| RJournal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Review
Volume 17, Number 4, April 2025, pages 181-186
Lipoprotein(a)-Lowering Drugs: A Mini Review
Masato Hamasakia, Kazuhiko Kotania, b
aDivision of Community and Family Medicine, Jichi Medical University, Shimotsuke-City, Tochigi 329-0498, Japan
bCorresponding Author: Kazuhiko Kotani, Division of Community and Family Medicine, Jichi Medical University, Shimotsuke-City, Tochigi 329-0498, Japan
Manuscript submitted February 1, 2025, accepted March 26, 2025, published online April 11, 2025
Short title: Lp(a)-Lowering Treatments
doi: https://doi.org/10.14740/jocmr6196
- Abstract
- Introduction
- Lipid-Lowering Drugs
- Estrogen-Related Drugs and Supplements
- Lp(a)-Specific Drugs
- Overview
- Conclusions
- References
| Abstract | ▴Top |
Lipoprotein(a) (Lp(a)) is a type of lipoprotein consisting of low-density lipoprotein with apoprotein(a) (apo(a)) and is a risk factor for cardiovascular disease (CVD). Lowering Lp(a) levels may improve CVD outcomes, but this has been challenging owing to the unique structure and metabolic pathway of Lp(a). Recently, several new treatments using apo(a)-targeting drugs have been developed to reduce Lp(a) levels. Here, we briefly summarize the treatments, including earlier attempts at reducing Lp(a). Some lipid-lowering drugs can reduce Lp(a) levels in a non-targeted manner; while the effect of statins varies, niacin and proprotein convertase subtilisin/kexin type 9 inhibitors exhibit a reduction of over 20% in Lp(a) levels. Estrogen-related drugs and certain supplements can reduce Lp(a) levels, which may promote a deeper understanding of the modulation of Lp(a) levels. An apo(a) antisense oligonucleotide, small interfering RNAs, and a small molecule Lp(a)-formation inhibitor have recently been developed as promising drugs that specifically reduce Lp(a) levels by approximately 80%. The treatment strategies for Lp(a) are set to be updated, although we are awaiting clinical evidence on the reduction of CVD events by new treatments and the effective threshold for Lp(a) levels for the prevention of CVD.
Keywords: Apo(a); Antisense oligonucleotide; Estrogen-related drug; Lp(a)-formation inhibitor; siRNA; Supplement
| Introduction | ▴Top |
Lipoprotein(a) (Lp(a)) is a unique type of lipoprotein consisting of low-density lipoprotein (LDL) and an apoprotein(a) (apo(a)) [1-5]. LDL, which carries apolipoprotein B (apoB), is known to be involved in atherogenesis, and apo(a) is a plasminogen-like protein that competes with plasminogen to inhibit its anti-thrombotic activity [1-3]. Apo(a) also stimulates a platelet-activating factor, which initiates angiogenesis, and binds to oxidized phospholipid (ox-PL)-rich LDL, which participates in the development of atherosclerosis [1, 3, 6, 7]. With such characteristics, Lp(a) exhibits pathophysiological relevance in cardiovascular disease (CVD) [8, 9].
Although the metabolism of Lp(a) is not completely understood, the majority of its synthesis and catabolism occurs in the liver, with only a minor part of the synthesis occurring in the intestine [10]. Apo(a) and apoB are produced in the nucleus, and LDL assembly occurs in the endoplasmic reticulum. Apo(a) binds to apoB of LDL on the cell surface [11], and Lp(a) is secreted from the liver into the bloodstream. Multiple pathways have been proposed for the catabolism of Lp(a), such as the binding of apo(a) to the LDL receptor or very-low-density lipoprotein receptor on hepatocytes and the binding of Lp(a) with ox-PL to CD36 or type B scavenger receptor on macrophages [12].
Blood Lp(a) levels show a skewed distribution towards higher concentrations, and Lp(a) < 30 mg/dL is often considered normal [4]. The Lp(a) level is primarily determined by genetic variations in apo(a) [3]. Genetic variation within the LPA gene results in the presence of kringle-like repeats, and a negative correlation has been observed between the length of this region and Lp(a) levels [3]. Single nucleotide polymorphisms (SNP) in the LPA gene affect kringle-like repeats, further affecting Lp(a) levels [13]. Lp(a) has also been positively associated with inflammation [4] because its expression of LPA gene is regulated by interleukin-6, an inflammatory molecule [14].
Even though cardiometabolic pathologies such as diabetes mellitus [15], renal kidney disease [16], and familial hypercholesterolemia [17] can modify blood Lp(a) levels, Lp(a) has been confirmed as an independent risk factor for CVD [8, 9]. In fact, guidelines recommend measuring Lp(a) levels to assess the risk of CVD [18, 19]. Lowering Lp(a) levels may reduce CVD risk [8, 9, 18, 19]. To meet this expectation, treatments that specifically control Lp(a) have recently emerged [20]. Such drugs greatly reduce Lp(a) levels using antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), or small molecule Lp(a)-formation inhibitors. To put this into perspective, we briefly summarize the treatments, including earlier attempts at reducing Lp(a).
| Lipid-Lowering Drugs | ▴Top |
Lipid-lowering drugs used in clinical practice include statins, fibrates, niacin, and other medications that mainly lower LDL-cholesterol (LDL-C) levels (Table 1) [14, 20-30]. Although these lipid-lowering therapies do not always focus on reducing Lp(a) levels, their effects on Lp(a) have been of interest. The functions of the drugs are partly known (Fig. 1).
 Click to view | Table 1. Effects of Lipid-Lowering Drugs and Lp(a)-Specific Drugs on Lp(a) |
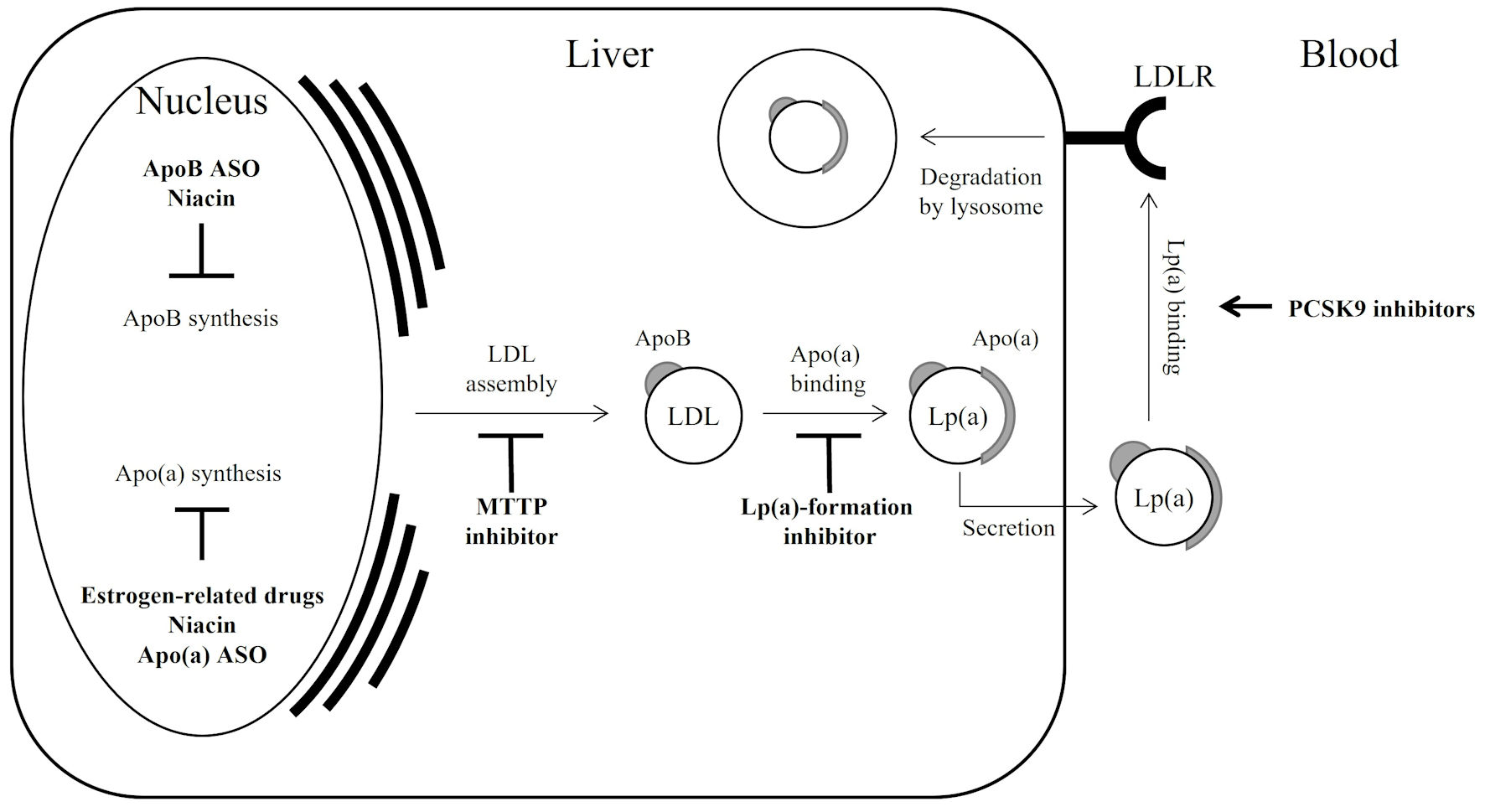 Click for large image | Figure 1. Lp(a) regulation by drugs; a simple schematic illustration. Bold arrow indicates acceleration; bold T-bar indicates inhibition. Since statins modulate both apoB synthesis and LDLR activation, resulting in a varied effect on Lp(a), they are not described in the figure. Apo(a): apolipoprotein(a); apoB: apolipoprotein B; ASO: antisense oligonucleotide; LDL: low-density lipoprotein; LDLR: low-density lipoprotein receptor; MTTP: microsomal triglyceride transfer protein; PCSK9: proprotein convertase subtilisin/kexin type 9; Lp(a): lipoprotein(a). |
The effects of lipid-lowering drugs on Lp(a) levels depend on the mechanism of cholesterol reduction. Statins, which control cholesterol synthesis in the liver and LDL receptor uptake of LDL-C from the blood, are popular lipid-lowering drugs [21, 31]. The effect of statins on Lp(a) levels is reported to vary; basically, Lp(a) levels are not largely changed by statins. Fibrates are generally used to reduce triglycerides, while some associations are suggested between triglycerides and Lp(a) levels; actually, Lp(a) levels are unchanged or slightly increased by fibrates [22, 32]. Niemann-Pick C1-Like1 inhibitors control cholesterol absorption, which can reduce Lp(a) levels by approximately 7% [24, 34, 35].
Recently used LDL-specific drugs that inhibit LDL formation or uptake are typically used in a condition such as severe hypercholesterolemia. As proprotein convertase subtilisin/kexin type 9 (PCSK9) degrades LDL receptors and then increases LDL in circulation, PCSK9 inhibitors protect LDL receptors and accelerate the uptake of LDL with Lp(a) [36], resulting in a > 20% reduction in Lp(a) levels [25, 26, 37].
Microsomal triglyceride transfer protein (MTTP) inhibitors and apoB ASO prevent LDL-formation, and the respective drugs show 13% and 32% reduction in Lp(a) levels [27, 38-40].
| Estrogen-Related Drugs and Supplements | ▴Top |
In the period when Lp(a) levels are not specifically reduced, estrogen-related drugs and certain supplements have been considered to modulate these levels (Table 2) [41-46]. Understanding the mechanisms behind Lp(a) levels can enhance our knowledge of how to modulate its levels effectively.
 Click to view | Table 2. Effects of Estrogen-Related Drugs and Supplements on Lp(a) |
Estrogen-related drugs have been reported to significantly reduce Lp(a) levels (Fig. 1). Hormone replacement therapy (HRT) reduces Lp(a) levels by 20% [41]. The transcription factor, estrogen receptor α, is upregulated by binding to estrogen response element sites near the LPA gene, leading to the suppression of apo(a) synthesis [41]. Tibolone is known for menopausal hormone therapy and also reduces Lp(a) levels by 25% [42]. Among the anti-estrogen drugs, tamoxifen and raloxifene reduced Lp(a) levels by 0.40 mg/dL in male patients with hypercholesterolemia [43, 44].
Observing Lp(a) reduction in some supplements (Table 2) [41-46], L-carnitine has been reported to reduce Lp(a) levels by 8.82 mg/dL in patients with diabetes mellitus and hypercholesterolemia [45]. L-carnitine may potentially reduce Lp(a) by attenuating the stimulation of fatty acid breakdown, which is necessary for bile acid production [45]. Coenzyme Q10 has also been shown to reduce Lp(a) levels by 3.54 mg/dL in patients with both diabetes mellitus and hypercholesterolemia [46]. The attenuation of inflammation by coenzyme Q10 can lead to the reduction in Lp(a) [46].
| Lp(a)-Specific Drugs | ▴Top |
The studies by Lp(a)-specific drugs are described in Table 1 [20, 28-30]. ASOs targeting apo(a) are designed to reduce apo(a) synthesis by inhibiting the silencing of apo(a) mRNA, which specifically reduces Lp(a), but not LDL (Fig. 1) [47, 48]. In a clinical trial [20], pelacarsen, an ASO conjugated with triantennary N-acetylgalactosamine specific to hepatocytes, using ligand-conjugated antisense technology, reduced Lp(a) levels by up to 47% in patients with high Lp(a) levels of ≥ 29 mg/dL (median: 82 mg/dL). Moreover, CVD risk reduction is assumed to be mediated by proinflammatory activation, as observed by a reduction in circulating monocytes [49]. Recently, a phase II study was conducted, and pelacarsen (20 mg per week) reduced Lp(a) levels by up to 80% over a 27-week period in CVD patients with high Lp(a) levels of ≥ 50 mg/dL (median: 87 mg/dL) [20]. The CVD outcomes, including the events and deaths, were not significantly affected by drug intervention because the intervention period was only 6 months, which appeared to be too short to observe the outcomes [20]. The clinical trial turned into a phase III study focusing on CVD outcomes over a period of several years. Similar apo(a)-targeting drugs using siRNAs are also being clinically tested. Olpasiran reduced Lp(a) levels by 40% in a phase II trial with high Lp(a) levels of ≥ 60 mg/dL (median: 104 mg/dL) [28]. The pre-clinical assessment of zerlasiran has been completed [50], showing a reduced Lp(a) levels by 90% in a phase II trial with high Lp(a) levels of ≥ 50 mg/dL (median: 85 mg/dL) [29].
Muvalaplin, a chemical compound, makes apo(a) non-functional [30] (Fig. 1). It binds the interaction site of apo(a) to apoB and inhibits the formation of Lp(a) [30, 51]. Muvalaplin was shown to reduce Lp(a) levels by 63-65% relative to placebo with high Lp(a) levels of ≥ 30 mg/dL (median: 58 mg/dL) [30]. The LDL-C level was not altered by muvalaplin treatment. Although this was only a phase I trial and did not reveal significantly lower Lp(a) levels, it is still of concern because it is the only oral Lp(a)-specific drug.
| Overview | ▴Top |
The overall summary of the treatments for Lp(a) reduction is listed in Table 1 (lipid-lowering drugs and Lp(a)-specific drugs) [14, 20-30] and Table 2 (estrogen-related drugs and supplements) [41-46]. In summary, recently developed Lp(a)-specific drugs, such as an apo(a) ASO, siRNAs, and Lp(a)-formation inhibitor, can have a greater effect on Lp(a) reduction. The fact that their effects are independent of LDL-C levels has received attention. Each 5-mg/dL reduction in Lp(a) levels is associated with a 2.5% relative CVD risk reduction [17, 52]. A great effect of Lp(a)-specific drugs to reduce CVD has been estimated, although up to now, a preceding trial using those drugs with a small population has not demonstrated their effect on CVD outcomes [20, 28, 29].
The threshold levels of Lp(a) for the development of CVD are also debatable [4]. Although Lp(a)-specific drugs have been used for patients with high Lp(a) levels [20, 28-30], the threshold (target) levels of Lp(a) for the prevention of CVD should be defined in the future. This would lead to a discussion regarding which patients are most likely to benefit from treatment and/or to whom treatments should be appropriately applied.
| Conclusions | ▴Top |
Here, an earlier attempt at lowering Lp(a) levels using lipid-lowering drugs, as well as estrogen-related drugs and supplements, has been reviewed. Recently developed Lp(a)-specific drugs, such as apo(a) ASO, siRNAs, and an Lp(a)-formation inhibitor, can have a greater effect on Lp(a) reduction. The effect of Lp(a) reduction on CVD outcomes is expected but remains unknown. Clinical trials using Lp(a)-specific drugs are ongoing, and additional data from such trials are needed to establish the benefits of treatment on CVD outcomes.
Acknowledgments
None to declare.
Financial Disclosure
No funding was received for this paper.
Conflict of Interest
MH also works at Eiken Chemical Co., Ltd. The other author declares no conflict of interest.
Author Contributions
Conceptualization: MH and KK. Writing - original draft preparation: MH. Writing - review and editing: KK. Supervision: KK. All authors have read and agreed to the published version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
Lp(a): lipoprotein (a); apo(a): apolipoprotein (a); apoB: apolipoprotein B; LDL: low-density lipoprotein; PCSK9: proprotein convertase subtilisin/kexin type 9; ASO: antisense oligonucleotide; siRNAs: small interfering RNAs
| References | ▴Top |
- Eaton DL, Fless GM, Kohr WJ, McLean JW, Xu QT, Miller CG, Lawn RM, et al. Partial amino acid sequence of apolipoprotein(a) shows that it is homologous to plasminogen. Proc Natl Acad Sci U S A. 1987;84(10):3224-3228.
doi pubmed - Berglund L, Ramakrishnan R. Lipoprotein(a): an elusive cardiovascular risk factor. Arterioscler Thromb Vasc Biol. 2004;24(12):2219-2226.
doi pubmed - Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein (a). J Lipid Res. 2016;57(8):1339-1359.
doi pubmed - Kotani K, Serban MC, Penson P, Lippi G, Banach M. Evidence-based assessment of lipoprotein(a) as a risk biomarker for cardiovascular diseases - Some answers and still many questions. Crit Rev Clin Lab Sci. 2016;53(6):370-378.
doi pubmed - Gencer B, Kronenberg F, Stroes ES, Mach F. Lipoprotein(a): the revenant. Eur Heart J. 2017;38(20):1553-1560.
doi pubmed - Leischik R, Dworrak B. [Lipoprotein(a): importance for the fibrinolytic system and thromboembolic complications]. Herz. 2006;31(2):144-152.
doi pubmed - Rubin J, Kim HJ, Pearson TA, Holleran S, Berglund L, Ramakrishnan R. The apolipoprotein(a) gene: linkage disequilibria at three loci differs in African Americans and Caucasians. Atherosclerosis. 2008;201:140-147.
- Cao YX, Jin JL, Guo YL, Sun D, Liu HH, Wu NQ, Xu RX, et al. Baseline and on-statin treatment lipoprotein(a) levels for predicting cardiovascular events in patients with familial hypercholesterolemia. Atherosclerosis. 2019;291:27-33.
doi pubmed - Watanabe J, Hamasaki M, Kotani K. Risk of cardiovascular disease with lipoprotein(a) in familial hypercholesterolemia: a review. Arch Med Sci Atheroscler Dis. 2020;5:e148-e152.
doi pubmed - Maranhao RC, Carvalho PO, Strunz CC, Pileggi F. Lipoprotein (a): structure, pathophysiology and clinical implications. Arq Bras Cardiol. 2014;103(1):76-84.
doi pubmed - Iannuzzo G, Tripaldella M, Mallardo V, Morgillo M, Vitelli N, Iannuzzi A, Aliberti E, et al. Lipoprotein(a) where do we stand? From the physiopathology to innovative therapy. Biomedicines. 2021;9(7):840.
- McCormick SPA, Schneider WJ. Lipoprotein(a) catabolism: a case of multiple receptors. Pathology. 2019;51(2):155-164.
doi pubmed - Coassin S, Kronenberg F. Lipoprotein(a) beyond the kringle IV repeat polymorphism: The complexity of genetic variation in the LPA gene. Atherosclerosis. 2022;349:17-35.
doi pubmed - Muller N, Schulte DM, Turk K, Freitag-Wolf S, Hampe J, Zeuner R, Schroder JO, et al. IL-6 blockade by monoclonal antibodies inhibits apolipoprotein (a) expression and lipoprotein (a) synthesis in humans. J Lipid Res. 2015;56(5):1034-1042.
doi pubmed - Qi Q, Qi L. Lipoprotein(a) and cardiovascular disease in diabetic patients. Clin Lipidol. 2012;7(4):397-407.
doi pubmed - Rosas S, Joffe M, Wolfe M, Brayman K, Rader DJ. Effects of renal replacement therapy on plasma lipoprotein(a) levels. Am J Nephrol. 2008;28(3):361-365.
doi pubmed - Hamasaki M, Kotani K. Lipoprotein(a) and familial hypercholesterolemia: a short review including the laboratory viewpoint. Cardiol Res. 2020;11(6):356-359.
doi pubmed - Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34(45):3478-3490a.
doi pubmed - Chemello K, Chan DC, Lambert G, Watts GF. Recent advances in demystifying the metabolism of lipoprotein(a). Atherosclerosis. 2022;349:82-91.
doi pubmed - Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, Shapiro MD, et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382(3):244-255.
doi pubmed - Yeang C, Hung MY, Byun YS, Clopton P, Yang X, Witztum JL, Tsimikas S. Effect of therapeutic interventions on oxidized phospholipids on apolipoprotein B100 and lipoprotein(a). J Clin Lipidol. 2016;10(3):594-603.
doi pubmed - Sahebkar A, Simental-Mendia LE, Watts GF, Serban MC, Banach M, Lipid, Blood Pressure Meta-analysis Collaboration G. Comparison of the effects of fibrates versus statins on plasma lipoprotein(a) concentrations: a systematic review and meta-analysis of head-to-head randomized controlled trials. BMC Med. 2017;15(1):22.
doi pubmed - Sahebkar A, Reiner Z, Simental-Mendia LE, Ferretti G, Cicero AF. Effect of extended-release niacin on plasma lipoprotein(a) levels: A systematic review and meta-analysis of randomized placebo-controlled trials. Metabolism. 2016;65(11):1664-1678.
doi pubmed - Sahebkar A, Simental-Mendia LE, Pirro M, Banach M, Watts GF, Sirtori C, Al-Rasadi K, et al. Impact of ezetimibe on plasma lipoprotein(a) concentrations as monotherapy or in combination with statins: a systematic review and meta-analysis of randomized controlled trials. Sci Rep. 2018;8(1):17887.
doi pubmed - Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722.
doi pubmed - Katsiki N, Vrablik M, Banach M, Gouni-Berthold I. Inclisiran, low-density lipoprotein cholesterol and lipoprotein (a). Pharmaceuticals (Basel). 2023;16(4):577.
doi pubmed - Rader DJ, Kastelein JJ. Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation. 2014;129(9):1022-1032.
doi pubmed - O'Donoghue ML, Rosenson RS, Lopez JAG, Lepor NE, Baum SJ, Stout E, Gaudet D, et al. The off-treatment effects of olpasiran on lipoprotein(a) lowering: OCEAN(a)-DOSE extension period results. J Am Coll Cardiol. 2024;84(9):790-797.
doi pubmed - Nissen SE, Wang Q, Nicholls SJ, Navar AM, Ray KK, Schwartz GG, Szarek M, et al. Zerlasiran-a small-interfering RNA targeting lipoprotein(a): a phase 2 randomized clinical trial. JAMA. 2024;332(23):1992-2002.
doi pubmed - Nicholls SJ, Nissen SE, Fleming C, Urva S, Suico J, Berg PH, Linnebjerg H, et al. Muvalaplin, an oral small molecule inhibitor of lipoprotein(a) formation: a randomized clinical trial. JAMA. 2023;330(11):1042-1053.
doi pubmed - Taylor F, Ward K, Moore TH, Burke M, Davey Smith G, Casas JP, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2011;1:CD004816.
doi pubmed - Tenenbaum A, Fisman EZ. Fibrates are an essential part of modern anti-dyslipidemic arsenal: spotlight on atherogenic dyslipidemia and residual risk reduction. Cardiovasc Diabetol. 2012;11:125.
doi pubmed - Koschinsky ML, Marcovina SM. Structure-function relationships in apolipoprotein(a): insights into lipoprotein(a) assembly and pathogenicity. Curr Opin Lipidol. 2004;15(2):167-174.
doi pubmed - Awad K, Mikhailidis DP, Katsiki N, Muntner P, Banach M, Lipid, Blood Pressure Meta-Analysis Collaboration G. Effect of ezetimibe monotherapy on plasma lipoprotein(a) concentrations in patients with primary hypercholesterolemia: a systematic review and meta-analysis of randomized controlled trials. Drugs. 2018;78(4):453-462.
doi pubmed - Tobaru T, Seki A, Asano R, Sumiyoshi T, Hagiwara N. Lipid-lowering and anti-inflammatory effect of ezetimibe in hyperlipidemic patients with coronary artery disease. Heart Vessels. 2013;28(1):39-45.
doi pubmed - Lambert G, Charlton F, Rye KA, Piper DE. Molecular basis of PCSK9 function. Atherosclerosis. 2009;203(1):1-7.
doi pubmed - Navarese EP, Kolodziejczak M, Schulze V, Gurbel PA, Tantry U, Lin Y, Brockmeyer M, et al. Effects of proprotein convertase Subtilisin/Kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(1):40-51.
doi pubmed - Nohara A, Otsubo Y, Yanagi K, Yoshida M, Ikewaki K, Harada-Shiba M, Jurecka A. Safety and efficacy of lomitapide in japanese patients with homozygous familial hypercholesterolemia (HoFH): results from the AEGR-733-301 long-term extension study. J Atheroscler Thromb. 2019;26(4):368-377.
doi pubmed - Nandakumar R, Matveyenko A, Thomas T, Pavlyha M, Ngai C, Holleran S, Ramakrishnan R, et al. Effects of mipomersen, an apolipoprotein B100 antisense, on lipoprotein (a) metabolism in healthy subjects. J Lipid Res. 2018;59(12):2397-2402.
doi pubmed - Chan DC, Barrett PH, Watts GF. Recent explanatory trials of the mode of action of drug therapies on lipoprotein metabolism. Curr Opin Lipidol. 2016;27(6):550-556.
doi pubmed - Godsland IF. Effects of postmenopausal hormone replacement therapy on lipid, lipoprotein, and apolipoprotein (a) concentrations: analysis of studies published from 1974-2000. Fertil Steril. 2001;75(5):898-915.
doi pubmed - Kotani K, Sahebkar A, Serban C, Andrica F, Toth PP, Jones SR, Kostner K, et al. Tibolone decreases Lipoprotein(a) levels in postmenopausal women: A systematic review and meta-analysis of 12 studies with 1009 patients. Atherosclerosis. 2015;242(1):87-96.
doi pubmed - Sahebkar A, Serban MC, Penson P, Gurban C, Ursoniu S, Toth PP, Jones SR, et al. The effects of tamoxifen on plasma lipoprotein(a) concentrations: systematic review and meta-analysis. Drugs. 2017;77(11):1187-1197.
doi pubmed - Ferretti G, Bacchetti T, Simental-Mendia LE, Reiner Z, Banach M, Sahebkar A. Raloxifene lowers plasma lipoprotein(a) concentrations: a systematic review and meta-analysis of randomized placebo-controlled trials. Cardiovasc Drugs Ther. 2017;31(2):197-208.
doi pubmed - Serban MC, Sahebkar A, Mikhailidis DP, Toth PP, Jones SR, Muntner P, Blaha MJ, et al. Impact of L-carnitine on plasma lipoprotein(a) concentrations: A systematic review and meta-analysis of randomized controlled trials. Sci Rep. 2016;6:19188.
doi pubmed - Sahebkar A, Simental-Mendia LE, Stefanutti C, Pirro M. Supplementation with coenzyme Q10 reduces plasma lipoprotein(a) concentrations but not other lipid indices: A systematic review and meta-analysis. Pharmacol Res. 2016;105:198-209.
doi pubmed - Viney NJ, Yeang C, Yang X, Xia S, Witztum JL, Tsimikas S. Relationship between "LDL-C", estimated true LDL-C, apolipoprotein B-100, and PCSK9 levels following lipoprotein(a) lowering with an antisense oligonucleotide. J Clin Lipidol. 2018;12(3):702-710.
doi pubmed - Greco MF, Sirtori CR, Corsini A, Ezhov M, Sampietro T, Ruscica M. Lipoprotein(a) lowering-from lipoprotein apheresis to antisense oligonucleotide approach. J Clin Med. 2020;9(7):2103.
doi pubmed - Stiekema LCA, Prange KHM, Hoogeveen RM, Verweij SL, Kroon J, Schnitzler JG, Dzobo KE, et al. Potent lipoprotein(a) lowering following apolipoprotein(a) antisense treatment reduces the pro-inflammatory activation of circulating monocytes in patients with elevated lipoprotein(a). Eur Heart J. 2020;41(24):2262-2271.
doi pubmed - Rider DA, Eisermann M, Loffler K, Aleku M, Swerdlow DI, Dames S, Hauptmann J, et al. Pre-clinical assessment of SLN360, a novel siRNA targeting LPA, developed to address elevated lipoprotein (a) in cardiovascular disease. Atherosclerosis. 2022;349:240-247.
doi pubmed - Ogorelkova M, Kraft HG, Ehnholm C, Utermann G. Single nucleotide polymorphisms in exons of the apo(a) kringles IV types 6 to 10 domain affect Lp(a) plasma concentrations and have different patterns in Africans and Caucasians. Hum Mol Genet. 2001;10(8):815-824.
doi pubmed - Tsimikas S, Narula J. Lipoprotein(a) and CT angiography: novel insights into high-risk plaque progression. J Am Coll Cardiol. 2022;79(3):234-237.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.