| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Case Report
Volume 17, Number 3, March 2025, pages 174-180
Differentiating Between Fibromuscular Dysplasia and Takayasu Arteritis in a Patient With Juvenile Renovascular Hypertension and Marked Hypokalemia Effectively Treated With Percutaneous Transluminal Renal Angioplasty
Ayako Ishiia, b, f, Keisuke Okamuraa, f, g, Shogo Morisakia, Yasunori Momotab, Akiko Yamashitab, Kenta Hatsusec, Keisuke Konod, Hideto Sakoa, Akihiro Udoa, Kenichiro Taniguchia, Tomoko Kosekia, Takuro Araia, Yoshie Yodogawaa, Yoshiko Obaa, Shiori Hirayamaa, Miki Inouea, Ichiro Imamurae
aDepartment of Cardiology and Cardiovascular Center, Imamura Hospital, Tosu, Saga, Japan
bDepartment of Neurology, Imamura Hospital, Tosu, Saga, Japan
cDepartment of Ophthalmology, Imamura Hospital, Tosu, Saga, Japan
dDepartment of Nephrology, Imamura Hospital, Tosu, Saga, Japan
eDepartment of Surgery, Imamura Hospital, Tosu, Saga, Japan
fThese authors contributed equally to this article.
gCorresponding Author: Keisuke Okamura, Department of Cardiology and Cardiovascular Center, Imamura Hospital, Tosu, Saga 841-0061, Japan
Manuscript submitted January 23, 2025, accepted March 6, 2025, published online March 9, 2025
Short title: FMD and TA Treated With PTRA
doi: https://doi.org/10.14740/jocmr6187
| Abstract | ▴Top |
Renovascular hypertension (RVHT) is most commonly caused by renal artery stenosis (RAS) secondary to arteriosclerosis. Other causes of RVHT include fibromuscular dysplasia (FMD) and other rare causes, such as Takayasu arteritis (TA). A male patient in his early 20s presented with hypertension. Laboratory findings were positive for hypokalemia as well as elevations in plasma renin activity and aldosterone concentration. Plain computed tomography revealed atrophy of the right kidney, and magnetic resonance angiography revealed right RAS. A diagnosis of RVHT was suspected, and he was admitted to the cardiovascular ward. After percutaneous transluminal renal angioplasty (PTRA) to treat the right RAS, a typical course was observed with decreased blood pressure, normalizing hypokalemia, and decreased plasma renin activity and aldosterone concentration (which previously were extremely elevated). As angiography showed no remarkable arteriosclerosis of other vessels and given the patient’s young age, FMD was suspected as the underlying cause of RVHT. However, the angiographic findings of RAS in the proximal renal artery and the lack of “string-of-beads” appearance were atypical for FMD. The patient had chronic inflammation, and further investigation revealed severe stenosis of the right carotid artery. The high C-reactive protein value and the thickened aortic wall in the computed tomography were the suggestive signs for TA. The patient was diagnosed with TA and started on steroid therapy. Although moderate stenosis remained after revascularization of the renal artery in this patient, hypertension improved markedly, demonstrating the effectiveness of PTRA. Given the diagnosis of TA as the underlying disease, the likelihood of recurrent RVHT due to restenosis of the renal artery remains high, and strict follow-up is thus required.
Keywords: Renovascular hypertension; Takayasu arteritis; Renin; Aldosterone; Percutaneous transluminal renal angioplasty
| Introduction | ▴Top |
Among patients with hypertension, the prevalence of renovascular hypertension (RVHT) caused by renal artery stenosis (RAS) [1, 2] is approximately 1% and has been reported to be as high as 10-40% among those with malignant or accelerated hypertension [3]. Patients with RVHT associated with atherosclerosis have a high mortality rate due to the accelerated development of cardiovascular diseases [4]. RAS reduces renal perfusion pressure and stimulates renin production in the juxtaglomerular apparatus of the kidney, leading to excess formation and release of angiotensin II and aldosterone. Angiotensin II induces contraction of vascular smooth muscles, and aldosterone triggers renal reabsorption of sodium, resulting in renin-dependent hypertension. RAS due to arteriosclerosis is the primary cause of RVHT, followed by fibromuscular dysplasia (FMD) and other rare vascular diseases such as Takayasu arteritis (TA) [2, 3].
We encountered a case of juvenile RVHT exhibiting a typical pathophysiological course before and after undergoing percutaneous transluminal renal angioplasty (PTRA), although the patient was later diagnosed with TA. In diagnosing juvenile RVHT, it is essential to differentiate between FMD and TA, regardless of the rarity of the latter diagnosis. TA is an inflammatory disease treated with systemic glucocorticoids and immunosuppressive therapy. The course and prognosis of this disease are greatly affected if TA is not well controlled.
| Case Report | ▴Top |
An Asian male (body mass index: 18.4 kg/m2, non-smoker, chanced drinker, estimated glomerular filtration rate: 90.7 mL/min/1.73 m2, caregiver) in his early 20s presented with muscle pain in the upper limbs. Physical examination was unremarkable, and the patient was given symptomatic treatment. Although the presenting symptoms eased, he returned for evaluation 2 weeks later upon noticing visual impairment in his left eye. The patient had a blood pressure of 183/122 mm Hg and a reduced potassium level of 2.6 mEq/L. He had no risk factors and no family history of arteriosclerosis. Fundoscopic examination revealed macular edema, exudative retinal detachment, and papilledema in the left eye. This presentation of juvenile hypertension with hypokalemia was indicative of secondary hypertension. The patient was thus admitted to the cardiovascular ward.
Laboratory tests revealed elevations in plasma renin activity (PRA; 94.6 ng/mL/h) and plasma aldosterone concentration (PAC; 290.6 pg/mL). Plain computed tomography (CT) showed no evidence of an adrenal mass but revealed a size difference between the right and left kidney, suggesting an atrophic change in the right kidney with a compensatory swelling of the left kidney (Fig. 1a). Identifying the right renal artery on renal artery ultrasound (RA-US) was difficult, and the RA-US assessment was limited by a lack of flow velocity. Non-contrast-enhanced magnetic resonance angiography (MRA) revealed poor visualization of the right renal artery (Fig. 1b). These findings were suggestive of RAS.
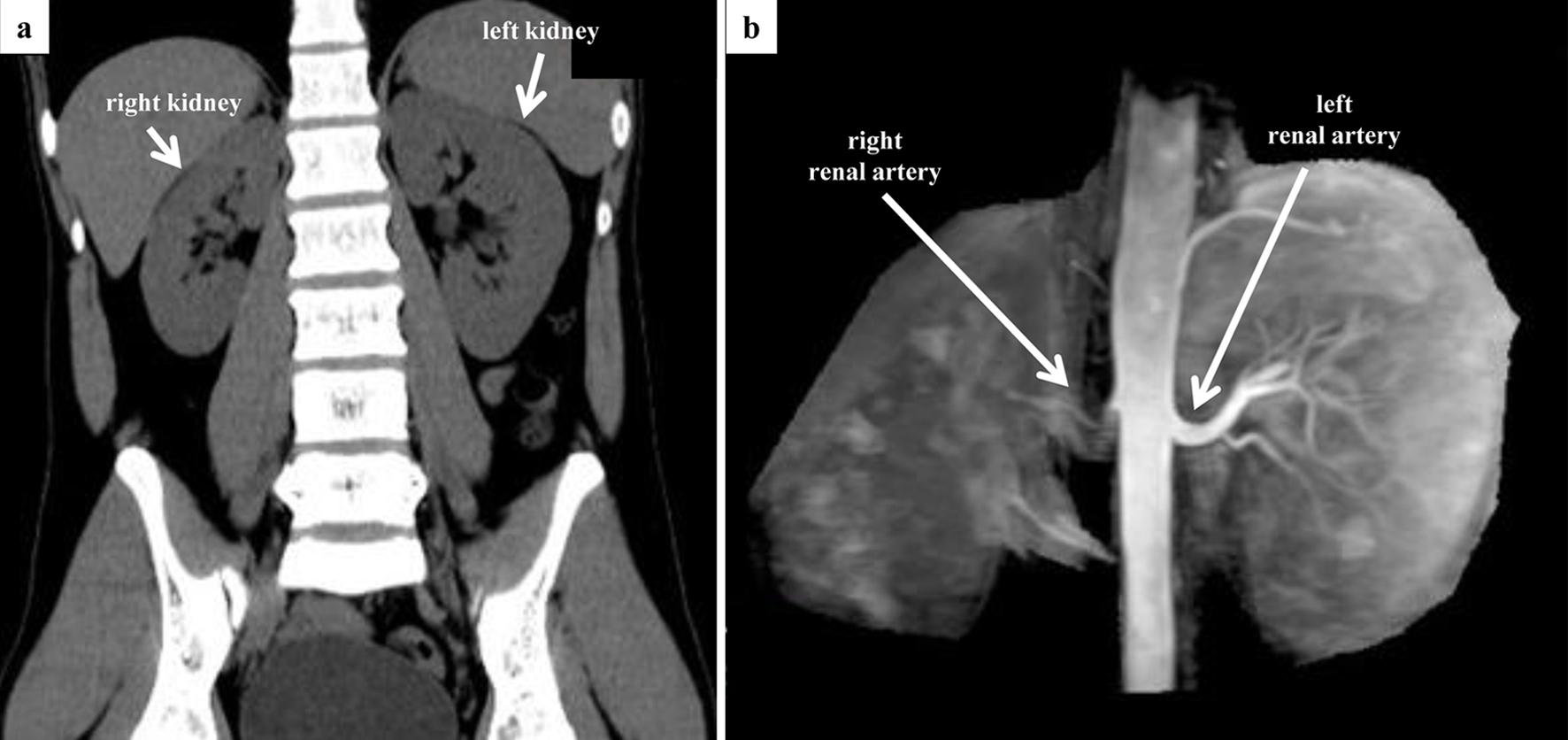 Click for large image | Figure 1. Preoperative CT and MRA evaluation. (a) Coronal view of abdominal CT. There is a difference in the size of the left and right kidney (right, 10.4 × 4.0 cm; left, 11.6 × 5.8 cm). No adrenal mass was observed. (b) Non-contrast-enhanced MRA. Although the entrance of the right renal artery is visible, visualization of the right renal artery itself is poor. CT: computed tomography; MRA: magnetic resonance angiography. |
We administered amlodipine (5 mg) and 1 week later performed percutaneous selective renal angiography, suspecting a typical RVHT (Fig. 2). Renal angiography confirmed a severe RAS, approximately 1.5 cm in length, at the ostium of the right renal artery (Fig. 2a). An ad hoc PTRA followed by balloon angioplasty was conducted, which improved the stenosis from 99% to 50% (Fig. 2b). Images obtained from intravascular ultrasound (IVUS) showed a circumscribed thickening of the arterial wall, with no calcification and unclear structure of the three layers of the tunica intima, tunica media, and tunica adventitia (Fig. 3).
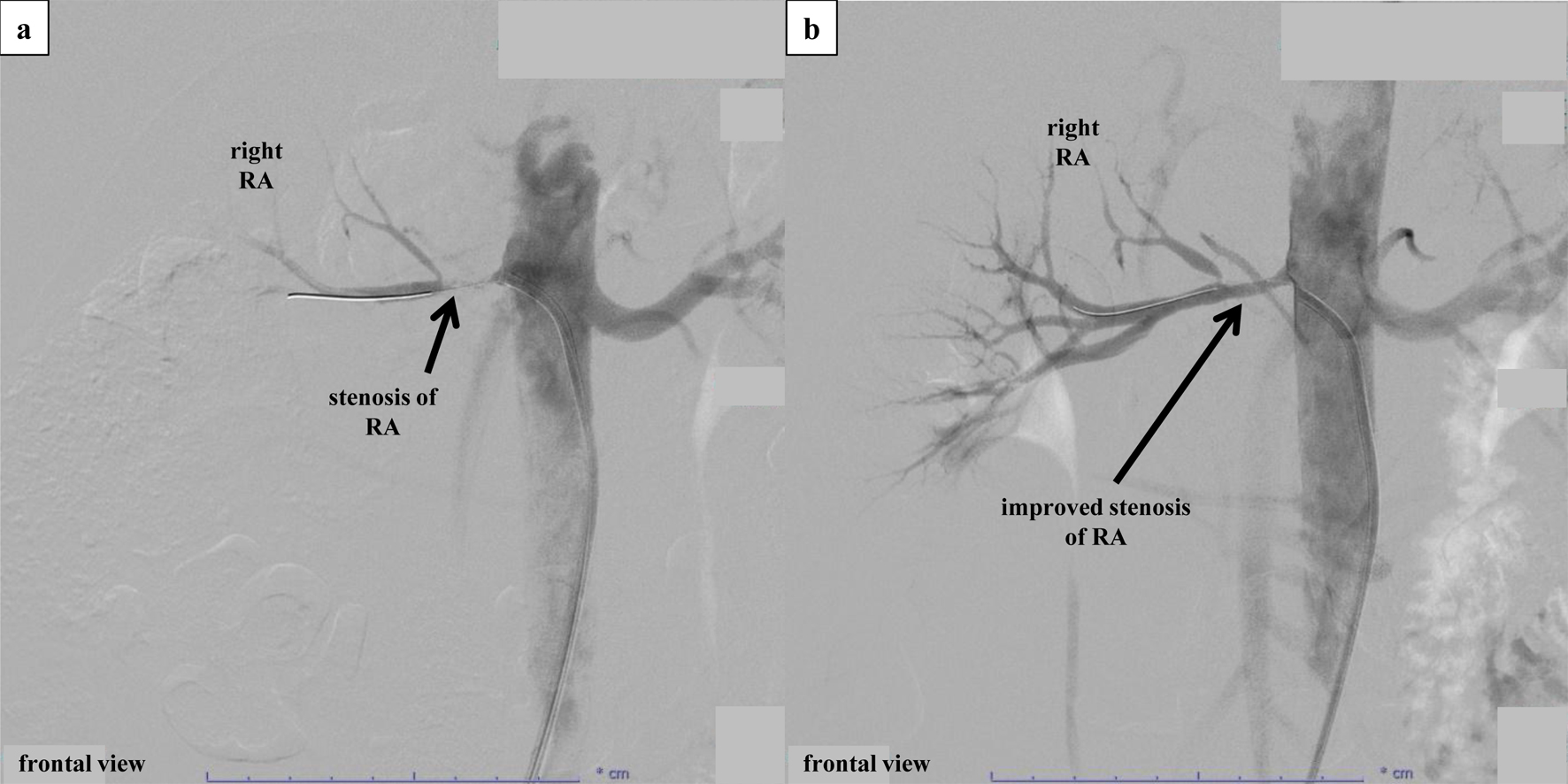 Click for large image | Figure 2. Percutaneous selective renal angiography and PTRA. (a) Percutaneous selective renal angiography before PTRA. Renal angiography was performed by approaching the renal artery from the right femoral artery. After performing contrast imaging of the left renal artery using a 6-French Mach 1® RDC1 catheter, severe stenosis at the ostium of the right renal artery was confirmed. Subsequently, PTRA was performed at the culprit location, and contrast imaging after insertion of an Aguru® wire (0.014 mm/180 cm) revealed a diffuse stenosis approximately 1.5 cm from the renal artery ostium. The lesion was observed using IVUS (Fig. 3a) before performing balloon angioplasty. After balloon angioplasty using a Sterling® (4.0 × 15 mm) catheter, a partial dissection-like lesion was observed while observing the culprit location via IVUS. Afterward, long inflation was performed on the entire stenosis using an NSE PTA® (4.0 × 40 mm) catheter. However, dilation to a low pressure of 3 atm was the limit due to the sensation of back pain in the patient. (b) Percutaneous selective renal angiography after PTRA. Although the dissection remained visible on IVUS (Fig. 3b), the right renal artery improved from 99% stenosis to 50% stenosis. IVUS: intravascular ultrasound; PTRA: percutaneous transluminal renal angioplasty. |
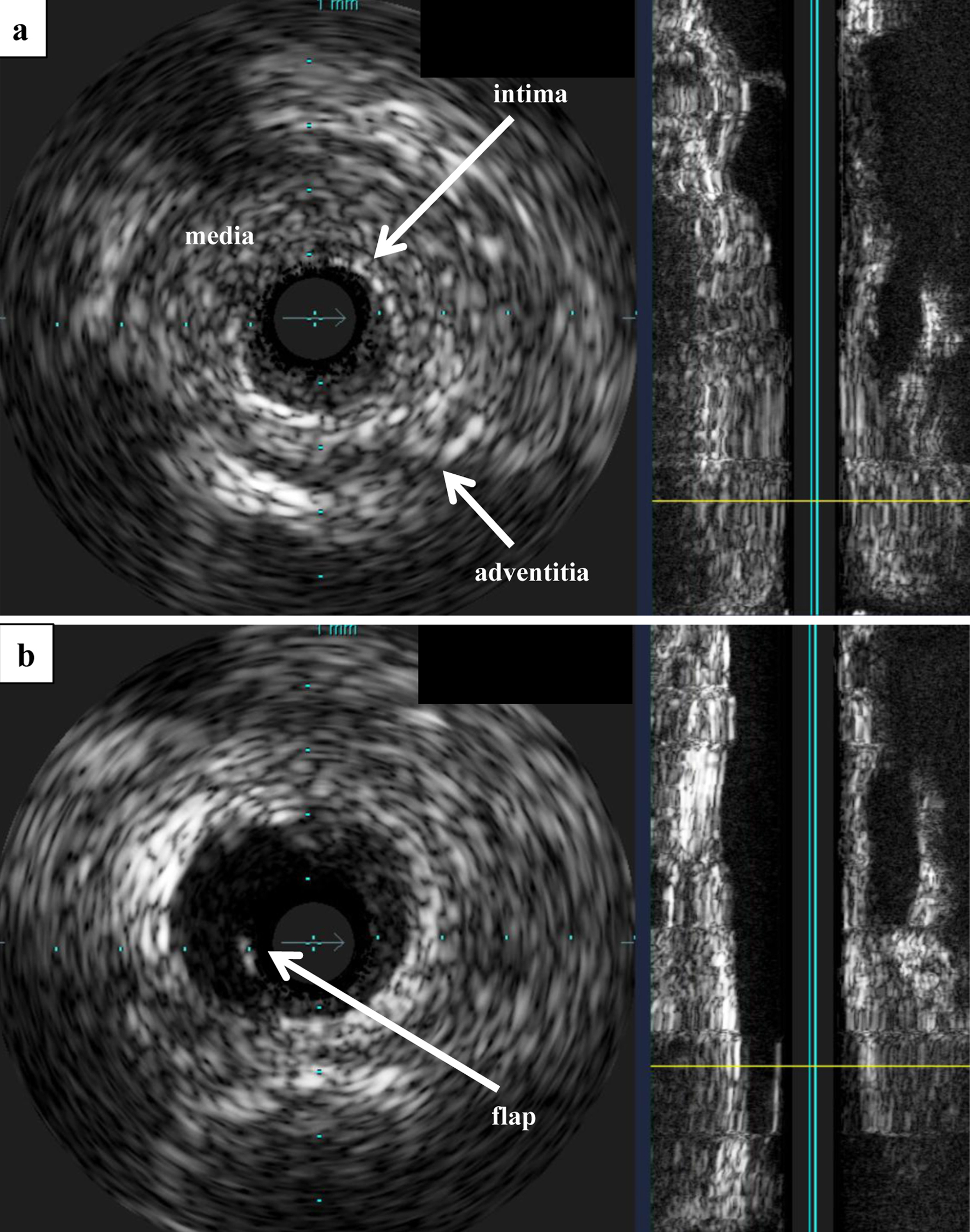 Click for large image | Figure 3. IVUS. (a) IVUS before PTRA. Observation of the lesion using Eagle Eye® IVUS revealed no calcification, and the three-layered structure of the tunica intima, tunica media, and tunica adventitia was unclear and concentrically thickened. (b) IVUS after PTRA. After balloon angioplasty, a flap that appeared to be partially dissected was observed. IVUS: intravascular ultrasound; PTRA: percutaneous transluminal renal angioplasty. |
Remarkable improvement in blood pressure occurred after PTRA, and systolic blood pressure was controlled at 120 - 130 mm Hg without the use of antihypertensive agents since the day after the procedure (Fig. 4). Hypokalemia normalized in 3 days. Prior to performing PTRA, the PRA (141.8 ng/mL/h) and PAC (353.7 pg/mL) were high. Beginning on the day after PTRA, both PRA and PAC gradually decreased, reaching normal ranges on day 3 post PTRA (7.9 ng/mL/h and 7.3 pg/mL, respectively). Blood flow in the right renal artery became detectable on RA-US, and an assessment of stenosis post PTRA revealed a peak systolic velocity (PSV) of 310 cm/s and a renal-aortic ratio (RAR) of 2.0. Fundoscopic examination performed 1 week after PTRA showed no changes in the papilledema, but the macular edema in the left eye had disappeared and exudative retinal detachment had improved.
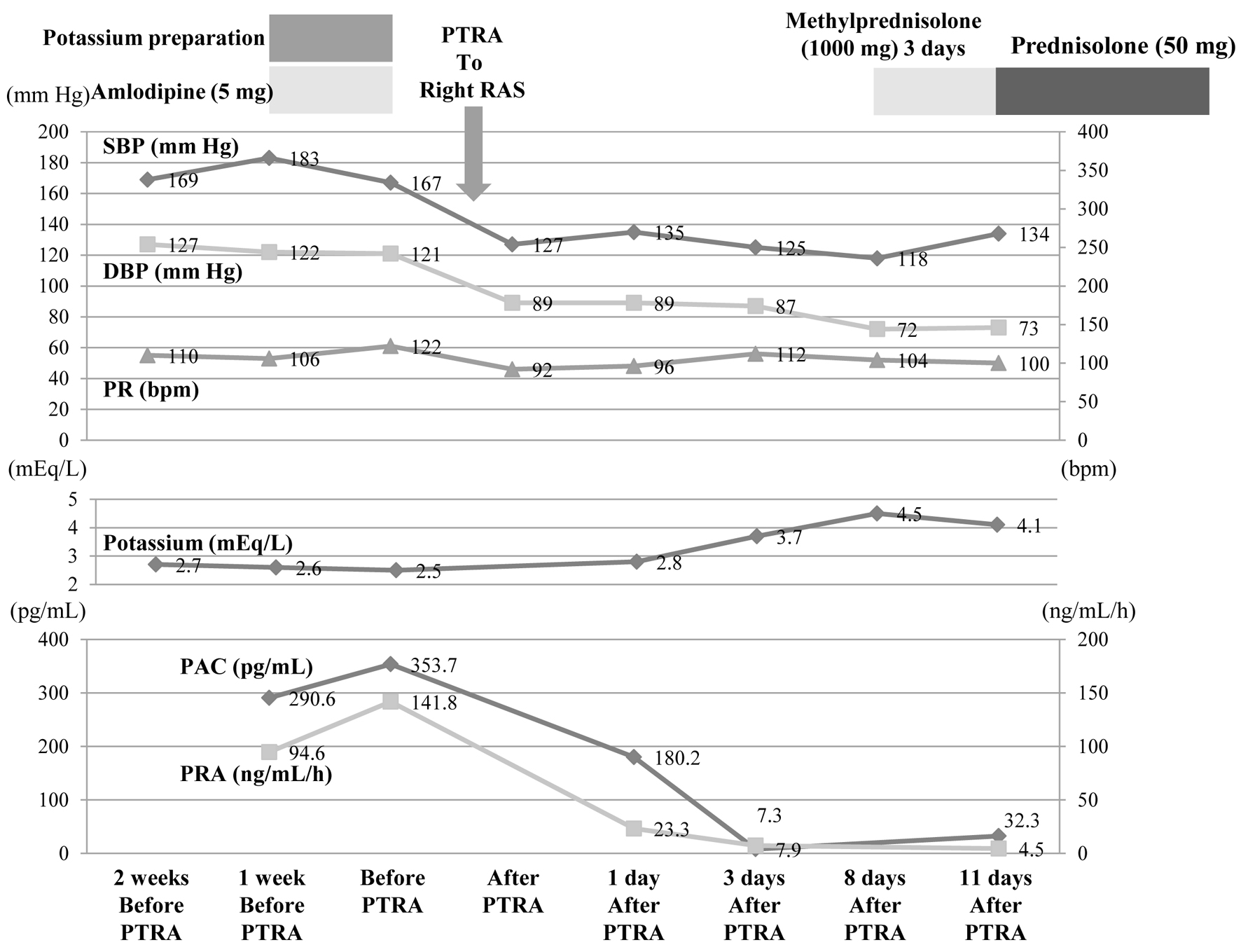 Click for large image | Figure 4. After PTRA was performed, SBP, DBP, PRA, and PAC decreased markedly, and potassium normalized. These changes have been maintained since then. DBP: diastolic blood pressure; PAC: plasma aldosterone concentration; PR: pulse rate; PRA: plasma renin activity; PTRA: percutaneous transluminal renal angioplasty; RAS: renal artery stenosis; SBP: systolic blood pressure. |
We suspected FMD as the underlying cause of RAS, given the patient’s age and the prevalence of FMD in this setting. However, the angiographic findings revealed stenosis of the proximal renal artery and did not exhibit the typical “string-of-beads” appearance, bringing into question the role of FMD as the etiology of RAS. Despite the normalization of blood pressure, the laboratory findings indicated continuing inflammation (white blood cell count, 10,000 - 14,000/µL; C-reactive protein, 1 - 4 mg/dL; erythrocyte sedimentation rate, 39 - 55 mm/h); therefore, additional imaging was conducted. Severe stenosis of the right common carotid artery (CCA) was observed on both ultrasound (Fig. 5) and MRA (Fig. 6a), with the “macaroni sign” noted on the ultrasound.
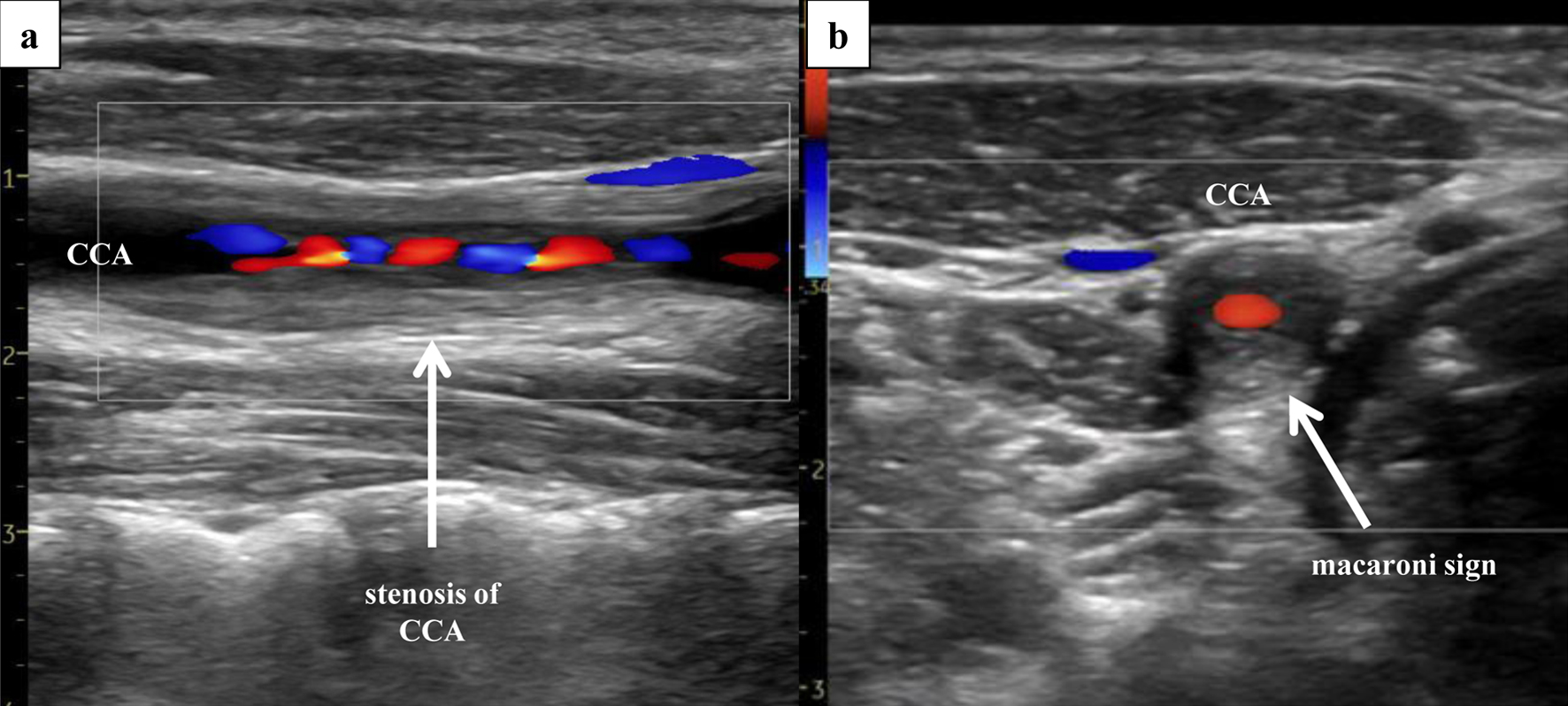 Click for large image | Figure 5. Right ultrasonography of the CCA. Ultrasonography of the CCA, performed 3 days after PTRA, revealed severe stenosis (area method, 97%; NASCET method, 66%; PSV, 310 cm/s) of the right CCA, with presence of the “macaroni sign”. (a) Sagittal section (Doppler). (b) Coronal section (Doppler). CCA: common carotid artery; PSV: peak systolic velocity; PTRA: percutaneous transluminal renal angioplasty. |
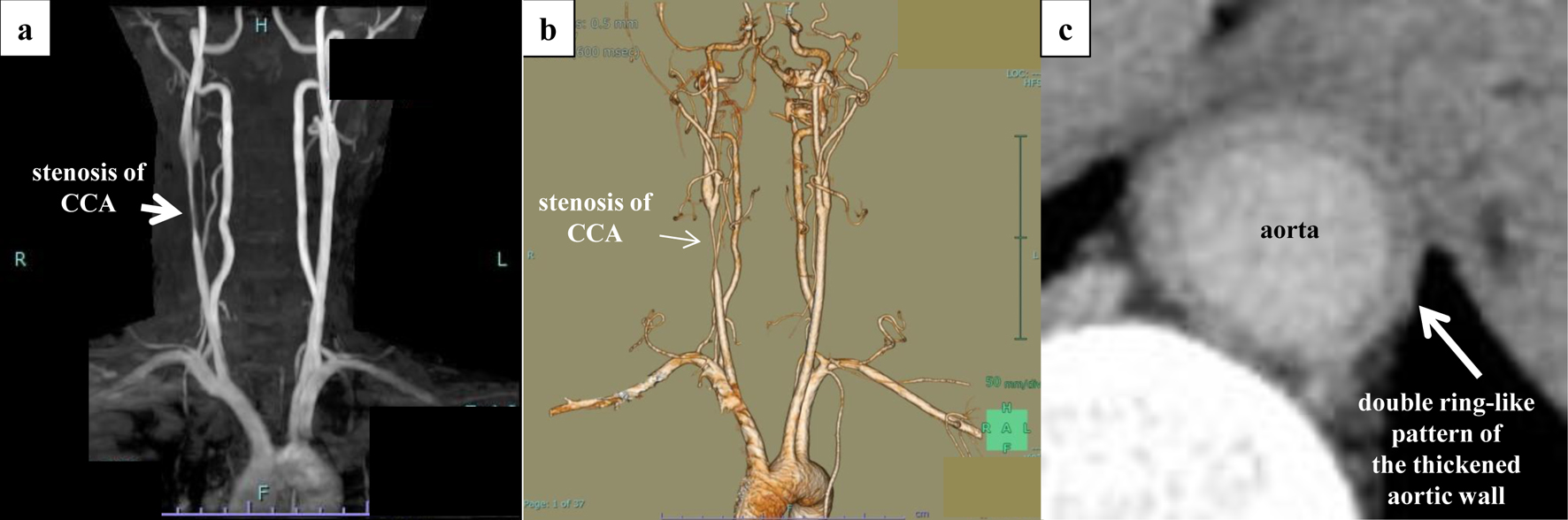 Click for large image | Figure 6. Aortic findings on non-contrast-enhanced MRA and contrast-enhanced CT. Severe stenosis of the right common carotid artery and inflammation within the wall of the descending aorta were confirmed. (a) Non-contrast-enhanced MRA. (b) Three-dimensional contrast-enhanced CT. (c) Late-phase CT shows a double ring-like pattern of the thickened aortic wall. CT: computed tomography; MRA: magnetic resonance angiography. |
Contrast-enhanced CT revealed stenosis of the right renal artery and CCA (Fig. 6b) and thickening of the aortic wall at the abdominal aorta involving the renal artery bifurcation (Fig. 6c). Vasculitis mimics were ruled out, and the patient was diagnosed with active-phase TA.
The patient was given oral aspirin (100 mg) and a 3-day pulse injection of methylprednisolone (1 g/day) after PTRA, followed by oral prednisolone (50 mg). The steroid was then gradually tapered.
| Discussion | ▴Top |
TA is a chronic inflammatory condition affecting the aorta and its major branches. The disease most commonly occurs in young women. The pathophysiology of TA involves immune-mediated processes in all layers of the arterial wall, including the tunica intima, tunica media, and tunica adventitia. Infiltration of inflammatory cells is accompanied by loss of smooth muscle cells and destruction of elastic fibers, indicating the presence of an inflammatory response, with signs of vascular remodeling, including intimal thickening and fibrosis [5]. Vascular remodeling leads to thickening of the vessel walls and, eventually, the development of luminal stenosis. The symptoms of TA are nonspecific; however, approximately 33-83% of patients exhibit hypertension, which is caused by RAS in 28-75% of cases [6-8]. Takayasu retinopathy occurs in 37% of patients and may impair vision [9]. CT angiography is the preferred imaging modality for identifying sites of stenosis and dilatation and assessing the degree of inflammation. The tunica media and tunica adventitia layers will appear contrast-enhanced due to inflammation, whereas the intimal layer, which has gel-forming mucin and undergoes swelling, will be poorly enhanced [10]. In this patient, contrast-enhanced CT showed a double ring-like pattern of thickened aortic wall at the abdominal aorta and including the renal artery, consistent with TA.
The main strategy in treating TA involves medical therapy using immunosuppressants, including glucocorticoids with or without disease-modifying antirheumatic drugs. Invasive therapy (endovascular therapy and open surgery) is often chosen in cases involving occlusion or severe stenosis. However, current guidelines recommend delaying invasive procedures until the disease activity is controlled with medical therapy [11, 12]. Balloon angioplasty or stent placement is used in patients with repeated recurrence of RAS.
This patient was ultimately diagnosed with TA. In juvenile RVHT, it is crucial to differentiate between FMD and TA, as immunosuppressive therapy is required in TA but unnecessary in FMD. The key difference between these two conditions is the evidence of inflammation in laboratory findings [13, 14]. Another significant feature is seen on imaging. Concentric arterial thickening with high attenuation indicates inflammation of the artery, a typical manifestation of TA that is seen on CT angiography. A “string-of-beads” appearance on CT angiography or catheter-based angiography would identify the presence of FMD. The location of the RAS is another factor to consider in diagnosis. A “string-of-beads” sign is commonly found in the middle to distal portion of the renal artery [2, 3]. Assessment with IVUS in catheter-based angiography provides detailed analysis of RAS. Detection of the site of the involved layer of the arterial wall reflects the histological type of FMD, although the low resolution of IVUS may at times prevent this imaging modality from clearly distinguishing the layers. If all three layers are affected, a diagnosis of FMD is less likely [15]. In this patient, angiography showed stenosis at the proximal renal artery and no “string-of-beads” appearance, and IVUS revealed a circumscribed thickening of the arterial wall. The CT angiography findings also were indicative of a large vessel vasculitis rather than FMD.
PTRA is performed for revascularization in RVHT, but rates of success differ in cases of TA versus FMD. The success rate for PTRA in treating RVHT due to FMD is high, with approximately 50-60% of cases being cured of hypertension and roughly 5-30% of cases having restenosis requiring a repeat procedure [13, 16-18]. Reported data indicate that RVHT caused by FMD cannot always be completely resolved, with only 6-30% of patients no longer needing antihypertensive drugs [13, 17].
The reported treatment outcomes of PTRA in TA are quite poor, with a very high rate of restenosis (78%) [19], requiring either repeated balloon angioplasty or stent placement. Restenosis is also seen in patients who have undergone stenting as a result of progressive intimal thickening due to fibrosis. Although the likelihood of in-stent restenosis is high, additional treatment with a drug-eluting balloon may be an effective option to consider [20].
With the intent of reducing the possibility of restenosis, guidelines recommend medical therapy prior to revascularization. We conducted PTRA before medical therapy as a means of emergency evacuation, as this patient had stenosis of 90% and an extremely activated renin-angiotensin-aldosterone system. In findings from a study of RA-US, an indicator of significant RAS is PSV ≥ 180 cm/s and RAR ≥ 3.5 [21], and emergency evacuation by PTRA should be considered in this setting.
After this patient underwent PTRA, his blood pressure, PRA, PAC, and fundoscopic findings normalized. The RA-US following PTRA showed a decrease in the RAR, but a high PSV persisted, suggesting a possible stenosis remaining at the culprit lesion. Given that hypertension and cardiovascular complications are determining factors in the prognosis of TA [22], this patient’s blood pressure and PRA as well as findings on RA-US and fundoscopic examination should be carefully monitored for early detection of recurrent RVHT.
Additionally, results of urinary analysis in this patient were positive for protein and occult blood before PTRA but negative for these findings after PTRA. One week after PTRA, the size difference between the kidneys was reduced on CT. Proteinuria and hematuria sometimes are positive in RVHT, indicating the presence of renal parenchymal disease and glomerulonephritis. Although fundoscopic findings showed mainly hypertensive changes without wreath-like arteriovenous anastomosis, a characteristic of late-phase TA [23], continued fundoscopic examination to monitor the patient’s progress is necessary.
Conclusion
This patient presented with RVHT caused by TA and underwent PTRA due to severe RAS. Although moderate RAS remained after PTRA, hypertension improved, demonstrating that PTRA is beneficial in this setting. Given that TA is the underlying disease in this case, however, the likelihood of restenosis of the renal artery is high, and strict follow-up is required.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
We received grant support from Otsuka Holdings for participating in a clinical trial of treating hypertension with an ultrasound renal denervation system.
Informed Consent
Permission to report was obtained from the patient.
Author Contributions
Ayako Ishii and Keisuke Okamura: study design, data collection, statistical analysis, data interpretation, manuscript preparation, and literature search; Shogo Morisaki, Yasunori Momota, Akiko Yamashita, Kenta Hatsuse, Keisuke Kono, Hideto Sako, Akihiro Udo, Kenichiro Taniguchi, Tomoko Koseki, Takuro Arai, Yoshie Yodogawa, Yoshiko Oba, Shiori Hirayama, Miki Inoue, and Ichiro Imamura: manuscript preparation and literature search.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
CCA: common carotid artery; CT: computed tomography; DBP: diastolic blood pressure; FMD: fibromuscular dysplasia; IVUS: intravascular ultrasound; MRA: magnetic resonance angiography; PAC: plasma aldosterone concentration; PR: pulse rate; PRA: plasma renin activity; PSV: peak systolic velocity; PTRA: percutaneous transluminal renal angioplasty; RAR: renal-aortic ratio; RAS: renal artery stenosis; RA-US: renal artery ultrasound; RVHT: renovascular hypertension; SBP: systolic blood pressure; TA: Takayasu arteritis
| References | ▴Top |
- Dworkin LD, Cooper CJ. Clinical practice. Renal-artery stenosis. N Engl J Med. 2009;361(20):1972-1978.
doi pubmed pmc - Plouin PF, Perdu J, La Batide-Alanore A, Boutouyrie P, Gimenez-Roqueplo AP, Jeunemaitre X. Fibromuscular dysplasia. Orphanet J Rare Dis. 2007;2:28.
doi pubmed pmc - Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344(6):431-442.
doi pubmed - Kalra PA, Guo H, Kausz AT, Gilbertson DT, Liu J, Chen SC, Ishani A, et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int. 2005;68(1):293-301.
doi pubmed - Nasu T. Takayasu’s truncoarteritis. Pulseless disease or aortitis syndrome. Acta Pathol Jpn. 1982;32(Suppl 1):117-131.
pubmed - Lupi-Herrera E, Sanchez-Torres G, Marcushamer J, Mispireta J, Horwitz S, Vela JE. Takayasu’s arteritis. Clinical study of 107 cases. Am Heart J. 1977;93(1):94-103.
doi pubmed - Hall S, Barr W, Lie JT, Stanson AW, Kazmier FJ, Hunder GG. Takayasu arteritis. A study of 32 North American patients. Medicine (Baltimore). 1985;64(2):89-99.
pubmed - Jain S, Sharma N, Singh S, Bali HK, Kumar L, Sharma BK. Takayasu arteritis in children and young Indians. Int J Cardiol. 2000;75(Suppl 1):S153-S157.
doi pubmed - Danda D, Goel R, Joseph G, Kumar ST, Nair A, Ravindran R, Jeyaseelan L, et al. Clinical course of 602 patients with Takayasu’s arteritis: comparison between childhood-onset versus adult onset disease. Rheumatology (Oxford). 2021;60(5):2246-2255.
doi pubmed pmc - Zhu FP, Luo S, Wang ZJ, Jin ZY, Zhang LJ, Lu GM. Takayasu arteritis: imaging spectrum at multidetector CT angiography. Br J Radiol. 2012;85(1020):e1282-e1292.
doi pubmed pmc - Park MC, Lee SW, Park YB, Lee SK, Choi D, Shim WH. Post-interventional immunosuppressive treatment and vascular restenosis in Takayasu’s arteritis. Rheumatology (Oxford). 2006;45(5):600-605.
doi pubmed - Hellmich B, Agueda A, Monti S, Buttgereit F, de Boysson H, Brouwer E, Cassie R, et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2020;79(1):19-30.
doi pubmed - Slovut DP, Olin JW. Fibromuscular dysplasia. N Engl J Med. 2004;350(18):1862-1871.
doi pubmed - Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003;349(2):160-169.
doi pubmed - Ogawa O, Watanabe R, Shimizu H, Masani F. Focal renal arterial fibromuscular dysplasia demonstrated via intravascular ultrasound image. Ann Vasc Dis. 2011;4(3):256-259.
doi pubmed pmc - Trinquart L, Mounier-Vehier C, Sapoval M, Gagnon N, Plouin PF. Efficacy of revascularization for renal artery stenosis caused by fibromuscular dysplasia: a systematic review and meta-analysis. Hypertension. 2010;56(3):525-532.
doi pubmed - Mousa AY, Campbell JE, Stone PA, Broce M, Bates MC, AbuRahma AF. Short- and long-term outcomes of percutaneous transluminal angioplasty/stenting of renal fibromuscular dysplasia over a ten-year period. J Vasc Surg. 2012;55(2):421-427.
doi pubmed - Alhadad A, Mattiasson I, Ivancev K, Gottsater A, Lindblad B. Revascularisation of renal artery stenosis caused by fibromuscular dysplasia: effects on blood pressure during 7-year follow-up are influenced by duration of hypertension and branch artery stenosis. J Hum Hypertens. 2005;19(10):761-767.
doi pubmed - Maksimowicz-McKinnon K, Clark TM, Hoffman GS. Limitations of therapy and a guarded prognosis in an American cohort of Takayasu arteritis patients. Arthritis Rheum. 2007;56(3):1000-1009.
doi pubmed - Yamamoto T, Shirai K, Okamura K, Urata H. Two years efficacy of paclitaxel-coated balloon dilation for in-stent renal artery restenosis due to Takayasu arteritis. Am J Case Rep. 2019;20:1089-1093.
doi pubmed pmc - Frauchiger B, Zierler R, Bergelin RO, Isaacson JA, Strandness DE, Jr. Prognostic significance of intrarenal resistance indices in patients with renal artery interventions: a preliminary duplex sonographic study. Cardiovasc Surg. 1996;4(3):324-330.
doi pubmed - Ishikawa K, Maetani S. Long-term outcome for 120 Japanese patients with Takayasu’s disease. Clinical and statistical analyses of related prognostic factors. Circulation. 1994;90(4):1855-1860.
doi pubmed - Numano F, Okawara M, Inomata H, Kobayashi Y. Takayasu’s arteritis. Lancet. 2000;356(9234):1023-1025.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.