| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 4, April 2025, pages 208-222
Relative, Conditional, and Overall Survival and Causes of Death in Patients With Glioblastoma: A Retrospective Longitudinal Cohort Study
Khaled Saada, q, r, Anas Elgenidyb, q, Eman F. Gada, Yasser Hamedc, Amir Aboelgheeta, Mazen M. Zidand, Nada A. Alsayedd, Mohammad Alzu’bie, Ahmed A. Abdelfattahf, Marwan H. Abdulrahimg, Shady Sapoorh, Doaa Ali Gamali, Usama El-Shokhaibyj, Hassan Ahmed Hashemc, Amira Elhoufeyk, l, Thamer A.M. Alruwailim, Hoda Atef Abdelsattar Ibrahimn, Kawashty Ragab Mohamedc, Khalid Hashim Mahmoudo, Mohamad-Hani Temsahp, Sandra Ahmedb
aDepartment of Pediatrics, Assiut University, Assiut, Egypt
bDepartment of Neurology, Cairo University, Cairo, Egypt
cDepartment of Neurology, Al-Azhar University, Assiut, Egypt
dFaculty of Medicine, Misr University for Science and Technology, Cairo, Egypt
eFaculty of Medicine, Hashemite University, Zarqa, Jordan
fFaculty of Medicine, Tanta University, Tanta, Egypt
gFaculty of Medicine, Cairo University, Cairo, Egypt
hFaculty of Medicine, Benha University, Benha, Egypt
iDepartment of Clinical Oncology, Faculty of Medicine, Assiut University, Assiut, Egypt
jDepartment of Neurosurgery, Al-Azhar University, Cairo, Egypt
kDepartment of Community Health Nursing, Alddrab University College, Jazan University, Jazan, Saudi Arabia
lDepartment of Community Health Nursing, Faculty of Nursing, Assiut University, Assiut, Egypt
mDepartment of Pediatrics, College of Medicine, Jouf University, Sakaka, Saudi Arabia
nDepartment of Pediatrics, Faculty of Medicine, Cairo University, Cairo, Egypt
oDepartment of Pediatrics, Faculty of Medicine, Shaqra University, Dawadmi, Saudi Arabia
pPediatric Intensive Care Unit, King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia
qThese authors contributed equally to this work and shared the first authorship.
rCorresponding Author: Khaled Saad, Faculty of Medicine, Assiut University, Assiut, Egypt
Manuscript submitted February 1, 2025, accepted April 3, 2025, published online April 11, 2025
Short title: Survival and CODs in Patients With Glioblastoma
doi: https://doi.org/10.14740/jocmr6192
| Abstract | ▴Top |
Background: Our goal in this manuscript was to perform a survival analysis and understand the causes of death (CODs) in patients with glioblastoma using the Surveillance, Epidemiology, and End Results (SEER) database.
Methods: A retrospective cohort study was conducted using version 8.3.9.2 of SEER*Stat software to extract data from the SEER 17 Plus database. Patients with World Health Organization (WHO) grade IV glioblastoma diagnosed between 2000 and 2019 were included to calculate overall survival (OS), relative survival (RS), and conditional survival. R software was used to calculate univariate and multivariate Cox regression models for age, sex, and race to identify their effect on survival.
Results: We included 45,071 patients with grade IV glioblastoma according to WHO 2016 classification. The observed 1-year, 3-year, and 5-year survival rates showed a decline to 40.1%, 9.8%, and 5.2%, respectively. Similarly, the relative 1-year, 3-year, and 5-year survival rates were 40.7%, 10.2%, and 5.4%, respectively. The conditional 3-year survival rates improved up to 16.9%, 42.9%, and 60.2% after 1, 3, and 5 years of survival, correspondingly, with females showing better estimates. The most common cancer CODs were the brain and other central nervous system (CNS) cancers. Among non-glioblastoma cancer CODs, breast cancer was the most common cause. Additionally, cardiovascular diseases, cerebrovascular diseases, and septicemia were the most common non-cancer CODs.
Conclusion: In this study, patients with glioblastoma showed a sharp decline in OS and RS over time after diagnosis. However, there was a notable improvement in conditional 3-year survival over time. Cardiovascular diseases emerged as the most common non-cancer COD, with lower survival rates in males and advanced age.
Keywords: Glioblastoma; Survival rate; Cause of death; SEER program; Non-cancer mortality
| Introduction | ▴Top |
Gliomas are tumors originating from glial cells or their precursors and include types such as astrocytomas. The World Health Organization (WHO) classifies gliomas into four grades (I to IV): grade I (pilocytic astrocytoma), grade II (diffuse astrocytoma), grade III (anaplastic astrocytoma), and grade IV (glioblastoma). Glioblastomas are further categorized into isocitrate dehydrogenase (IDH)-wild-type, which develops de novo without evidence of a less malignant precursor lesion, and IDH-mutant-type, which arises from preexisting lower-grade astrocytomas [1].
Glioblastoma is known as the most aggressive, malignant primary brain tumor. In the United States of America (USA), the age-adjusted incidence rate of glioblastoma is 3.22 per 100,000 persons, constituting about 15% of all brain tumors and 48.3% of malignant brain tumors [2]. Research indicates no significant trend in increasing incidence rates in the USA or Canada, though incidence varies across different regions worldwide [3].
There are limited available data to distinguish between glioblastoma-specific survival and overall survival (OS) in glioblastoma patients. Non-cancer-related deaths can affect OS since OS is defined as the duration from randomization to death from any cause. Relative survival (RS) represents the ratio of the OS in patients with cancer to the expected survival of a comparable cancer-free population. In contrast, cancer-specific survival focuses solely on deaths caused by cancer, excluding other competing causes of death (CODs) from its endpoint. There is a high risk of competing non-cancer events for glioblastoma patients with a high rate of associated comorbidities, especially with advanced age. Few studies reported cancer itself as a predominant COD in these patients, with a significant subset related to other causes. Most of these deaths are due to heart and cerebrovascular diseases and infections. Given the high proportion of elderly glioblastoma patients in registries and trials, it is difficult to distinguish between glioblastoma-specific and non-glioblastoma deaths and the efficacy of drugs in survival and quality of life improvement [4].
Our primary objective in this study was to estimate OS, RS, and conditional survival to determine if patients’ survival rates improved over the years following diagnosis and to investigate both cancer-specific mortality and non-cancer reasons for death in glioblastoma patients. Utilizing the Surveillance, Epidemiology, and End Results (SEER) database, we conducted comprehensive analyses to thoroughly understand the clinical characteristics, prognosis, and their associations with risk factors, aiming to enhance outcomes for glioblastoma patients.
| Materials and Methods | ▴Top |
Ethics approval and consent to participate
This study was conducted in accordance with the ethical guidelines outlined by the World Medical Association’s Declaration of Helsinki. Approval for all procedures was obtained from the Ethical Committee of Assiut Faculty of Medicine, Egypt (10-2024). All data were collected from the anonymized SEER database. The study’s execution did not require informed consent (the need for consent was waived).
Study population
In this retrospective cohort study, we employed SEER*Stat software version 8.3.9.2 to collect data from the SEER 17 Plus database, an authoritative population-based cancer registry that covers nearly 34.6% of the US population [5]. The study included patients diagnosed between 2000 and 2019. We utilized the International Classification of Diseases for Oncology, Third Edition (ICD-O-3), applying the codes 9440-9442 to identify glioblastoma cases (previously classified as WHO grade IV) and topographic location codes C71.0-C71.9 to specify brain tumor locations [6]. These codes were used for case identification in epidemiological and registry-based research. We excluded patients who had an unknown survival time. Similarly, we excluded patients who were missing critical clinical data. Additionally, we excluded other histology and other grades.
Outcomes and their measures
We evaluated both cancer-specific and non-cancer CODs in our study, as well as calculated the standardized mortality ratios (SMRs) for each COD. To pinpoint the most clinically significant CODs among patients with glioblastoma and to inform follow-up care decisions, we categorized non-cancer CODs by age groups: overall, under 18, 18 - 44, 45 - 59, 60 - 74, and over 75 years. CODs were determined using the SEER COD recode, which is based on death certificate information and employs the ICD-10 codes. Other assessed outcomes in this study were OS, RS, and conditional survival. OS was the percentile of cancer patients who were still alive after a certain duration after being diagnosed, and it is not considered because of death, so it also included non-cancer CODs [7]. The expected survival rate was calculated using a cohort of individuals without cancer. This calculation was based on the assumption that multiple CODs act independently and compete with each other concurrently [7, 8]. Conditional survival was defined as the probability that a patient survives an additional specified period, given that they have already survived a certain number of years following an initial cancer diagnosis [9].
Statistical analysis
We employed SEER*Stat software version 8.3.9.2 to calculate SMRs for all CODs following a glioblastoma diagnosis. SMRs were determined by dividing the number of observed deaths by the number of expected deaths. Here, observed deaths referred to glioblastoma patients who succumbed to a specific cause, while expected deaths represented the anticipated number of deaths from the same cause in a demographically similar population without glioblastoma. Adjustments were made for demographic variables such as age and race. The data was stratified into three intervals based on the time from diagnosis to death: less than 1 year, 1 to 5 years, and more than 5 years, each accompanied by their corresponding 95% confidence intervals (CIs).
Simultaneously, we calculated OS, RS, and conditional survival using survival analysis modules within SEER*Stat. Univariate and multivariate Cox proportional hazards regression models were constructed using R software version 3.5 to evaluate the impact of variables, including age groups (< 18, 18 - 44, 45 - 59, 60 - 74, ≥ 75 years), sex, and race. Outcome measures are presented with their corresponding 95% CIs to indicate the precision of the estimates. Statistical significance was defined as a P value less than 0.05. All statistical tests were two-sided.
| Results | ▴Top |
Patients and clinical characteristics
From 2000 to 2019, a total of 45,071 patients with glioblastoma grade IV, according to the WHO 2016 classification, were included in our analysis (Table 1). About 40.4% of patients were in the 60 - 74 years age group. The majority were male (26,139; 58.0%) and White individuals (40,037; 88.8%). The mean survival time of the included patients was 13.8 months (standard deviation (SD), 21.7 months). Throughout the study period, the majority of the included patients (40,568; 90.1%) died. Within this group, a significant portion of patients (38,089; 85.5%) died from glioblastoma grade IV, whereas a smaller cohort of patients (1,934; 4.3%) died from causes unrelated to the primary tumor. Notably, the frontal lobe (12,347; 27.4%) and temporal lobe (11,023; 24.5%) were the most common sites affected by glioblastoma grade IV.
 Click to view | Table 1. Baseline Characteristics of Patients With Glioblastoma |
Survival analysis
The observed 1-year, 3-year, and 5-year survival rates of our patients declined over time to 40.1%, 9.8%, and 5.2%, respectively. Likewise, the relative 1-year, 3-year, and 5-year survival rates decreased to 40.7%, 10.2%, and 5.4%, respectively (Table 2). In male participants, the observed 3-year and 5-year survival rates were 9.5% and 4.9%, respectively, whereas female participants had better observed 3-year and 5-year survival rates of 10.3% and 5.6%, respectively. White patients had the lowest survival rates among all included patients. For the older cohorts, the 3-year and 5-year observed survival rates were the lowest among all age groups. The conditional survival estimates of glioblastoma grade IV improved over time since the primary diagnosis. After surviving 1, 3, and 5 years, the conditional 3-year survival estimates increased to 16.9%, 42.9%, and 60.2%, respectively. Female patients had better conditional survival than male patients. Older patients had the lowest conditional survival of any age bracket.
 Click to view | Table 2. Survival of Glioblastoma Patients With Conditional Survival According to Age |
Prognostic factors (age, sex, race)
We conducted univariate Cox regression analyses to recognize the significant factors that influence the survival of our patients. Age, sex, and race were investigated to determine whether they had a significant impact on survival (P < 0.05), followed by a multivariate Cox regression analysis (Table 3). Older patients displayed a significantly lower probability of survival than younger patients. Male patients had a lower likelihood of survival than female patients. White patients showed the lowest survival rate among all races. Multivariate Cox proportional hazards analysis demonstrated that age, sex, and race were independent prognostic factors influencing survival outcomes in patients with glioblastoma grade IV.
 Click to view | Table 3. Univariate and Multivariate Analysis of Age, Race, and Sex as Prognostic Factors |
CODs
Of the included patients, 39,937 (88.6%) died during the follow-up period. Most deaths (63.2%) occurred within 1 year of diagnosis, while 34.8% occurred from 1 to 5 years, and 2% occurred after 5 years. Among all age groups, patients aged between 60 - 74 years (40.9%) had the highest number of deaths throughout the study. Of the total deaths, 35,235 (88.23%) were from brain and other nervous system cancers, 657 (1.65%) were from other cancers, and 4,045 (10.13%) were from non-cancer causes. Figure 1 displays the most common COD in glioblastoma graded by latency period.
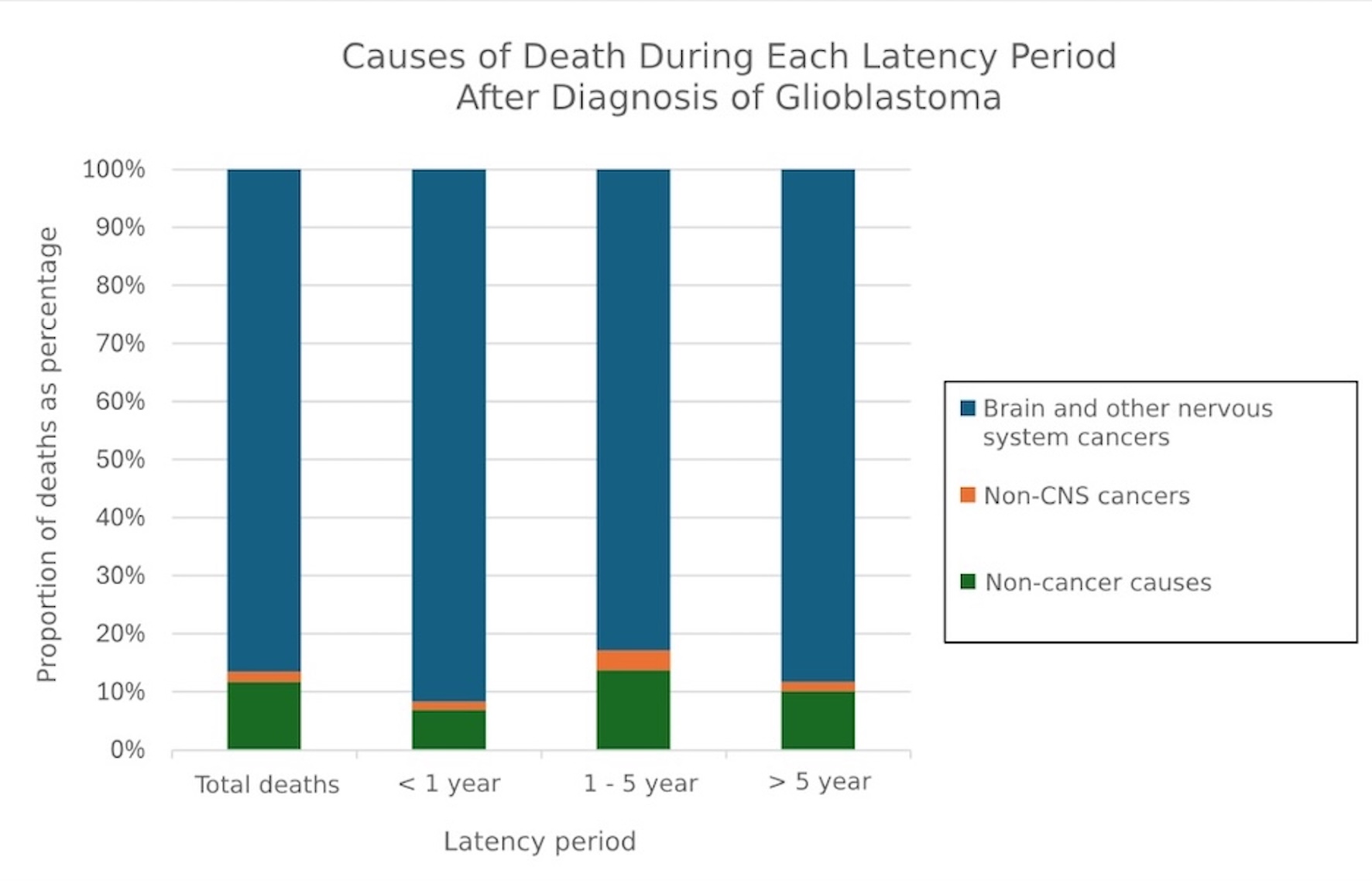 Click for large image | Figure 1. Causes of death after glioblastoma diagnosis graded by latency period. CNS: central nervous system. |
Cancer CODs
The most common non-glioblastoma grade IV cancer causes were breast cancer (SMR, 2.26; 95% CI, 1.46 - 3.33), followed by cancers of other endocrine organs including the thymus (SMR, 63.28; 95% CI, 36.86 - 101.32), small intestine (SMR, 15.42; 95% CI, 6.20 - 31.77), soft tissue including heart (SMR, 5.04; 95% CI, 2.03 - 10.39), and bones and joints (SMR, 10.65; 95% CI, 2.90 - 27.26). Other causes of cancer are demonstrated in Table 4.
 Click to view | Table 4. SMRs for Each Cancer COD Following Diagnosis of Glioblastoma |
Non-cancer CODs
The most common non-cancer CODs were cardiovascular diseases (SMR, 2.97; 95% CI, 2.73 - 3.23), cerebrovascular diseases (SMR, 5.11; 95% CI, 4.39 - 5.92), and septicemia (SMR, 8.97; 95% CI, 7.26 - 10.96) during all latency periods (Table 5). Infectious and parasitic diseases, including human immunodeficiency virus (HIV), were noted as a considerable COD (SMR, 5.94; 95% CI, 4.41 - 7.83). This may be attributed to HIV’s tendency to target microglial cells and macrophages in the CNS. Figure 2 shows the most common non-cancer COD in glioblastoma.
 Click to view | Table 5. SMRs for Each COD Following Diagnosis of Glioblastoma |
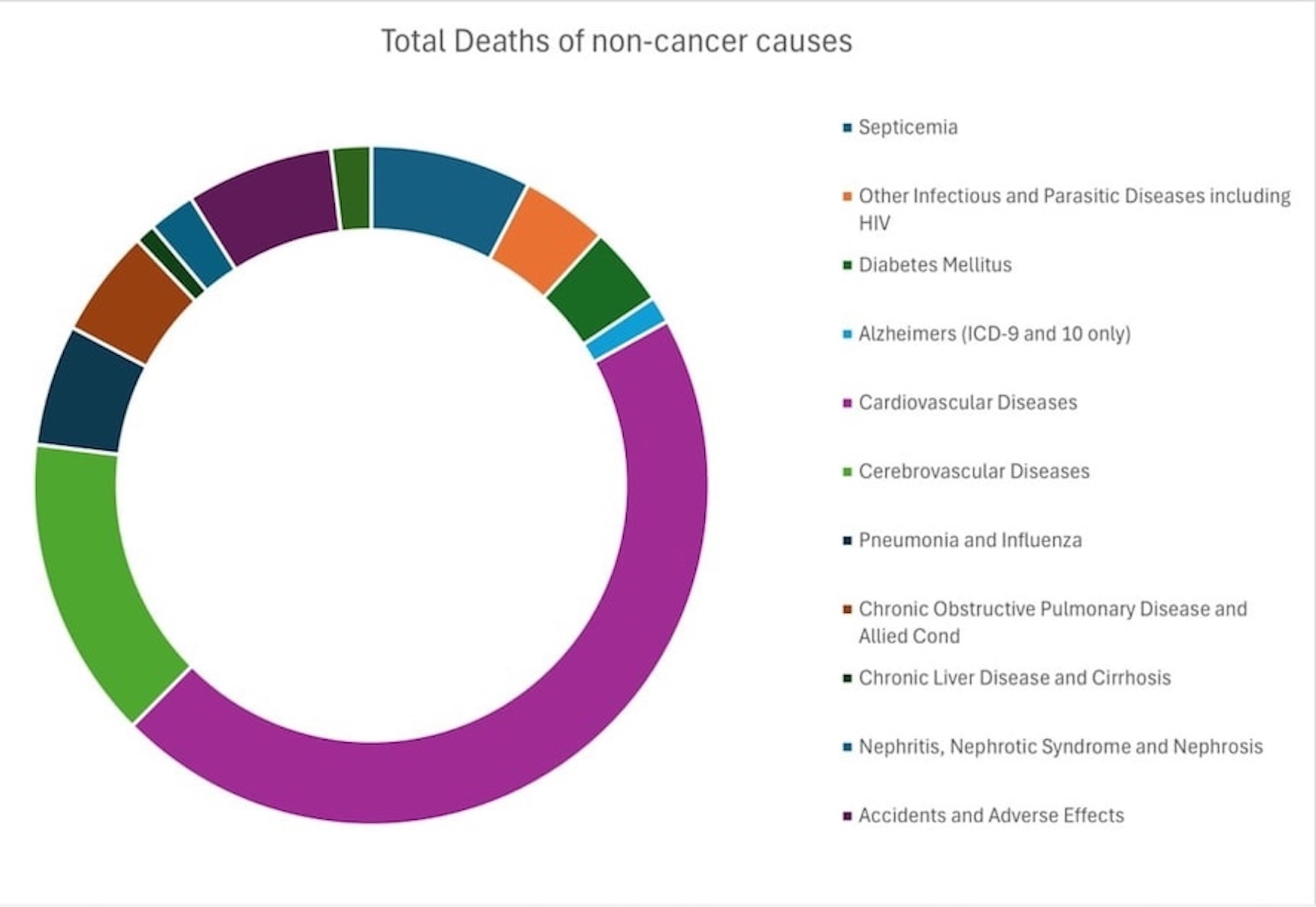 Click for large image | Figure 2. Non-cancer causes of death in glioblastoma. |
Non-cancer CODs within 1 year after glioblastoma grade IV diagnosis
A total of 25,235 deaths occurred within 1 year after glioblastoma grade IV diagnosis; 21,833 (86.5%) patients died of brain and other nervous system cancers, 427 (1.7%) died of other cancers, and 2,975 (11.8%) died of non-cancer causes. Within this year, cardiovascular diseases, cerebrovascular diseases, and septicemia remained the leading CODs with SMRs of 3.47 (95% CI, 3.15 - 3.81), 5.77 (95% CI, 4.83 - 6.84), and 9.94 (95% CI, 7.67 - 12.67), respectively.
In the first year after glioblastoma grade IV diagnosis, the most common non-cancer COD in all age groups, except for those younger than 18 years and patients aged 18 - 44, was cardiovascular diseases. There were no significant differences in the risk of death in patients younger than 18 years compared to the general population. Patients aged 18 - 44 had higher risks of dying from cerebrovascular diseases (SMR, 24.52; 95% CI, 5.06 - 71.67) and other parasitic and infectious diseases, including HIV (SMR, 9.96; 95% CI, 1.21 - 35.99). Notably, participants older than 75 years had the highest risk of death from heart diseases (SMR, 3.00; 95% CI, 2.59 - 3.47) compared to any other age group within this year after diagnosis. Tables 6-10 show the CODs for all age subgroups.
 Click to view | Table 6. SMRs for Each COD Following Glioblastoma Diagnosis in Patients Less Than 18 Years |
 Click to view | Table 7. SMRs for Each COD Following Glioblastoma Diagnosis in Patients Aged 18 - 44 |
 Click to view | Table 8. SMRs for Each COD Following Glioblastoma Diagnosis in Patients Aged 45 - 59 |
 Click to view | Table 9. SMRs for Each COD Following Glioblastoma Diagnosis in Patients Aged 60 - 74 |
 Click to view | Table 10. SMRs for Each COD Following Glioblastoma Diagnosis in Patients Older Than 75 |
Non-cancer CODs from 1 to 5 years after glioblastoma grade IV diagnosis
A total of 13,910 deaths (34.8% of all deaths) occurred from 1 to 5 years after diagnosis of glioblastoma grade IV; 12,746 (91.6%) patients died of brain and other nervous system cancers, 203 (1.5%) died of other cancers, and 961 (6.9%) died of non-cancer causes. The most common non-cancer CODs were cardiovascular diseases (SMR, 2.22; 95% CI, 1.84 - 2.65), cerebrovascular diseases (SMR, 3.92; 95% CI, 2.76 - 5.41), and treatment-related adverse effects (SMR, 3.05; 95% CI, 2.09 - 4.30).
Within 1 to 5 years of glioblastoma grade IV diagnosis, cardiovascular diseases were the most common non-cancer COD in all age groups except participants who were diagnosed below 18 years and those over 75 years. Compared with the general population, patients younger than 18 years had a significantly higher risk of death from pneumonia and influenza (SMR, 402.27; 95% CI, 10.18 - 2,241.30). Patients aged 18 - 44 had higher risks of dying from cardiovascular diseases (SMR, 5.88; 95% CI, 2.54 - 11.58), influenza, and pneumonia (SMR, 31.62; 95% CI, 6.52 - 92.41). Patients diagnosed with glioblastoma grade IV aged 45 - 59 and 60 - 74 had a higher risk of death from cardiovascular and cerebrovascular diseases, accidents, and adverse effects. Interestingly, there were no significant differences in the risk of death in patients older than 75 years compared to the general population.
Non-cancer CODs more than 5 years after glioblastoma grade IV diagnosis
A total of 792 patients, accounting for 2.0% of all deaths, died more than 5 years after being diagnosed with grade IV glioblastoma; 656 (82.8%) patients died of brain and other nervous system cancers, 27 (3.4%) died of other cancers, and 109 (13.8%) died of non-cancer causes. The most common non-cancer CODs were cerebrovascular diseases (SMR, 3.56; 95% CI, 1.63 - 6.76), followed by septicemia (SMR, 4.67; 95% CI, 1.27 - 11.95), influenza and pneumonia (SMR, 4.02; 95% CI, 1.09 - 10.29). More than 5 years after diagnosis of glioblastoma grade IV, the leading COD in patients aged 18 - 44 was cerebrovascular diseases (SMR, 30.17; 95% CI, 9.80 - 70.40).
| Discussion | ▴Top |
Glioblastoma remains one of the most formidable challenges in neuro-oncology due to its aggressive nature and limited treatment options [9]. Our study utilized data from the SEER database to comprehensively analyze the clinical characteristics, outcomes, and CODs in patients diagnosed with glioblastoma grade IV between 2000 and 2019.
We examined the distribution of glioblastoma cases across different demographic groups. Remarkably, most patients were aged between 60 and 74 years, reflecting the increasing incidence of glioblastoma with advancing age. Male patients constituted the larger proportion of the cohort, which is consistent with previous observations suggesting a male predilection for glioblastoma and previous studies’ results [10, 11].
Previous studies have shown that glioblastoma patients experience a gender disparity in outcomes, with females often having a better prognosis than males [12, 13]. Survival analysis reveals a substantial decline in both OS and RS over time, reflecting the disease’s aggressive nature. Compared to males, females experienced a slight but consistently increased survival. This suggests a possible sex-specific difference either in the mechanism of the disease or response to therapy. Survival disparities existed among the various racial groups, with White patients having the lowest survival rates. More research is needed to better understand the significant causes of disparities, whether social or biological. The survival estimates were based on conditional survival curves, which demonstrate that survival prospects improve with time after diagnosis, especially for those who survive 6 - 10 years after diagnosis. Conditional survival curves also clearly show that older patients had lower conditional survival rates compared with younger patients. Young people, particularly those in the adolescent and young adult category, had the highest SMRs for death from cancer and non-cancer causes, underscoring the importance of age-relative considerations in glioblastoma treatment decision-making and survivorship care planning. Older patients had an increased risk of death from cerebrovascular disease, highlighting the importance of managing this age-related comorbidity and optimizing outcomes in this population, which is supported by existing data [14, 15]. Cardiovascular diseases were the most commonly identified non-glioblastoma COD, just as they are in the general population [16, 17]. Notably, this emphasizes the importance of systems that provide cardiovascular risk reduction for all patients with glioblastoma, particularly given the increased likelihood of cardiac mortality following diagnosis. Colorectal cancer was the next most common non-glioblastoma COD. This could be in part due to common modifiable risk factors for cardiovascular disease and colorectal cancer, such as dyslipidemia, smoking, and a sedentary lifestyle.
Our analysis also revealed an increased risk of death from septicemia, particularly in the first 5 years following diagnosis, suggesting a potential association with cancer-related treatments such as surgery, chemotherapy, and radiotherapy. It is crucial to ensure careful supervision and immediate action whenever any signs of infection are spotted among people suffering from glioblastoma under treatment. Furthermore, our study identified suicide as a notable risk within the first 5 years of glioblastoma diagnosis, highlighting the need for integrated mental health support and psychosocial interventions as part of comprehensive glioblastoma care.
Our study’s limitations include its retrospective nature, which may introduce biases and limitations in adjusted factors. The large sample size may also lead to statistically significant findings that may not always translate into clinical significance. Additionally, the study period limited the assessment of long-term outcomes, particularly for patients diagnosed later in the study period.
Furthermore, the ICD-O-3 classification we utilized does not incorporate molecular features such as IDH mutation status, which are now essential in the WHO 2021 classification. Prior to 2021, glioblastoma encompassed both IDH-wild-type and IDH-mutant cases. Given the constraints of our dataset, our study relies on the historical classification system. However, we acknowledge that the 2021 WHO Classification of CNS tumors has redefined glioblastoma strictly as an IDH-wild-type, CNS WHO grade IV tumor in adults, while IDH-mutant cases are now categorized separately as astrocytoma, WHO grade IV.
Our analysis of CODs revealed that the majority of deaths were attributable to glioblastoma itself due to the aggressive nature of the disease. However, a notable proportion of deaths were attributed to non-cancer causes, with cardiovascular diseases, cerebrovascular diseases, and septicemia emerging as the most common non-cancer CODs. Importantly, the risk of non-cancer mortality varied across different age groups, with older patients exhibiting a higher risk of death from cardiovascular and cerebrovascular conditions.
Strength of the study
Our study may raise the awareness of healthcare providers about glioblastoma and its management, including an assessment of other associations such as CODs. Overall, our study provides valuable insights into the clinical characteristics, outcomes, and CODs in patients with glioblastoma grade IV. These findings point to the urgent need for novel therapeutic approaches to improve survival outcomes and mitigate the burden of non-cancer comorbidities in this patient population. It is also essential to integrate holistic and personalized approaches to glioblastoma management, cardiovascular risk assessment, infectious disease management, and psychosocial support into routine care to optimize outcomes and enhance the quality of life for glioblastoma patients across the continuum of their disease journey.
Recommendation
Further research is warranted to elucidate the underlying mechanisms driving disparities in survival outcomes across demographic groups and to inform personalized treatment strategies for glioblastoma patients.
Conclusion
In this study, patients diagnosed with WHO grade IV glioblastoma showed a sharp decline in OS and RS over time after diagnosis. However, there was a notable improvement in conditional 3-year survival over time. Cardiovascular diseases emerged as the most common non-cancer COD, with lower survival rates in males and advanced age. Accordingly, age, race, and sex were considered independent prognostic factors influencing survival outcomes in patients with glioblastoma grade IV.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
None to declare.
Author Contributions
AE, KS, MMZ, AEl, UE, SA, AA, and EFG conceived the idea and supervised the project. AE, NAA, MA, AAA, MHA, SS, KRM, and DAG collected and analyzed the data. MMZ, HAAE, HAH, TAMA, and AE drafted the manuscript. All authors revised and agreed to the final version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
CODs: causes of death; OS: overall survival; RS: relative survival; SEER: Surveillance, Epidemiology, and End Results; SMRs: standardized mortality ratios
| References | ▴Top |
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820.
doi pubmed - Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019;21(Suppl 5):v1-v100.
doi pubmed - Davis FG, Smith TR, Gittleman HR, Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Glioblastoma incidence rate trends in Canada and the United States compared with England, 1995-2015. Neuro Oncol. 2020;22(2):301-302.
doi pubmed - Best B, Nguyen HS, Doan NB, Gelsomino M, Shabani S, Ahmadi Jazi G, Sadati M, et al. Causes of death in glioblastoma: insights from the SEER database. J Neurosurg Sci. 2019;63(2):121-126.
doi pubmed - Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, Kelly SP, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889-3897.
doi pubmed - Dong X, Noorbakhsh A, Hirshman BR, Zhou T, Tang JA, Chang DC, Carter BS, et al. Survival trends of grade I, II, and III astrocytoma patients and associated clinical practice patterns between 1999 and 2010: A SEER-based analysis. Neurooncol Pract. 2016;3(1):29-38.
doi pubmed - Mariotto AB, Noone AM, Howlader N, Cho H, Keel GE, Garshell J, Woloshin S, et al. Cancer survival: an overview of measures, uses, and interpretation. J Natl Cancer Inst Monogr. 2014;2014(49):145-186.
doi pubmed - Agnew P. Estimating virulence from relative survival. bioRxiv. 2019.
doi - Yu XQ, Baade PD, O'Connell DL. Conditional survival of cancer patients: an Australian perspective. BMC Cancer. 2012;12:460.
doi pubmed - Hansen S, Rasmussen BK, Laursen RJ, Kosteljanetz M, Schultz H, Norgard BM, Guldberg R, et al. Treatment and survival of glioblastoma patients in Denmark: The Danish Neuro-Oncology Registry 2009-2014. J Neurooncol. 2018;139(2):479-489.
doi pubmed - Fuentes-Raspall R, Solans M, Roca-Barcelo A, Vilardell L, Puigdemont M, Del Barco S, Comas R, et al. Descriptive epidemiology of primary malignant and non-malignant central nervous tumors in Spain: Results from the Girona Cancer Registry (1994-2013). Cancer Epidemiol. 2017;50(Pt A):1-8.
doi pubmed - Trifiletti DM, Alonso C, Grover S, Fadul CE, Sheehan JP, Showalter TN. Prognostic implications of extent of resection in glioblastoma: analysis from a large database. World Neurosurg. 2017;103:330-340.
doi pubmed - Sun T, Plutynski A, Ward S, Rubin JB. An integrative view on sex differences in brain tumors. Cell Mol Life Sci. 2015;72(17):3323-3342.
doi pubmed - Li K, Lu D, Guo Y, Wang C, Liu X, Liu Y, Liu D. Trends and patterns of incidence of diffuse glioma in adults in the United States, 1973-2014. Cancer Med. 2018;7(10):5281-5290.
doi pubmed - McKinnon C, Nandhabalan M, Murray SA, Plaha P. Glioblastoma: clinical presentation, diagnosis, and management. BMJ. 2021;374:n1560.
doi pubmed - Brandes AA, Scelzi E, Salmistraro G, Ermani M, Carollo C, Berti F, Zampieri P, et al. Incidence of risk of thromboembolism during treatment high-grade gliomas: a prospective study. Eur J Cancer. 1997;33(10):1592-1596.
doi pubmed - Kreisl TN, Toothaker T, Karimi S, DeAngelis LM. Ischemic stroke in patients with primary brain tumors. Neurology. 2008;70(24):2314-2320.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.