| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 5, May 2025, pages 270-284
Anti-Breast Cancer Effects of Thymoquinone-Chemotherapeutic Combinations: A Systematic Review of the Latest In Vitro and In Vivo Studies
Nur Qodira, b, Legiranb, e, Zen Hafyb, Didit Pramudithoc, Muhammad Baharul Imand, Fara Syafirad, Raehan Satya Deanasad, Putri Mahirah Afladhantid
aDivision of Surgical Oncology, Department of Surgery, Faculty of Medicine, Universitas Sriwijaya, Palembang, Indonesia
bDepartment of Biomedical Science, Faculty of Medicine, Universitas Sriwijaya, Palembang, Indonesia
cDivision of Surgical Urology, Department of Surgery, Faculty of Medicine, Universitas Sriwijaya, Palembang, Indonesia
dFaculty of Medicine, Universitas Sriwijaya, Palembang, Indonesia
eCorresponding Author: Legiran, Department of Biomedical Science, Faculty of Medicine, Universitas Sriwijaya, Palembang, Indonesia
Manuscript submitted March 5, 2025, accepted April 28, 2025, published online May 28, 2025
Short title: TQ-Chemotherapeutic Combinations for Breast Cancer
doi: https://doi.org/10.14740/jocmr6230
| Abstract | ▴Top |
Background: Breast cancer is a leading malignancy among women globally, with chemotherapy as a cornerstone of treatment. However, the side effects and toxicity associated with chemotherapy necessitate the exploration of adjunctive therapies to improve efficacy and reduce adverse effects. Thymoquinone (TQ) has shown potential anti-cancer properties. This systematic review aimed to evaluate the effectiveness of TQ in combination with chemotherapeutic agents in treating breast cancer.
Methods: This study thoroughly reviewed and synthesized existing research following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines. The selected databases, including PubMed, ProQuest, ScienceDirect, Epistemonikos, and Google Scholar, were searched over the past 10 years. Eligibility criteria were based on the PICOS framework, focusing on experimental studies involving TQ-chemotherapy combinations. Data extraction and quality assessment were performed using SYRCLE and SCIRAP tools. This review included 18 in vitro and six in vivo studies.
Results: Findings revealed that TQ enhances the efficacy of chemotherapeutic agents by inducing apoptosis, enhancing autophagy, inhibiting tumor growth, and regulating cancer cell signaling pathways as well as multiple phases of the cell cycle. Additionally, TQ reduced chemotherapy-related toxicity, such as heart, blood, liver, and kidney damage, and also improved patient tolerance. Nanoparticle-based delivery systems further amplified these synergistic effects.
Conclusions: The TQ-chemotherapy combination shows significant potential as a therapy for breast cancer, enhancing treatment efficacy while mitigating side effects. Future clinical studies are needed to establish its safety and therapeutic applicability.
Keywords: Anti-cancer effect; Breast cancer; Thymoquinone; Nigella sativa; Systematic review
| Introduction | ▴Top |
Breast cancer is a leading malignancy among women globally, significantly impacting public health and socioeconomic structures [1]. According to the World Health Organization (WHO), it constitutes about 24.5% of all cancers diagnosed in women, with annual case numbers rising [2]. Currently, chemotherapy remains a vital part of breast cancer treatment, often combined with surgery and radiation therapy [3]. Several drugs have also been developed for breast cancer management, such as antibody-drug conjugates (ADCs) and immune checkpoint inhibitors (ICIs) [4-6]. Chemotherapy, while effective in targeting tumor tissues, is associated with significant side effects that cause discomfort and burden for patients. These drugs also affect normal cells, leading to adverse effects that can hinder patient adherence to cancer therapy [3, 7]. Therefore, there is a critical need for alternative or adjunctive therapies that can provide practical anti-cancer effects with minimal toxicity [8].
One potential solution is integrating natural compounds with established chemotherapeutic drugs. Thymoquinone (TQ) is recognized for its medicinal properties and potential anti-cancer effect [9, 10]. The ability of TQ to induce apoptosis, inhibit cell proliferation, enhance immune response, and reduce cell viability in cancer cells makes it a candidate for combination therapy in breast cancer treatment [11]. It is supported by evidence that cancer is a multifactorial disease influenced by metabolic dysregulation and inflammation, with markers such as the neutrophil-to-lymphocyte ratio underscoring the pivotal role of immune modulation in treatment [12, 13]. Leveraging TQ may lead to more effective and less toxic breast cancer treatments, with studies exploring its synergistic effects with chemotherapeutic agents to enhance efficacy and reduce side effects [14]. TQ, a primary compound in black cumin (Nigella sativa), can regulate redox systems and inhibit cell proliferation, migration, and tumor growth through various signaling pathways [15, 16]. Additionally, TQ may alleviate chemotherapy-induced complications, such as kidney damage, enhancing its therapeutic benefits [15, 17].
The extensive research on TQ has laid a robust groundwork for its anti-cancer properties, particularly in breast cancer therapy. Despite the valuable insights from these studies, there are still gaps in understanding TQ’s full therapeutic potential and mechanisms of action of TQ when used in combination with standard chemotherapeutic agents [18, 19]. This systematic review analyzed the recent effects of TQ-chemotherapeutic combinations in breast cancer treatment. It combines the latest in vitro and in vivo findings to provide a comprehensive understanding of TQ’s efficacy and mechanism of action in treating breast cancer. This study is also intended to be a reference for advancing to clinical trials.
| Materials and Methods | ▴Top |
Source of data and search strategy
The PRISMA 2020 guidelines were used to structure this systematic review [20]. The data source comprised accessible studies from five databases: PubMed, ProQuest, ScienceDirect, Epistemonikos, and Google Scholar, utilizing a combination of search terms: “((“Thymoquinone”) OR (“TQ”) OR (“Nigella sativa”) OR (“Cuminum”) OR (“Black cumin”) OR (“Black caraway”) OR (“Kalonji”) OR (“Black seed”)) AND (“Breast cancer”) AND (“In vivo”) AND (“In vitro”)”. The approval of the Institutional Review Board and adherence to ethical guidelines concerning human or animal subjects are irrelevant to this study.
Inclusion and exclusion criteria
The studies included in this systematic review must meet specific criteria based on the PICOS framework: P (population): in vitro and in vivo studies; I (intervention): treatment with TQ-chemotherapeutic combinations; C (comparison): negative (saline or untreated cell) and positive (chemotherapeutic alone); O (outcome): anti-cancer effects; S (study): experimental studies.
Clinical trials, protocols, conference proceedings, news articles, editorials, posters, review articles, presentations, and studies without a control group were excluded. Moreover, studies without full-text access, non-English publications, and those published before 2015 were excluded.
Data extraction and quality assessment
Basic data were extracted, including 1) the corresponding author of the selected study, 2) the year of publication, and 3) the country where the study was conducted. Subsequently, the table of characteristic results was divided into two tables: one for in vitro studies and another for in vivo studies. Data extraction and quality assessment were performed independently by four investigators (MBI, RSD, FS, and PMA) using the Systematic Review Center for Laboratory Animal Experimentation (SYRCLE) risk of bias tool for in vivo studies [21] and Science in Risk Assessment and Policy (SCIRAP) tool to evaluate the methodological quality of in vitro toxicity studies [22-25]. Furthermore, the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) Working Group’s Guideline Development Tool (GRADEpro GDT) was utilized [26, 27].
| Results | ▴Top |
Study selection
Through the use of several databases, a total of 2,138 articles were obtained. After removing 436 duplicate entries, 1,702 articles were filtered by examining their titles and abstracts, excluding 1,594 articles. Additionally, specific inclusion and exclusion criteria were applied, leading to the elimination of 87 articles. Consequently, this systematic review focused its analysis on 21 original articles [28-48]. The process and results of the literature screening are shown in Figure 1.
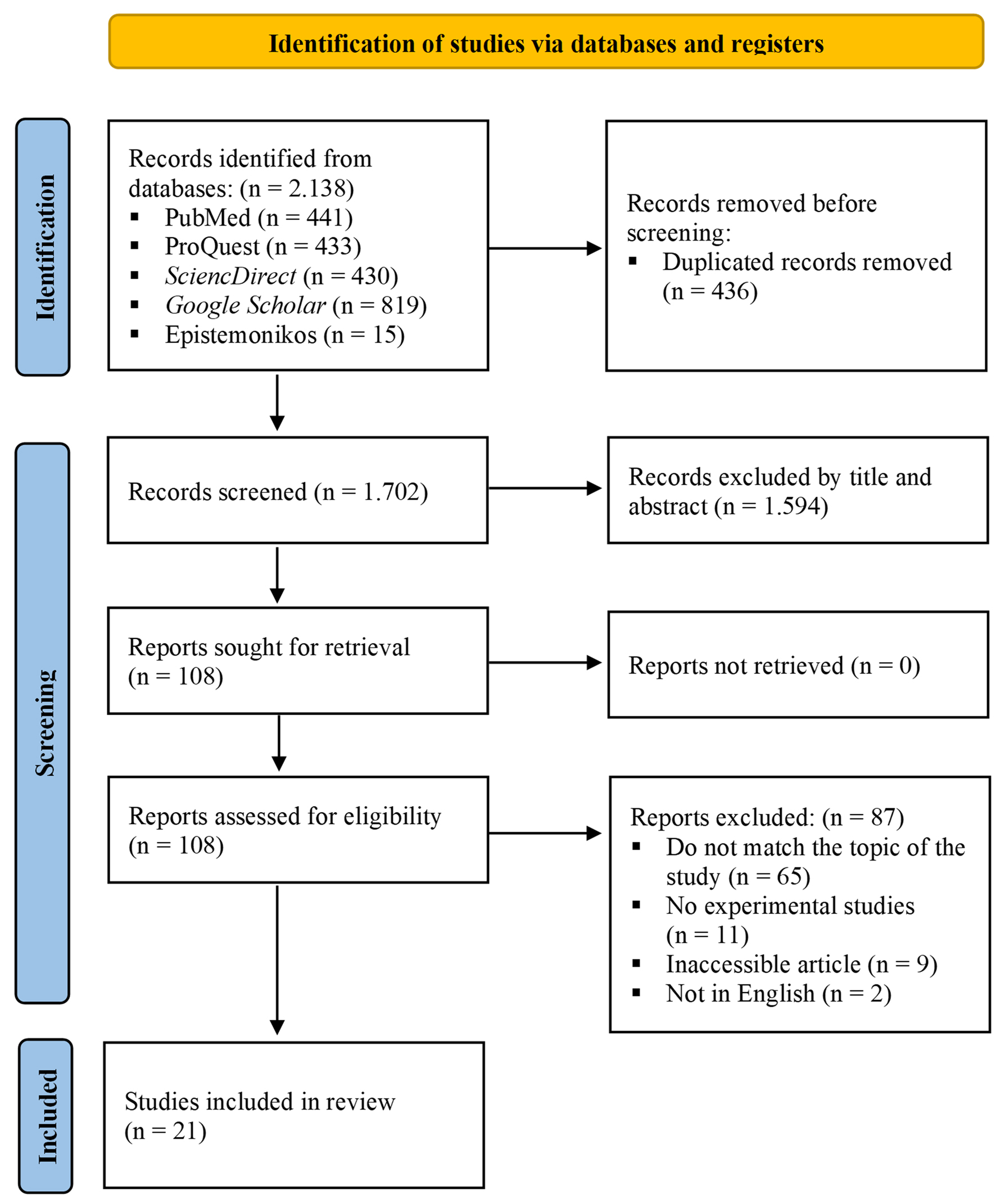 Click for large image | Figure 1. Flowchart illustrating the article selection procedure in accordance with the PRISMA 2020 guidelines for performing a systematic review. |
Characteristics of the studies
All included studies used an in vivo and in vitro study, published in English, and occurred between 2015 and 2023. The characteristics and primary outcomes are presented in Table 1 [28-37, 40, 42-47] for in vitro studies and Table 2 [29-31, 38, 39, 41] for in vivo studies. The in vitro studies examined data concerning cell viability, the apoptosis/DNA fragmentation rate, autophagy rate, necrotic rate, gene expression, tumor stem cell detection, cell cycle distribution, wound healing rate/cancer cell migration, cell invasion, and side effects of TQ and chemotherapeutic agent treatments. Meanwhile, the in vivo studies examined data concerning inhibitory effect, tumor gene expression, CD4+ and CD8+ expression, myeloid cell count, apoptotic effect, and antiangiogenic effect.
 Click to view | Table 1. Characteristics and Main Outcomes of In Vitro Studies Included |
 Click to view | Table 2. Characteristics and Main Outcomes of In Vivo Studies Included |
Quality evidence and risk of bias
This review uses the SCIRAP tool to evaluate the quality of in vitro study reports analyzed via Microsoft Excel™. The review focused on five main areas: test compounds and controls, test systems, administration of test compounds, data collection and analysis, and funding/competing interests, covering 23 topics. Of the 18 studies reviewed, two did not meet solubility and control vehicle criteria [28, 46], while 14 exhibited a high risk of bias related to contamination screening [28, 29, 32-37, 40, 43-47]. Additionally, seven studies lacked detail on metabolic competence [28, 32, 34, 37, 45, 46, 48], one did not specify cell passages [28], two had a high risk of bias associated with compound administration [28, 37], and one study presented a high risk of bias related to funding and competing interests [48]. Nonetheless, over 60% of the studies demonstrated a low bias risk, particularly in data collection and analysis (Supplementary Material 1, jocmr.elmerjournals.com).
In vivo studies’ bias risk was assessed using the SYRCLE tool, supported by RevMan 5.4. All studies showed a high risk of bias related to blinding (performance bias) and an unclear risk regarding allocation concealment. Among the six studies, three exhibited a high risk of bias in random housing and blinding (detection bias) [29-31]. Three studies also showed an unclear risk of bias concerning blinding (detection bias) [38, 39, 41] and sequence generation [29-31]. However, all studies showed a low risk of bias regarding baseline characteristics, incomplete outcome data, selective reporting, and other biases (Supplementary Material 2 and 3, jocmr.elmerjournals.com). The certainty of evidence was analyzed using GRADE criteria with the GRADEpro GDT tool, focusing on five aspects: risk of bias, inconsistency, indirectness, imprecision, and other considerations presented descriptively [26, 27]. Four results were rated high certainty [29, 38, 39, 41], while two were rated moderate certainty [30, 31]. Further details are available in Supplementary Material 4 (jocmr.elmerjournals.com).
Overall outcomes of the studies
The in vitro studies summarized in Table 1 reveal important interactions between TQ and conventional chemotherapeutic agents. Eleven studies on cell viability demonstrated that TQ combined with chemotherapeutics consistently resulted in the lowest cell viability [28-31, 33, 34, 36, 37, 42-44]. Among 10 studies assessing half-maximal inhibitory concentration (IC50), six indicated that TQ effectively reduced the required concentration of chemotherapeutics, enhancing their anticancer efficacy [28, 31, 34, 37, 40, 45]. However, four studies found no significant enhancement in potency, although the TQ-chemotherapy combinations still yielded the lowest IC50 values [35, 36, 46, 47]. One study noted that TQ could overcome resistance in paclitaxel (PTX)-resistant cancer cell lines [47]. Furthermore, nine studies evaluated the combination index (CI), with five showing a synergistic effect between TQ and chemotherapeutics [28, 31, 40, 45, 46], particularly when TQ was in carrier form [35, 36, 43]. In contrast, one study observed an antagonistic effect in its free-drug form [47].
In a review of nine studies on apoptosis rates, eight showed that TQ combined with chemotherapeutic agents significantly increased apoptosis compared to either treatment alone or untreated cells [32, 33, 35, 40, 42, 43, 46, 47]. At the same time, one study noted that TQ alone also heightened apoptosis [29]. The TQ-chemotherapeutic combination further enhanced DNA fragmentation compared to chemotherapeutics alone [48] and showed notable improvements in wound healing and cell invasion [43]. Among four studies on autophagy, three indicated that chemotherapeutics enhanced autophagy when combined with TQ [35, 46, 47]. In contrast, one study reported that TQ significantly increased autophagy compared to chemotherapy alone and untreated cells [40].
Six studies analyzing the cell cycle found that TQ combined with conventional chemotherapeutic agents significantly increased cell death across several phases, including pre-G, sub-G0, S, sub-G1, and G1 [33, 40, 43, 44, 47, 48]. Additionally, four studies on tumor stem cells and breast cancer stem cells (BCSCs) showed that TQ significantly decreased CD44+/CD24- when combined with chemotherapeutics compared to either treatment alone [35, 40, 47, 48].
Five studies on gene expression demonstrated that TQ significantly regulates genes in breast cancer treatment when combined with chemotherapeutic agents. TQ upregulated apoptosis-related genes, cytokines, p53 signaling pathway [29, 37], and tumor suppressor genes such as p21 and BRCA [29]. It inhibited phosphorylated Akt (p-5473-Akt), increased phosphatase and TENsin homolog (PTEN) expression [44], and decreased SNAIL-1, TWIST-1, and cyclin D1 levels [44, 48]. Additionally, TQ enhanced the B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax)/Bcl-2 ratio [32, 37] and boosted the activities of caspases 3, 7, 9, 12, and peroxisome proliferator-activated receptor (PPAR) [29, 32, 37].
The in vivo studies summarized in Table 2 indicate that TQ significantly enhances the efficacy of conventional chemotherapeutic agents. Five studies found that the combination of TQ and chemotherapy notably inhibited tumor growth compared to other treatments [29, 31, 38, 39, 41]. Two studies revealed TQ’s crucial role in regulating breast cancer-related genes by reducing Bcl-2 levels, increasing P53 levels, and enhancing IL-2 expression while suppressing Notch 1 and vascular endothelial growth factor (VEGF) [38, 41]. Safety analyses showed that TQ mitigated chemotherapy-related side effects, reducing heart toxicity [38] and increasing splenocyte counts [39]. One study noted liver and kidney minimal toxicity with TQ-lipid nanocapsules (LNCs) compared to docetaxel (DTX) [31]. Additionally, the TQ-chemotherapy combination increased CD4+ T cells compared to chemotherapy alone and showed varying effects on CD8+ T cells [39, 41]. The combination also enhanced the presence of granulocytic and monocytic cells [39], and improved apoptosis rates [41] and antiangiogenic effects compared to either treatment alone [30].
| Discussion | ▴Top |
Breast cancer has overtaken lung cancer as the most frequently diagnosed cancer and is the fifth leading cause of cancer-related deaths globally [2]. To date, research into effective therapies to reduce mortality from this disease continues to grow and remains a significant concern. One of the challenges in its management is that each chemotherapy drug has its limitations and potential side effects. This systematic review analyzed the recent effects of TQ-chemotherapeutic combinations in breast cancer treatment. The findings from the conducted identification and analysis reveal that combining TQ with chemotherapeutic agents consistently led to the lowest levels of cell viability. In addition, this study found that most studies showed that TQ effectively reduced the concentration of chemotherapy required and showed a synergistic effect between TQ and chemotherapy agents.
Multiple studies across various cancer cell lines have documented a synergistic effect of TQ, indicating its potential to enhance the efficacy of conventional chemotherapy [49, 50]. For instance, the TQ-cisplatin combination significantly increased cytotoxicity against oral squamous cell carcinoma, helping to address cisplatin’s limitations, such as drug resistance and toxicity [51]. Mahmood and Hamaamin (2022) highlighted TQ’s ability to induce apoptosis and inhibit cell proliferation, supporting its combined use with other chemotherapeutic agents [52]. Additionally, research by Celebioglu et al (2022) demonstrated that combining TQ with etoposide reduced cell viability and exhibited a potentially synergistic effect, improving therapeutic outcomes [53].
However, some studies indicate that TQ may not enhance the potency of chemotherapeutic agents and can even exhibit antagonistic effects in breast cancer cell lines. For instance, Bashmail et al (2020) found that TQ increased PTX’s anti-breast cancer activity despite mathematical antagonism, potentially due to its reduction of BCSCs (CD44+/CD24-), associated with drug resistance. TQ increased apoptotic and necrotic cell death in T47D cells with PTX and induce autophagy in MCF-7 cells [47]. Moreover, Motaghed et al (2014) reported that TQ’s combination with certain chemotherapies could diminish efficacy, especially in estrogen receptor-positive breast cancer, likely by downregulating the epidermal growth factor receptor (EGFR) pathway [54]. These findings underscore the complexity of integrating natural compounds like TQ with conventional chemotherapy in breast cancer treatment.
This review emphasizes that TQ synergizes with chemotherapeutic agents to enhance therapeutic outcomes by improving DNA fragmentation, inducing autophagy, inhibiting tumor growth, and promoting apoptosis. TQ supports apoptotic body formation and increases histone H2A.X phosphorylation, an early indicator of DNA damage [55, 56]. It can induce necrosis by activating reactive oxygen species (ROS) and modulating key signaling pathways like p38 MAPK, which are vital in mediating cell death responses [57-59]. In breast cancer models, TQ depletes tumor-associated stem cells through autophagy, sensitizing them to apoptosis and improving treatment efficacy [46, 60]. Furthermore, TQ downregulates anti-apoptotic proteins like Bcl-2, promotes apoptosis across various cancer cell lines, and affects their migratory and invasive abilities, underscoring its therapeutic potential [46, 60-62].
This study demonstrated that TQ combined with chemotherapeutics significantly enhanced the inhibitory effects on wound healing and cell invasion in in vitro models. Consistent with previous research, TQ effectively inhibits breast cancer cell migration and invasion by downregulating epithelial-to-mesenchymal transition (EMT) markers such as Twist1 and Zeb1 while upregulating E-cadherin, which is essential for maintaining epithelial integrity [63, 64]. TQ also inhibits EMT in prostate cancer by negatively regulating the transforming growth factor-beta (TGF-β)/Smad2/3 signaling pathway [65] and reduces renal cancer cell migration and invasion by downregulating matrix metalloproteinase-2 (MMP-2) and urokinase-type plasminogen activator (u-PA) [66]. Additionally, TQ increased antiangiogenic effects by inhibiting key signaling pathways, such as VEGF, reducing pro-inflammatory cytokines, and directly affecting endothelial cells [67, 68].
Current evidence indicates that TQ effectively inhibits tumor cells during various phases of development. The cell cycle’s progression through the G1, S, G2, and M phases is regulated by cyclin-dependent kinases (CDKs) and cyclins [69, 70]. Recent studies show that TQ induces cell cycle arrest in breast cancer cell lines, particularly in triple-negative breast cancer, affecting G0/G1, G1/S, or G2/M phases depending on the cell type [16, 71]. Our findings support previous research that TQ induces G2/M arrest in doxorubicin-resistant breast cancer and spindle carcinoma cells, which is linked to decreased levels of cyclin B1 and cell division cycle 25/Cdc25 phosphatase, along with increased p53 expression [72].
Our review highlights that the combination of TQ with chemotherapeutic agents significantly decreases the population of CD44+/CD24- BCSCs, a phenotype linked to chemoresistance and poor prognosis. This reduction is vital since CD44+/CD24- cells contribute to tumor initiation and recurrence due to their stem-like properties [73]. By targeting this population, TQ may help overcome the challenges posed by cancer stem cells, potentially leading to improved patient prognostic outcomes [74].
This study found that TQ can overcome resistance in PTX-resistant cancer cell lines, addressing a significant challenge in breast cancer treatment. TQ sensitizes resistant cells by modulating key signaling pathways related to cell survival and apoptosis. For instance, Khan et al (2023) reported that TQ inhibits the Akt signaling pathway, promoting pro-apoptotic protein expression while downregulating anti-apoptotic proteins like Bcl-2 and X-linked inhibitor of apoptosis protein (XIAP) [75]. Additionally, Woo et al (2013) emphasized TQ’s role in inducing ROS production, which contributes to cancer cell apoptosis, suggesting a mechanism to counteract resistance [55]. Furthermore, Abdelfadil et al (2013) demonstrated that TQ induces apoptosis in various cancer cell lines via the p38 mitogen-activated protein kinase (MAPK) pathway [76]. Collectively, these findings indicate that TQ enhances the efficacy of existing chemotherapeutics and offers a promising strategy to overcome drug resistance, potentially improving patient outcomes.
The in vivo results of this study indicate that TQ plays a vital role in regulating breast cancer-related genes by reducing Bcl-2 levels, increasing P53 levels, and suppressing genes like Notch1 and VEGF while enhancing interleukin 2 (IL-2) expression. Consistent with in vitro findings, TQ influences gene expression and cell behavior in breast cancer by inducing apoptosis and modulating the p53 signaling pathway and tumor suppressor genes like p21 and BRCA. TQ inhibits p-5473-Akt and increases PTEN expression while decreasing SNAIL-1 and TWIST-1 levels, thereby enhancing the Bax/Bcl-2 ratio and increasing caspase-3 activity. By downregulating Bcl-2, TQ promotes apoptosis and restores normal apoptotic pathways disrupted in cancer [77, 78], while its upregulation of P53 further supports tumor suppression [60, 79]. Additionally, the suppression of Notch1 and VEGF expression highlights TQ’s ability to inhibit tumor growth and angiogenesis [52], and the enhancement of IL-2 suggests a potential boost in immune responses against tumors [43]. Collectively, these findings underscore TQ’s potential as a therapeutic agent in breast cancer treatment by modulating key regulatory pathways involved in tumor survival and progression.
This study found that the combination of TQ and conventional chemotherapy enhances anti-breast cancer effects by increasing helper T cells and myeloid cells. The elevation of CD4+ and CD8+ T cells indicates a strong adaptive immune response vital for effectively targeting and eliminating tumor cells [80, 81]. TQ likely contributes to this immune enhancement by promoting T-cell activation and proliferation, strengthening the body’s defenses against breast cancer [82]. Additionally, the increase in myeloid cells suggests that TQ facilitates the recruitment of these immune cells to the tumor site, amplifying the anti-tumor response [83, 84]. Granulocytic and monocytic cells are crucial for inflammation and immune surveillance, supporting tumor suppression [85]. These findings highlight TQ’s potential as an immunomodulatory agent that targets cancer cells directly and boosts the immune system, presenting a promising strategy for combination therapies that integrate cytotoxic effects and immune activation [86].
The combination therapy of TQ and conventional chemotherapy for breast cancer has shown potential safety benefits and reduced side effects in various studies. This review indicates that TQ mitigates chemotherapy-related toxicities by lowering cardiac injury markers like lactate dehydrogenase (LDH) and creatine kinase-MB (CK-MB), which are the indicators of heart toxicity. This cardioprotective effect is crucial, given the frequent cardiotoxicity associated with specific chemotherapeutic agents, especially anthracyclines [87]. Additionally, TQ may help protect immune cells during treatment, although specific evidence regarding splenocyte counts is limited. TQ also exhibits minimal toxicity to the liver and kidneys, evidenced by normal liver enzyme, blood urea nitrogen (BUN), and creatinine levels, highlighting its potential as a safe adjunct therapy. These findings underscore TQ’s role in enhancing chemotherapy efficacy while improving safety profiles, making it a promising candidate for combination therapies that minimize adverse effects and maximize therapeutic benefits (Table 3) [28, 29, 31, 34-41, 43, 45-47, 54, 88].
 Click to view | Table 3. Summary of Beneficial and Adverse Effects of TQ-Chemotherapy Combination in Clinical Practice |
This systematic review has certain limitations, as it includes only in vivo and in vitro (preclinical) studies, which highlights the need for clinical studies. Additionally, the data encompass a wide range of variables, complicating the ability to perform a thorough meta-analysis for all variables. Furthermore, more than 50% of the in vivo studies exhibit a high risk of bias related to blinding and random assessments. However, despite these limitations, this systematic review has significantly contributed to the provision of preclinical evidence regarding the efficacy of TQ in treating breast cancer.
Over the next 5 years, we anticipate progression to phase I/II clinical trials, particularly targeting triple-negative breast cancer subtypes, where therapeutic options remain inadequate. Advancements in pharmaceutical technology may address TQ’s hydrophobicity and stability limitations through sophisticated liposomal or polymer-based delivery systems. TQ may set a precedent for integrating traditional medicinal compounds into contemporary oncology protocols. However, critical questions regarding optimal dosing regimens, specific chemotherapeutic combinations, and patient stratification criteria must be resolved through rigorously designed clinical trials with comprehensive safety and efficacy endpoints.
Conclusions
The systematic review suggests that based on in vitro and in vivo studies, the TQ exhibits significant anti-cancer properties, particularly in breast cancer treatment. TQ effectively induces apoptosis, enhances autophagy, inhibits tumor growth, and regulates cancer cell signaling pathways as well as multiple phases of the cell cycle. Combined with chemotherapeutic agents, TQ enhances efficacy, reduces required drug dosages, and mitigates side effects such as healthy cell toxicity. Furthermore, advanced delivery systems such as nanoparticles and lipid carriers amplify these effects, ensuring targeted action and improved therapeutic outcomes. Despite its promising role in improving breast cancer treatment outcomes and reducing chemotherapy toxicity, further clinical trials are needed to validate its safety, efficacy, and mechanisms in human applications.
| Supplementary Material | ▴Top |
Suppl 1. Risk-of-bias and applicability concerns graph of in vitro studies.
Suppl 2. Risk-of-bias and applicability concerns summary table of in vivo studies.
Suppl 3. Risk-of-bias and applicability concerns graph of in vivo studies.
Suppl 4. Certainty of evidence from in vivo studies.
Acknowledgments
The authors would like to thank the Doctoral Program, Department of Biomedical Science, Faculty of Medicine, Universitas Sriwijaya, for the support of the research.
Financial Disclosure
This study received no external funding.
Conflict of Interest
All the authors declare that there is no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
NQ was involved in developing the research concept and conducting the systematic review. MBI, FS, RSD, and PMA significantly contributed to the article’s literature search, quality assessment, and language enhancement. L, ZH, and DP reviewed, edited, and approved the final manuscript, while all authors endorsed the draft and affirmed its originality.
Data Availability
The authors declare that data supporting the findings of this study are available within the article and its supplementary information files.
Abbreviations
ACNP: aragonite calcium carbonate (CaCO3) nanoparticle; ADCs: antibody-drug conjugates; ALT: alanine transaminase; AST: aspartate transaminase; Bax: Bcl-2-associated X protein; Bcl-2: B-cell lymphoma 2; B-NE: borage nanoemulsion; Brca 1: breast cancer susceptibility gene 1; BUN: blood urea nitrogen; CDK: cyclin-dependent kinase; Cdkn1a: cyclin-dependent kinase inhibitor 1a; Cis: cisplatin; CK-MB: creatine kinase-MB; CLNCs: chitosan-coated lipid nanocapsule; CTNNB1: cadherin-associated protein beta-1; Cyclo: cyclophosphamide; DOX: doxorubicin; DTX: docetaxel; ECis: early cisplatin; EMT: epithelial-mesenchymal transition; Fasl: Fas ligand; 5-FU: 5-fluorouracil; GCB: gemcitabine; GSH: glutathione; Gstp: glutathione S-transferase p; Hes1: hairy and enhancer-of-split 1; Hic 1: hypermethylated in cancer 1; ICIs: immune checkpoint inhibitors; IFN-γ: interferon gamma; IL-6: interleukin-6; IL-2: interleukin-2; Jag1: jagged 1; LCis: late cisplatin; LDH: lactate dehydrogenase; LLCNs: lyotropic liquid crystalline nanoassemblies; LNCs: lipid nanocapsules; MDA: malondialdehyde; NE: nanoemulsion; NF-κB: nuclear factor-kappa B; NS: normal saline; PBS: phosphate buffer saline; p-5473-Akt: phosphorylated Akt; PI3K/Akt: phosphatidylinositol 3-kinase/protein kinase B; PLGA: poly lactic-co-glycolic acid; PPAR: peroxisome proliferator-activated receptor; PTEN: phosphatase and TENsin homolog; PTX: paclitaxel; RBC: red blood cell; SOD: superoxide dismutase; TNF-α: tumor necrosis factor alpha; TQ: thymoquinone; Trail: tumor necrosis factor-related apoptosis-inducing ligand; ULNCs: drug-loaded uncoated lipid nanocapsule; VEGF: vascular endothelial growth factor; WBC: white blood cell
| References | ▴Top |
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263.
doi pubmed - Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288-300.
doi pubmed - Rizzo A, Schipilliti FM, Di Costanzo F, Acquafredda S, Arpino G, Puglisi F, Del Mastro L, et al. Discontinuation rate and serious adverse events of chemoimmunotherapy as neoadjuvant treatment for triple-negative breast cancer: a systematic review and meta-analysis. ESMO Open. 2023;8(6):102198.
doi pubmed - Caputo R, Buono G, Piezzo M, Martinelli C, Cianniello D, Rizzo A, Pantano F, et al. Sacituzumab Govitecan for the treatment of advanced triple negative breast cancer patients: a multi-center real-world analysis. Front Oncol. 2024;14:1362641.
doi pubmed - Rizzo A, Cusmai A, Acquafredda S, Giovannelli F, Rinaldi L, Misino A, Palmiotti G. KEYNOTE-522, IMpassion031 and GeparNUEVO: changing the paradigm of neoadjuvant immune checkpoint inhibitors in early triple-negative breast cancer. Future Oncol. 2022;18(18):2301-2309.
doi pubmed - Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, et al. Breast cancer. Nat Rev Dis Primers. 2019;5(1):66.
doi pubmed - Li S, So TH, Tang G, Tan HY, Wang N, Ng BFL, Chan CKW, et al. Chinese herbal medicine for reducing chemotherapy-associated side-effects in breast cancer patients: a systematic review and meta-analysis. Front Oncol. 2020;10:599073.
doi pubmed - Mollazadeh H, Afshari AR, Hosseinzadeh H. Review on the potential therapeutic roles of nigella sativa in the treatment of patients with cancer: involvement of apoptosis: - black cumin and cancer. J Pharmacopuncture. 2017;20(3):158-172.
doi pubmed - Khan MA, Chen HC, Tania M, Zhang DZ. Anticancer activities of Nigella sativa (black cumin). Afr J Tradit Complement Altern Med. 2011;8(5 Suppl):226-232.
doi pubmed - Almajali B, Al-Jamal HAN, Taib WRW, Ismail I, Johan MF, Doolaanea AA, Ibrahim WN. Thymoquinone, as a novel therapeutic candidate of cancers. Pharmaceuticals (Basel). 2021;14(4):369.
doi pubmed - Sahin TK, Ayasun R, Rizzo A, Guven DC. Prognostic value of neutrophil-to-eosinophil ratio (NER) in cancer: a systematic review and meta-analysis. Cancers (Basel). 2024;16(21):3689.
doi pubmed - Vitale E, Rizzo A, Santa K, Jirillo E. Associations between "Cancer Risk", "Inflammation" and "Metabolic Syndrome": A Scoping Review. Biology (Basel). 2024;13(5):352.
doi pubmed - Majdalawieh AF, Fayyad MW. Recent advances on the anti-cancer properties of Nigella sativa, a widely used food additive. J Ayurveda Integr Med. 2016;7(3):173-180.
doi pubmed - Hannan MA, Rahman MA, Sohag AAM, Uddin MJ, Dash R, Sikder MH, Rahman MS, et al. Black cumin (Nigella sativa L.): a comprehensive review on phytochemistry, health benefits, molecular pharmacology, and safety. Nutrients. 2021;13(6):1784.
doi pubmed - Gomathinayagam R, Ha JH, Jayaraman M, Song YS, Isidoro C, Dhanasekaran DN. Chemopreventive and anticancer effects of thymoquinone: cellular and molecular targets. J Cancer Prev. 2020;25(3):136-151.
doi pubmed - Majdalawieh AF, Fayyad MW, Nasrallah GK. Anti-cancer properties and mechanisms of action of thymoquinone, the major active ingredient of Nigella sativa. Crit Rev Food Sci Nutr. 2017;57(18):3911-3928.
doi pubmed - Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, Damanhouri ZA, et al. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac J Trop Biomed. 2013;3(5):337-352.
doi pubmed - Baig WA, Alwosaibai K, Al-Jubran KM, Chaudhry TM, Al-Dowish N, Alsaffar F, Alam MA. Synergistic anti-cancer effects of Nigella sativa seed oil and conventional cytotoxic agent against human breast cancer. Drug Metab Pers Ther. 2022;37(3):315-321.
doi pubmed - Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160.
doi pubmed - Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43.
doi pubmed - Cicera NFL, Luciene F de L, Denise BC, Sara T de SM, Jessica P de S, Enaide SS, et al. Systematic review: Medicinal use and scientific elucidation of the Piper genus for the treatment of symptoms and inflammatory diseases. J Med Plants Res. 2020;14(2):62-72.
- Duchman KR, Lemmex DB, Patel SH, Ledbetter L, Garrigues GE, Riboh JC. The Effect of Non-Steroidal Anti-Inflammatory Drugs on Tendon-to-Bone Healing: A Systematic Review with Subgroup Meta-Analysis. Iowa Orthop J. 2019;39(1):107-119.
pubmed - Almeida N, Silva FRP, Carneiro ALB, Lima ES, Barcellos JFM, Furtado SC. Libidibia ferrea (juca) anti-inflammatory action: A systematic review of in vivo and in vitro studies. PLoS One. 2021;16(11):e0259545.
doi pubmed - SciRAP.org. Instructions for evaluating reliability and relevance of in vivo and in vitro toxicity studies using the SciRAP tool. 2018;1-5.
- Hooijmans CR, de Vries RBM, Ritskes-Hoitinga M, Rovers MM, Leeflang MM, IntHout J, Wever KE, et al. Facilitating healthcare decisions by assessing the certainty in the evidence from preclinical animal studies. PLoS One. 2018;13(1):e0187271.
doi pubmed - GRADEpro [Internet]. [cited 2024 Dec 10]. Available from: https://www.gradepro.org/.
- Soni P, Kaur J, Tikoo K. Dual drug-loaded paclitaxel-thymoquinone nanoparticles for effective breast cancer therapy. J Nanoparticle Res. 2015;17(18):1-12.
- Sakalar C, Izgi K, Iskender B, Sezen S, Aksu H, Cakir M, Kurt B, et al. The combination of thymoquinone and paclitaxel shows anti-tumor activity through the interplay with apoptosis network in triple-negative breast cancer. Tumour Biol. 2016;37(4):4467-4477.
doi pubmed - Zafar S, Akhter S, Ahmad I, Hafeez Z, Alam Rizvi MM, Jain GK, Ahmad FJ. Improved chemotherapeutic efficacy against resistant human breast cancer cells with co-delivery of Docetaxel and Thymoquinone by Chitosan grafted lipid nanocapsules: Formulation optimization, in vitro and in vivo studies. Colloids Surf B Biointerfaces. 2020;186:110603.
doi pubmed - Zafar S, Akhter S, Garg N, Selvapandiyan A, Kumar Jain G, Ahmad FJ. Co-encapsulation of docetaxel and thymoquinone in mPEG-DSPE-vitamin E TPGS-lipid nanocapsules for breast cancer therapy: Formulation optimization and implications on cellular and in vivo toxicity. Eur J Pharm Biopharm. 2020;148:10-26.
doi pubmed - El-Far AH, Godugu K, Noreldin AE, Saddiq AA, Almaghrabi OA, Al Jaouni SK, Mousa SA. Thymoquinone and costunolide induce apoptosis of both proliferative and doxorubicin-induced-senescent colon and breast cancer cells. Integr Cancer Ther. 2021;20:15347354211035450.
doi pubmed - Zheng M, Mei Z, Junaid M, Tania M, Fu J, Chen HC, Khan MA. Synergistic role of thymoquinone on anticancer activity of 5-fluorouracil in triple negative breast cancer cells. Anticancer Agents Med Chem. 2022;22(6):1111-1118.
doi pubmed - Anandan I, Gokul T, Elango A, Vivekkumar P. A study on concurring effect of paclitaxel and thymoquinone on MCF-7 cell line-In vitro. Natl J Physiol Pharm Pharmacol. 2023;14(04):776-781.
- Bawadud RS, Alkhatib MH. Growth and invasion inhibition of T47D ductal carcinoma cells by the association of docetaxel with a bioactive agent in neutral nanosuspension. Bioimpacts. 2023;13(2):145-157.
doi pubmed - Loo YS, Zahid NI, Madheswaran T, Ikeno S, Nurdin A, Mat Azmi ID. Coencapsulation of gemcitabine and thymoquinone in citrem-phosphatidylcholine hexosome nanocarriers improves in vitro cellular uptake in breast cancer cells. Mol Pharm. 2023;20(9):4611-4628.
doi pubmed - Mousavinasab F, Hashemipour N, Soghala S, Talebi M. Assessment of the synergistic effect of thymoquinone and cisplatin on MCF-7 breast cancer cells. Med Biomed J. 2024;1(1):6-18.
- El-Ashmawy NE, Khedr EG, Ebeid EM, Salem ML, Zidan AA, Mosalam EM. Enhanced anticancer effect and reduced toxicity of doxorubicin in combination with thymoquinone released from poly-N-acetyl glucosamine nanomatrix in mice bearing solid Ehrlish carcinoma. Eur J Pharm Sci. 2017;109:525-532.
doi pubmed - Gomaa S, Aboshafey A, Aladawy A. Combination treatment of thymoquinone-loaded gold nanoparticles and cisplatin potentiates anti-tumor activity and immunomodulatory effects in breast cancer model. Egypt J Exp Biol. 2018;14(1):21-31.
- Bashmail HA, Alamoudi AA, Noorwali A, Hegazy GA, G AJ, Choudhry H, Al-Abd AM. Thymoquinone synergizes gemcitabine anti-breast cancer activity via modulating its apoptotic and autophagic activities. Sci Rep. 2018;8(1):11674.
doi pubmed - Mosalam EM, Zidan AA, Mehanna ET, Mesbah NM, Abo-Elmatty DM. Thymoquinone and pentoxifylline enhance the chemotherapeutic effect of cisplatin by targeting Notch signaling pathway in mice. Life Sci. 2020;244:117299.
doi pubmed - Zidan AA, El-Ashmawy NE, Khedr EG, Ebeid EM, Salem ML, Mosalam EM. Loading of doxorubicin and thymoquinone with F2 gel nanofibers improves the antitumor activity and ameliorates doxorubicin-associated nephrotoxicity. Life Sci. 2018;207:461-470.
doi pubmed - Ibiyeye KM, Nordin N, Ajat M, Zuki ABZ. Ultrastructural changes and antitumor effects of doxorubicin/thymoquinone-loaded CaCO(3) nanoparticles on breast cancer cell line. Front Oncol. 2019;9:599.
doi pubmed - Khan A, Aldebasi YH, Alsuhaibani SA, Khan MA. Thymoquinone augments cyclophosphamide-mediated inhibition of cell proliferation in breast cancer cells. Asian Pac J Cancer Prev. 2019;20(4):1153-1160.
doi pubmed - Odeh F, Naffa R, Azzam H, Mahmoud IS, Alshaer W, Al Bawab A, Ismail S. Co-encapsulation of thymoquinone with docetaxel enhances the encapsulation efficiency into PEGylated liposomes and the chemosensitivity of MCF7 breast cancer cells to docetaxel. Heliyon. 2019;5(11):e02919.
doi pubmed - Alkhatib MH, Bawadud RS, Gashlan HM. Incorporation of docetaxel and thymoquinone in borage nanoemulsion potentiates their antineoplastic activity in breast cancer cells. Sci Rep. 2020;10(1):18124.
doi pubmed - Bashmail HA, Alamoudi AA, Noorwali A, Hegazy GA, Ajabnoor GM, Al-Abd AM. Thymoquinone enhances paclitaxel anti-breast cancer activity via inhibiting tumor-associated stem cells despite apparent mathematical antagonism. Molecules. 2020;25(2):426.
doi pubmed - Bawadud R, Alkhatib M, HM G. Combination of docetaxel with thymoquinone in nanoemulsion impedes the migration of breast cancer stem cells. Int J Pharm Investig. 2020;10(2):211-216
- Kwan K, Han AY, Mukdad L, Barragan F, Selim O, Alhiyari Y, St John M. Anticancer effects of thymoquinone in head and neck squamous cell carcinoma: A scoping review. Laryngoscope Investig Otolaryngol. 2023;8(4):876-885.
doi pubmed - Jung T, Cheon C. Synergistic and additive effects of herbal medicines in combination with chemotherapeutics: a scoping review. Integr Cancer Ther. 2024;23:15347354241259416.
doi pubmed - Alaufi OM, Noorwali A, Zahran F, Al-Abd AM, Al-Attas S. Cytotoxicity of thymoquinone alone or in combination with cisplatin (CDDP) against oral squamous cell carcinoma in vitro. Sci Rep. 2017;7(1):13131.
doi pubmed - Mahmood NMA, Hamaamin KS. Thymoquinone’s potential role in cancer chemotherapy: a mini review. Al-Rafidain J Med Sci. 2022;3:64-70.
- Nur Celebioglu H, Becit M, Caglayan A, Aydın Dilsiz S. Effects of thymoquinone and etoposide combination on cell viability and genotoxicity in human cervical cancer hela cells. Istanbul J Pharm. 2022;52(3):258-264.
- Motaghed M. Cytotoxic, cytostatic and anti-estrogenic effect of thymoquinone on estrogen receptor-positive breast cancer MCF7 cell line. Am J Life Sci. 2015;3(2):7.
- Woo CC, Hsu A, Kumar AP, Sethi G, Tan KH. Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: the role of p38 MAPK and ROS. PLoS One. 2013;8(10):e75356.
doi pubmed - Goyal SN, Prajapati CP, Gore PR, Patil CR, Mahajan UB, Sharma C, Talla SP, et al. Therapeutic Potential and Pharmaceutical Development of Thymoquinone: A Multitargeted Molecule of Natural Origin. Front Pharmacol. 2017;8:656.
doi pubmed - Velho-Pereira R, Kumar A, Pandey BN, Jagtap AG, Mishra KP. Radiosensitization in human breast carcinoma cells by thymoquinone: role of cell cycle and apoptosis. Cell Biol Int. 2011;35(10):1025-1029.
doi pubmed - Yu SM, Kim SJ. The thymoquinone-induced production of reactive oxygen species promotes dedifferentiation through the ERK pathway and inflammation through the p38 and PI3K pathways in rabbit articular chondrocytes. Int J Mol Med. 2015;35(2):325-332.
doi pubmed - El-Najjar N, Chatila M, Moukadem H, Vuorela H, Ocker M, Gandesiri M, Schneider-Stock R, et al. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis. 2010;15(2):183-195.
doi pubmed - Yildirım IH, Azzawri AA, Duran T. Thymoquinone induces apoptosis via targeting the Bax/BAD and Bcl-2 pathway in breast cancer cells. Dicle Tip Derg. 2019;46:411-417.
- Park EJ, Chauhan AK, Min KJ, Park DC, Kwon TK. Thymoquinone induces apoptosis through downregulation of c-FLIP and Bcl-2 in renal carcinoma Caki cells. Oncol Rep. 2016;36(4):2261-2267.
doi pubmed - He P, He Y, Ma J, Liu Y, Liu C, Baoping Y, Dong W. Thymoquinone induces apoptosis and protective autophagy in gastric cancer cells by inhibiting the PI3K/Akt/mTOR pathway. Phytother Res. 2023;37(8):3467-3480.
doi pubmed - Imani S, Wei C, Cheng J, Khan MA, Fu S, Yang L, Tania M, et al. MicroRNA-34a targets epithelial to mesenchymal transition-inducing transcription factors (EMT-TFs) and inhibits breast cancer cell migration and invasion. Oncotarget. 2017;8(13):21362-21379.
doi pubmed - Khan MA, Tania M, Wei C, Mei Z, Fu S, Cheng J, Xu J, et al. Thymoquinone inhibits cancer metastasis by downregulating TWIST1 expression to reduce epithelial to mesenchymal transition. Oncotarget. 2015;6(23):19580-19591.
doi pubmed - Kou B, Liu W, Zhao W, Duan P, Yang Y, Yi Q, Guo F, et al. Thymoquinone inhibits epithelial-mesenchymal transition in prostate cancer cells by negatively regulating the TGF-beta/Smad2/3 signaling pathway. Oncol Rep. 2017;38(6):3592-3598.
doi pubmed - Liou YF, Hsieh YS, Hung TW, Chen PN, Chang YZ, Kao SH, Lin SW, et al. Thymoquinone inhibits metastasis of renal cell carcinoma cell 786-O-SI3 associating with downregulation of MMP-2 and u-PA and suppression of PI3K/Src signaling. Int J Med Sci. 2019;16(5):686-695.
doi pubmed - Asaduzzaman Khan M, Tania M, Fu S, Fu J. Thymoquinone, as an anticancer molecule: from basic research to clinical investigation. Oncotarget. 2017;8(31):51907-51919.
doi pubmed - Homayoonfal M, Asemi Z, Yousefi B. Potential anticancer properties and mechanisms of thymoquinone in osteosarcoma and bone metastasis. Cell Mol Biol Lett. 2022;27(1):21.
doi pubmed - Sheikhnia F, Rashidi V, Maghsoudi H, Majidinia M. Potential anticancer properties and mechanisms of thymoquinone in colorectal cancer. Cancer Cell Int. 2023;23(1):320.
doi pubmed - Grison A, Atanasoski S. Cyclins, cyclin-dependent kinases, and cyclin-dependent kinase inhibitors in the mouse nervous system. Mol Neurobiol. 2020;57(7):3206-3218.
doi pubmed - Stallaert W, Taylor SR, Kedziora KM, Taylor CD, Sobon HK, Young CL, Limas JC, et al. The molecular architecture of cell cycle arrest. Mol Syst Biol. 2022;18(9):e11087.
doi pubmed - Mostofa AGM, Hossain MK, Basak D, Bin Sayeed MS. Thymoquinone as a potential adjuvant therapy for cancer treatment: evidence from preclinical studies. Front Pharmacol. 2017;8:295.
doi pubmed - Opyrchal M, Salisbury JL, Zhang S, McCubrey J, Hawse J, Goetz MP, et al. Aurora-A mitotic kinase induces endocrine resistance through down-regulation of ERα expression in initially ERα+ breast cancer cells. PLoS One. 2014;9(5):e96995.
- Seo AN, Lee HJ, Kim EJ, Kim HJ, Jang MH, Lee HE, Kim YJ, et al. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer. 2013;109(10):2705-2713.
doi pubmed - Khan M, Lam SK, Yan S, Feng Y, Chen C, Ko FC, Ho JC. The anti-neoplastic impact of thymoquinone from Nigella sativa on small cell lung cancer: in vitro and in vivo investigations. J Cancer Res Ther. 2024;20(4):1224-1231.
doi pubmed - Abdelfadil E, Cheng YH, Bau DT, Ting WJ, Chen LM, Hsu HH, Lin YM, et al. Thymoquinone induces apoptosis in oral cancer cells through p38beta inhibition. Am J Chin Med. 2013;41(3):683-696.
doi pubmed - Dastjerdi MN, Mehdiabady EM, Iranpour FG, Bahramian H. Effect of thymoquinone on P53 gene expression and consequence apoptosis in breast cancer cell line. Int J Prev Med. 2016;7:66.
doi pubmed - Li Q, Ren P, Shi P, Chen Y, Xiang F, Zhang L, Wang J, et al. MicroRNA-148a promotes apoptosis and suppresses growth of breast cancer cells by targeting B-cell lymphoma 2. Anticancer Drugs. 2017;28(6):588-595.
doi pubmed - Hernandez Borrero LJ, El-Deiry WS. Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. Biochim Biophys Acta Rev Cancer. 2021;1876(1):188556.
doi pubmed - Boieri M, Malishkevich A, Guennoun R, Marchese E, Kroon S, Trerice KE, Awad M, et al. CD4+ T helper 2 cells suppress breast cancer by inducing terminal differentiation. J Exp Med. 2022;219(7):e20201963.
doi pubmed - Chen X, Ghanizada M, Mallajosyula V, Sola E, Capasso R, Kathuria KR, Davis MM. Differential roles of human CD4(+) and CD8(+) regulatory T cells in controlling self-reactive immune responses. Nat Immunol. 2025;26(2):230-239.
doi pubmed - Wu TC, Xu K, Banchereau R, Marches F, Yu CI, Martinek J, Anguiano E, et al. Reprogramming tumor-infiltrating dendritic cells for CD103+ CD8+ mucosal T-cell differentiation and breast cancer rejection. Cancer Immunol Res. 2014;2(5):487-500.
doi pubmed - Su X, Xu Y, Fox GC, Xiang J, Kwakwa KA, Davis JL, Belle JI, et al. Breast cancer-derived GM-CSF regulates arginase 1 in myeloid cells to promote an immunosuppressive microenvironment. J Clin Invest. 2021;131(20):e145296.
doi pubmed - Neophytou CM, Pierides C, Christodoulou MI, Costeas P, Kyriakou TC, Papageorgis P. The role of tumor-associated myeloid cells in modulating cancer therapy. Front Oncol. 2020;10:899.
doi pubmed - Boieri M, Marchese E, Pham QM, Azin M, Steidl LE, Malishkevich A, Demehri S. Thymic stromal lymphopoietin-stimulated CD4(+) T cells induce senescence in advanced breast cancer. Front Cell Dev Biol. 2022;10:1002692.
doi pubmed - Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2(4):361-370.
doi pubmed - Lu X, Zhao Y, Chen C, Han C, Xue L, Xing D, Huang O, et al. BNP as a marker for early prediction of anthracycline-induced cardiotoxicity in patients with breast cancer. Oncol Lett. 2019;18(5):4992-5001.
doi pubmed - Friyoga Syahril, Wirdah A, Nur Qodir, Irfanuddin, Irsan Saleh, Yenny Dian Andayani, et al. Effectiveness of nigella sativa addition against TNF-alpha in stage iii and iv breast cancer undergoing doxorubicin and cyclophosphamide chemotherapy at Dr. Mohammad Hoesin General Hospital, Palembang, Indonesia. Biosci Med J Biomed Transl Res. 2023;8(2):4009-4014.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.