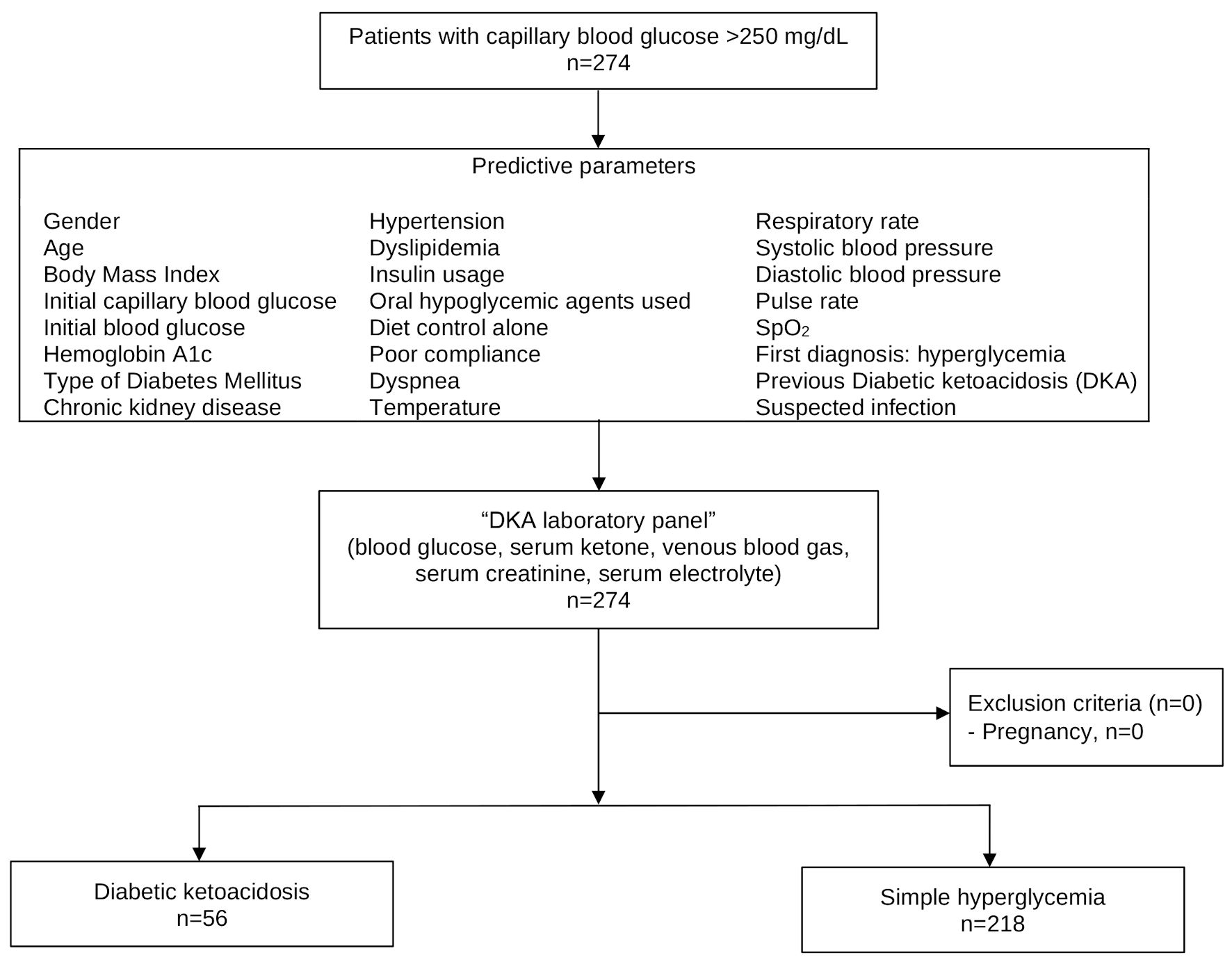 Click for large image | Figure 1. Study flow diagram. |
In this study, the majority of patients were female (52.9%) with a mean age of 58.8 ± 15.0 years. Patients with DKA were generally younger, had a higher average initial CBG level, and predominantly had type 1 DM, receiving insulin for treatment. They also exhibited poorer compliance, more frequently presented with an increased respiratory rate, were often suspected of having concurrent infections, and had a history of previous DKA (Table 1).
 Click to view | Table 1. Baseline Characteristics of Patients With Diabetic Ketoacidosis and Simple Hyperglycemia |
Significant predictors
Using the stepwise backward elimination method with manual entry, six predictors (seven parameters) were identified for predicting DKA: initial CBG (multivariable risk ratio (mRR): 1.00; 95% confidence interval (CI): 1.00, 1.00; P = 0.043), type 1 DM (mRR: 2.93; 95% CI: 1.00, 8.52; P = 0.049), type 2 DM (mRR: 2.14; 95% CI: 0.83, 5.53; P = 0.117), insulin usage (mRR: 2.79; 95% CI: 1.63, 4.77; P < 0.001), poor compliance (mRR: 2.43; 95% CI: 1.45, 4.05; P = 0.001), respiratory rate (mRR: 1.04; 95% CI: 1.01, 1.06; P = 0.001), and suspected infection (mRR: 2.51; 95% CI: 1.32, 4.78; P = 0.005), respectively (Table 2).
 Click to view | Table 2. Final multivariable risk ratio regression analysis for the predictive model |
Model performances
Six predictors were identified, including initial CBG, type of DM, insulin usage, poor compliance, respiratory rate, and suspected infection. The discriminative ability, assessed using the AuROC, was 0.8777 (95% CI: 0.8294, 0.9259), indicating excellent discrimination (Fig. 2). The calibration plot showed good agreement between observed and predicted risks (Fig. 3). The bootstrap AuROC was close to the apparent value (0.8770; 95% CI: 0.8330, 0.9310), with a bootstrap shrinkage of 0.991 (Table 3). Details of the equation to create the application named “1-DKA Alert” prediction model are as follows:
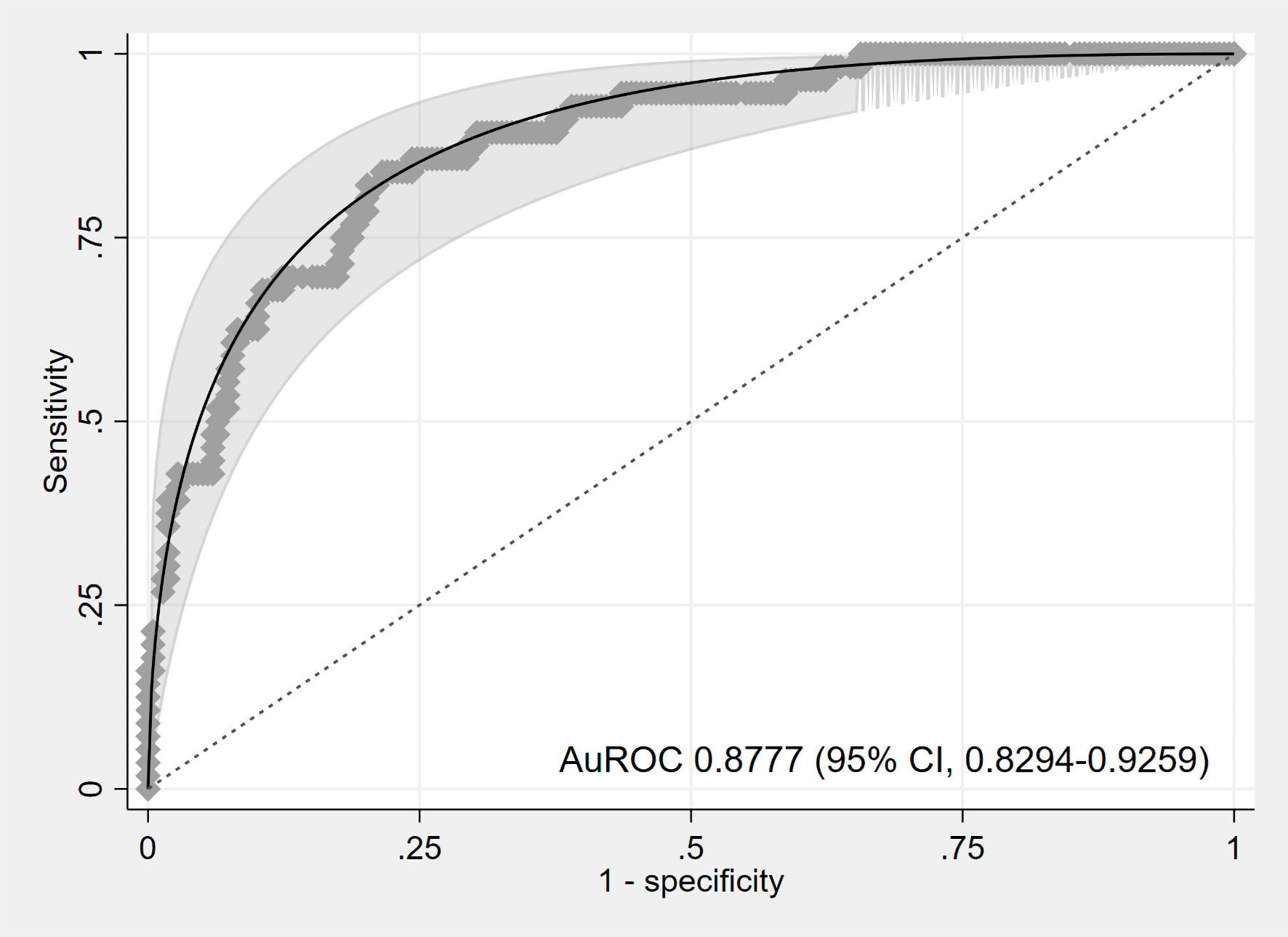 Click for large image | Figure 2. Area under the receiver operating characteristic (AuROC) curve. CI: confidence interval. |
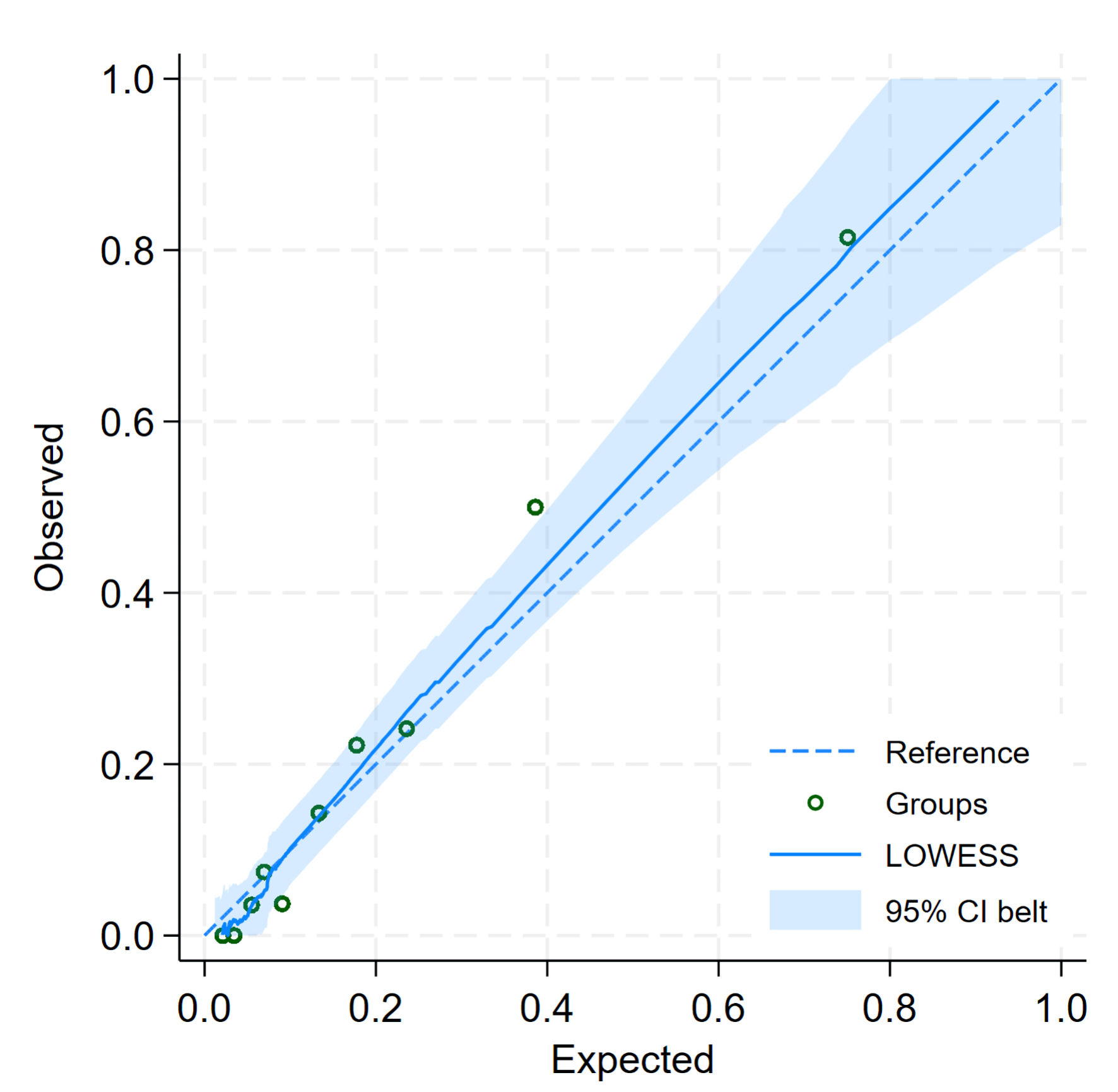 Click for large image | Figure 3. Calibration plot for predicted and observed probabilities of diabetic ketoacidosis. CI: confidence interval. |
 Click to view | Table 3. Internal Validation Using the Bootstrap Resampling Method |
Utilization of the “1-DKA Alert” prediction model is illustrated in the DCA graph (Fig. 4). The model demonstrates a NB starting at a DKA prevalence of 5%, which continues to increase progressively across higher prevalence levels of DKA.
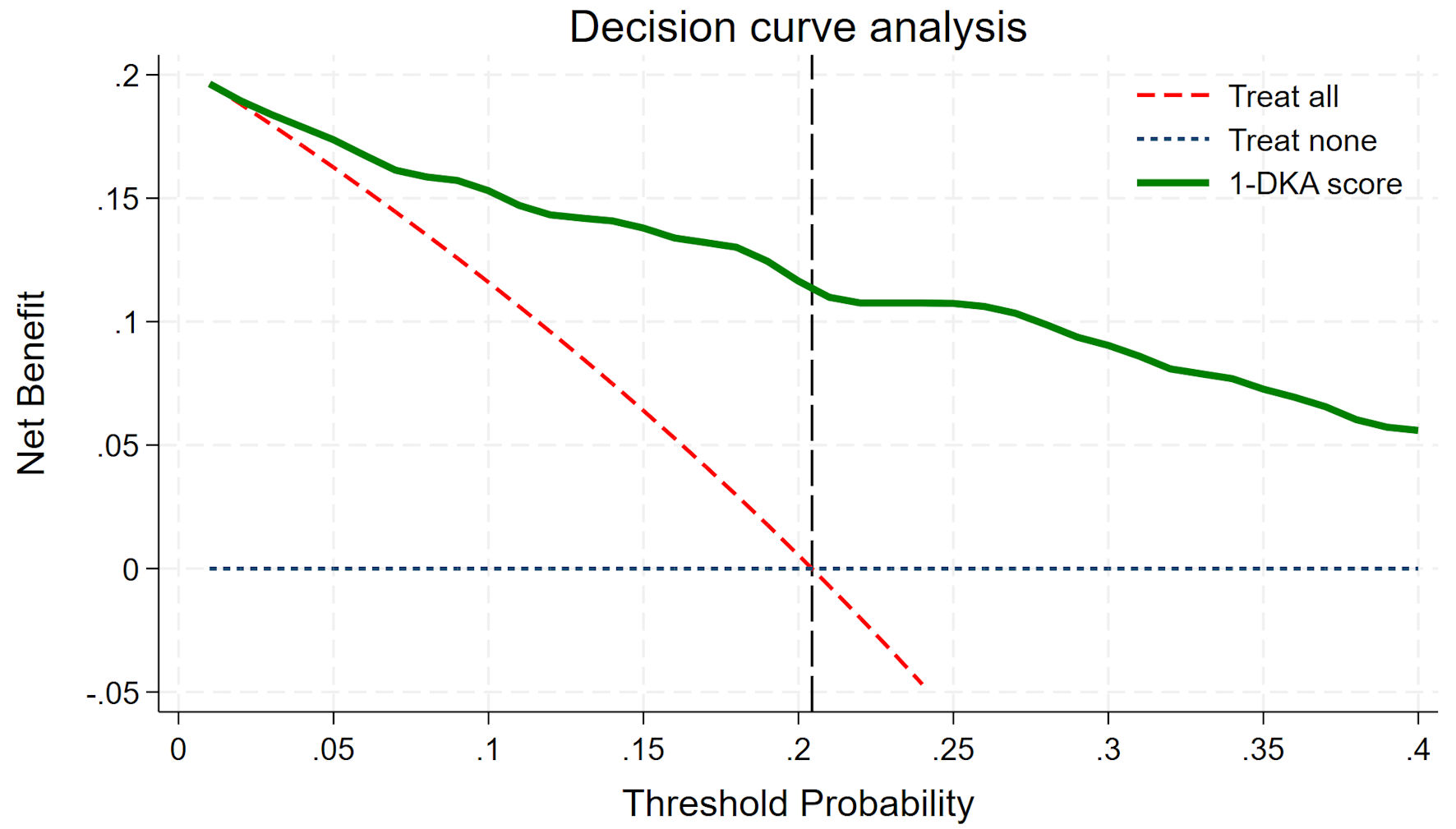 Click for large image | Figure 4. Decision curve analysis. DKA: diabetic ketoacidosis. |
Cut-point threshold selection
Using the confusion matrix concept, we determined the optimal predicted probability cut-off to maintain a false negative below 5% and a false positive below 10%. We began with the dataset’s DKA prevalence of 20.4%, corresponding to a linear combination (xb) of -1.6. However, the false negative at this threshold was 5.1%, exceeding the predefined limit. Adjusting the cut-off to 19.4% (xb = -1.65) minimized the false negative to 4.7%. Additionally, we explored alternative cut-off points at 15% (xb = -1.9) and 25% (xb = -1.4) to assess their impact on the model’s performance (Fig. 5, Table 4).
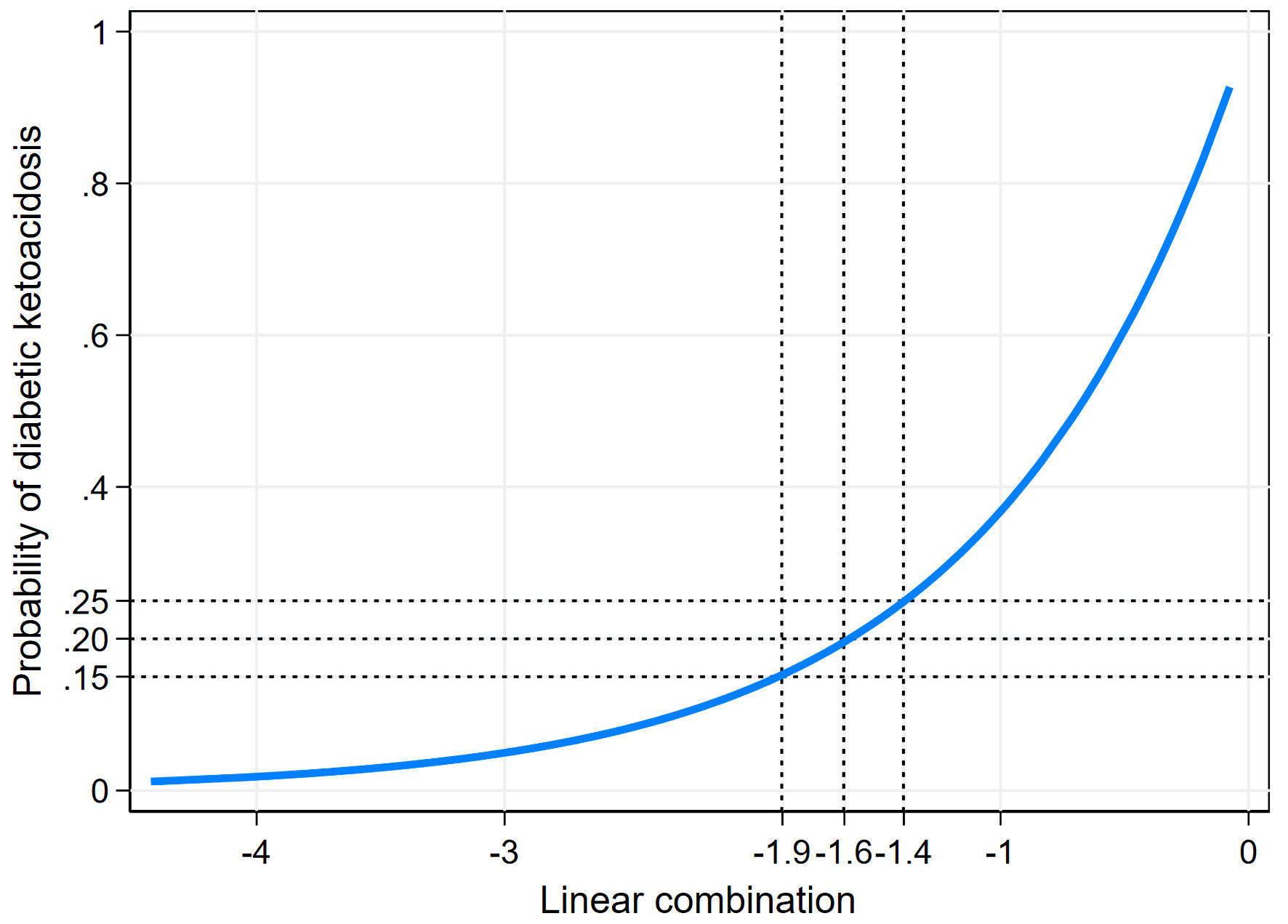 Click for large image | Figure 5. Risk curve showing predicted probability of diabetic ketoacidosis across linear combination values. |
 Click to view | Table 4. Accuracy and Diagnostic Indices at Different Probability Cut-Points for Predicting DKA |
| Discussion | ▴Top |
Previous studies have reported a DKA prevalence as low as 1-5% [2]. In contrast, this study observed a significantly higher prevalence of 20.4%, consistent with findings from a study conducted in Hat Yai, Thailand [21]. This discrepancy may be due to differences in patient demographics, as the earlier study [2] involved a different ethnic population. Additionally, previous studies [2] may have assessed DKA prevalence across entire hospitals, whereas this study specifically focused on ED patients, where DKA prevalence is naturally higher. Another possible explanation is that prior studies may not have consistently performed the full DKA laboratory panel on all cases, potentially leading to an underestimation of DKA diagnoses. As shown in Figure 4, the DCA demonstrates that the “1-DKA Alert” provides a NB even at a low prevalence (5%), with the benefit increasing as prevalence rises. This suggests that the model can be effectively generalized across both low- and high-prevalence settings.
For walk-in patients from other hospitals with limited education, determining the type of DM can be challenging. We classified patients based on age, body type, age at diagnosis, and medications brought. In Thailand, the majority (95.0%) have type 2 DM [22], while type 1 DM typically presents at a younger age, often before 20, with a lean body type [23, 24]. If hyperglycemia is observed at the initial diagnosis without a prior history of diabetes, the patient is classified as undiagnosed.
The primary concern regarding the implementation of the “1-DKA Alert” application is its potential time-consuming nature, particularly in verifying medical history, diabetes type, medication compliance, and insulin usage. However, our experience demonstrates that this system is both highly efficient and practical in the ED setting, requiring only 1 - 3 min for completion.
Although machine learning methods are more popular and perform outstandingly in both discrimination and calibration, the small study size makes traditional statistical modeling a more suitable approach for model derivation in this study. Furthermore, the simplicity, predictor interpretability, deployment availability, and reproducibility of traditional statistical methods, specifically multivariable cluster-robust variance correction risk ratio regression, outweigh those of machine learning.
If the “1-DKA Alert” is not utilized, detecting all 56 true DKA cases would require performing the full DKA laboratory panel on all 274 patients, meaning five patients must be tested to identify one true case. While this guarantees no missed cases (false negatives = 0%), it is highly resource-intensive and inefficient. By applying the “1-DKA Alert” with a predicted probability threshold of 19.4%, targeted testing can reduce the number of patients requiring the DKA laboratory panel to 85 cases, while still detecting 43 true DKA cases. This results in an improved efficiency of two patients tested per true case detected, as reflected in the confusion matrix (Table 4), which demonstrates the balance between sensitivity and specificity in model performance.
The benefits of using the “1-DKA Alert” include: 1) Absolute reduction in tests: The number of tests decreases by 189 cases (from 274 to 85), leading to an estimated cost saving of 141,750 Thai Baht (THB) (about $4,218.40) at 750 THB ($22.30) per test. This cost saving is calculated based on data collected over a 4-month period. 2) Relative reduction in tests: The total number of tests is reduced by 69.0% ((189/274) × 100). Although the “1-DKA Alert” significantly optimizes resource utilization while maintaining clinical safety, its representativeness, generalizability, and transportability should be further evaluated in an external validation dataset.
Limitations and biases
This study utilized the DKA diagnostic criteria established by the ADA in 2009 [1], which specifies blood glucose > 250 mg/dL. However, in 2024, these criteria were revised [7], lowering the blood glucose threshold to ≥ 200 mg/dL or a prior history of diabetes, along with revised parameters of pH < 7.3 and/or bicarbonate concentration < 18 mmol/L. The change reflects findings from several studies reporting an increase in euglycemic DKA cases. The researchers obtained ethics committee approval for this study, and data collection was completed prior to these guideline changes. External validation in a new diagnostic criteria domain should be performed.
Assessing poor compliance may introduce bias, especially when medical records lack documentation. In such cases, particularly for walk-in patients from other hospitals, compliance is assessed by asking the patient or reliable relatives about their physician’s feedback. However, this approach is prone to self-reporting bias, as patients may claim good compliance even if they are non-adherent (social desirability bias). If a patient admits to poor compliance, it is likely reliable, as individuals tend to portray themselves favorably. However, when a patient reports good compliance or is uncertain, misclassification bias may occur, leading to potential overestimation of adherence. This limitation should be considered when interpreting the study findings.
Among patients presenting with hyperglycemia as their first diagnosis, the type of diabetes was classified as “undiagnosed”. Additionally, as previously mentioned, we assumed their compliance to be “poor” since they had never undergone a check-up. Despite exhibiting an increased respiratory rate and a suspected infection, the “1-DKA Alert” tended to underpredict DKA in this group.
In contrast, the prevalence of type 1 DM is higher in the pediatric population than in the adult population. The proportion of HbA1c, poor drug compliance, drug dose, and other predictors might be less available compared to adult patients. External validation using a full model approach in both pediatric and adult populations may be valuable. However, hyperglycemia in the pediatric population is rare, while type 1 DM presenting with DKA is common. Therefore, investigating all pediatric patients with hyperglycemia is worthwhile and reasonable.
Although the model achieved a false negative below the pre-defined threshold (< 5%), it produced a false positive of 15.3%, exceeding the target of < 10%. This overestimation remains a key limitation, necessitating further refinement to enhance specificity while maintaining clinical utility.
This study did not identify any cases of hyperosmolar hyperglycemic state (HHS) due to its very low incidence [25, 26]. In cases where predicting HHS is necessary, a research design incorporating a polynomial outcome may be appropriate. This would include: 1) simple hyperglycemia, 2) DKA, and 3) HHS, which would require a different statistical approach.
Conclusions
The “1-DKA Alert” demonstrates excellent discriminative ability and high calibration performance, providing DKA prediction with a low false-negative, suggesting its potential for use in clinical practice for patients with CBG >250 mg/dL. However, further studies are necessary for external validation in different domains.
Acknowledgments
This study was supported by the Lampang Medical Education Center. The authors would like to thank the Emergency Medicine residents, staff, and registered nurses at the Department of Emergency Medicine, Lampang Hospital.
Financial Disclosure
This study was supported by the Lampang Medical Education Center.
Conflict of Interest
The authors reported no contents in the article as conflict of interests.
Informed Consent
Informed consent was waived due to the observational nature of the study.
Author Contributions
Conceptualization: Wanwisa Peamyao, Thanin Lokeskrawee, Suppachai Lawanaskol, Jayanton Patumanond. Data curation: Wanwisa Peamyao. Formal analysis: Suwapim Chanlaor, Wanwisa Bumrungpagdee, Chawalit Lakdee, Thanin Lokeskrawee, Suppachai Lawanaskol, Jayanton Patumanond. Methodology: Thanin Lokeskrawee, Suppachai Lawanaskol, Jayanton Patumanond. Supervision: Thanin Lokeskrawee, Suppachai Lawanaskol, Jayanton Patumanond. Writing - original draft: Wanwisa Peamyao, Thanin Lokeskrawee, Suppachai Lawanaskol, Jayanton Patumanond.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author. To access this application of “1-DKA Alert”, we have provided the information at the link (https://www.tharathipdevelop.com/dka/form).
| References | ▴Top |
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343.
doi pubmed - Davis TME, Davis W. Incidence and associates of diabetic ketoacidosis in a community-based cohort: the Fremantle Diabetes Study Phase II. BMJ Open Diabetes Res Care. 2020;8(1):e000983.
doi pubmed - Safari S, Rahmani F, Soleimanpour H, Ebrahimi Bakhtavar H, Mehdizadeh Esfanjani R. Can APACHE II score predict diabetic ketoacidosis in hyperglycemic patients presenting to emergency department? Anesth Pain Med. 2014;4(4):e21365.
doi pubmed - Novida H, Setiyawan F, Adi Soelistijo S. A prediction model of mortality in patients hospitalized with diabetic ketoacidosis in a Tertiary Referral Hospital in Surabaya, Indonesia. Indian J Forensic Med Toxicol. 2021;15(2):2519-2526.
- Almazrouei R, Rahman Siddiqua A, Alanqar A, Govender R, Al-Shamsi S. Development and validation of a nomogram to predict diabetes ketoacidosis resolution time in a tertiary care hospital in the United Arab Emirates. Diabetes Res Clin Pract. 2024;213:111763.
doi pubmed - Jung Y, Lau A, Bednarczyk J. Immune checkpoint inhibitor-associated diabetic ketoacidosis and insulin-dependent diabetes: a case report. J Med Case Rep. 2024;18(1):611.
doi pubmed - Umpierrez GE, Davis GM, ElSayed NA, Fadini GP, Galindo RJ, Hirsch IB, Klonoff DC, et al. Hyperglycemic crises in adults with diabetes: a consensus report. Diabetes Care. 2024;47(8):1257-1275.
doi pubmed - Nuntapaitoon M, Sirisawadi S, Asawakarn S, Tummaruk P. Accuracy of portable human glucose meter (Accu-chek® Performa) for blood glucose measurement in newborn piglets. Thai J Vet Med. 2019;49(1):37-42.
- Paul S, Debnath S, Singh AK, Mishra S, Rajotiya S, Singh M, et al. Epidemiologic pattern and factors associated with adverse outcomes of diabetic ketoacidosis in medical intensive care units of a tertiary care centre in India. Endocrine and Metabolic Science. 2024;16:100204.
- Alhamdani YF, Almadfaa LO, AlAgha AE. Clinical variables influencing the severity of diabetes ketoacidosis. Saudi Med J. 2024;45(5):502-509.
doi pubmed - Hammersen J, Tittel SR, Warncke K, Fritsch M, Placzek K, Pacaud D, Karges B, et al. Previous diabetic ketoacidosis as a risk factor for recurrence in a large prospective contemporary pediatric cohort: Results from the DPV initiative. Pediatr Diabetes. 2021;22(3):455-462.
doi pubmed - Umpierrez GE, Kitabchi AE. Diabetic ketoacidosis: risk factors and management strategies. Treat Endocrinol. 2003;2(2):95-108.
doi pubmed - American Diabetes Association. Standards of care in diabetes-2023 abridged for primary care providers. Clin Diabetes. 2022;41(1):4-31.
doi pubmed - Wan EYF, Yu EYT, Mak IL, Youn HM, Chan KS, Chan EWY, Wong ICK, et al. Diabetes with poor-control HbA1c is cardiovascular disease 'risk equivalent' for mortality: UK Biobank and Hong Kong population-based cohort study. BMJ Open Diabetes Res Care. 2023;11(1):e003075.
doi pubmed - Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA, Clinical Guidelines Committee of the American College of Physicians, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168(8):569-576.
doi pubmed - Riley RD, Snell KI, Ensor J, Burke DL, Harrell FE, Jr., Moons KG, Collins GS. Minimum sample size for developing a multivariable prediction model: PART II - binary and time-to-event outcomes. Stat Med. 2019;38(7):1276-1296.
doi pubmed - Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5(9):1315-1316.
doi pubmed - Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565-574.
doi pubmed - Vickers AJ, van Calster B, Steyerberg EW. A simple, step-by-step guide to interpreting decision curve analysis. Diagn Progn Res. 2019;3:18.
doi pubmed - TRIPOD checklist: Prediction Model Development [Internet]. [cited 2024 Nov 21]. Available from: https://www.tripod-statement.org/wp-content/uploads/2020/01/Tripod-Checlist-Prediction-Model-Development.pdf.
- Na Rungsee P, Promwang N. Incidence of diabetic ketoacidosis in the emergency room of Hatyai Hospital. J Med Health Sci. 2022;29(3):81-92.
- The Diabetes Association of Thailand, The Endocrine Society of Thailand. Clinical Practice Guidelines for Diabetes. Bangkok: Srimoung Printing; 2023.
- Dejkhamron P, Santiprabhob J, Likitmaskul S, Deerochanawong C, Rawdaree P, Tharavanij T, Reutrakul S, et al. Young-onset diabetes patients in Thailand: Data from Thai Type 1 Diabetes and Diabetes diagnosed Age before 30 years Registry, Care and Network (T1DDAR CN). J Diabetes Investig. 2022;13(5):796-809.
doi pubmed - Ozougwu O. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J Physiol Pathophysiol. 2013;4(4):46-57.
- Karslioglu French E, Donihi AC, Korytkowski MT. Diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome: review of acute decompensated diabetes in adult patients. BMJ. 2019;365:l1114.
doi pubmed - Anthanont P, Khawcharoenporn T, Tharavanij T. Incidences and outcomes of hyperglycemic crises: a 5-year study in a tertiary care center in Thailand. J Med Assoc Thai. 2012;95(8):995-1002.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.