| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 000, Number 000, May 2025, pages 000-000
Comparative Efficacy of Tirzepatide vs. Semaglutide in Reducing Body Weight in Humans: A Systematic Review and Meta-Analysis of Clinical Trials and Real-World Data
Ahmad Bin Aamira , Rabia Latifb, d
, Jood Faisal Alqoofic, Fatimah Abdulkarim Almarzoqc, Joory Osamah Fallatahc, Ghala Abdullah Hassanc, Fatimah Abbas Abdullah Al Abu Saabc
aPunjab Medical College, Faisalabad Medical University, Faisalabad, Pakistan
bDepartment of Physiology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
cCollege of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
dCorresponding Author: Rabia Latif, Department of Physiology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam 31441, Saudi Arabia
Manuscript submitted March 7, 2025, accepted April 21, 2025, published online May 13, 2025
Short title: Tirzepatide and Semaglutide
doi: https://doi.org/10.14740/jocmr6231
| Abstract | ▴Top |
Background: The aim of the study was to compare the effectiveness of tirzepatide versus semaglutide in producing weight loss.
Methods: A systematic search was conducted in databases PubMed, Scopus, and Web of Science on January 22, 2025, using search terms (“tirzepatide,” “semaglutide,” and “weight loss”) and their alternatives, which yielded 751 studies in total. After deduplication, title/abstract and full text screening was conducted, and studies were assessed based on the eligibility criteria. After extracting the data, a meta-analysis (MA) was performed through RStudio. Heterogeneity among studies was evaluated with Cochran’s Q and I2 tests. A random-effect model was used to calculate pooled “mean differences” (MDs). Study quality was estimated by Newcastle-Ottawa Quality Assessment Scale (NOS) and Cochrane risk of bias (RoB) version 2 tool, and publication bias was estimated through forest plots and the Egger’s test.
Results: A total of two randomized controlled trials (RCTs) and five retrospective cohorts were included in this MA. MA results showed that compared with the semaglutide, tirzepatide could produce significantly greater weight loss (MD = 4.23; 95% confidence interval (CI): 3.22 - 5.25; P < 0.01). Subgroup analysis showed a dose- and duration-dependent significantly superior therapeutic effect of tirzepatide (> 10 mg dose: MD = 6.50, 95% CI: 5.93 - 7.08, P < 0.01 vs. ≤ 10 mg: MD = 3.89, 95% CI: 2.12 - 5.65, P < 0.01) (> 6 months duration: MD = 5.00, 95% CI: 3.48 - 6.52, P < 0.01 vs. ≤ 6 months: MD = 3.50, 95% CI: 2.24 - 4.75, P < 0.01). The supremacy of tirzepatide was maintained in both types of studies: RCTs and retrospective cohorts. No publication bias was found through forest plots visually or Egger’s test (Egger’s regression asymmetry test P value 0.94). Study quality estimated by NOS revealed the quality of each study as “good” (≥ 7 points) and that estimated by the Cochrane RoB tool revealed “low” RoB.
Conclusion: The pooled analysis provides evidence that tirzepatide is better than semaglutide in reducing body weight, regardless of study design. A dose-response relationship exists, and the weight loss magnitude increases with the dose or duration of tirzepatide. The studies that provide this evidence are of high quality and have a low RoB.
Keywords: Semaglutide (Ozempic, Rybelsus, Wegovy); Tirzepatide (LY3298176, Zepbound, Mounjaro); Glucagon-like peptides; Body weight; Weight loss; Anti-obesity agents
| Introduction | ▴Top |
Tirzepatide was initially authorized by the US Food and Drug Administration (FDA) in May 2022 for use in type 2 diabetes mellitus (T2DM; trade name: Mounjaro), later for obesity or overweight (trade name: Zepbound) in adults in November 2023 [1]. Tirzepatide is a twin glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide-1 receptor agonist (GLP-1 RA) class [2]. Both GIP and GLP-1 are natural incretins and enhance insulin release and insulin sensitivity while reducing stomach clearing and digestive tract movements [3]. Hence, tirzepatide can reduce body weight alongside decreasing blood sugar. Tirzepatide has relatively more affinity for the GIP receptor (GIPR) than the GLP-1 receptor (GLP-1R), reflecting a biased action. Tirzepatide’s sensitivity for the GIPR is equivalent to endogenous GIP, but its sensitivity for the GLP-1R is five times less compared to endogenous GLP-1 [2]. Tirzepatide seems to be a promising and versatile addition in the FDA-approved drugs’ list of obesity management [4]. Semaglutide is a GLP-1 RA and was approved by FDA in June 2021 as an anti-obesity drug [5]. Several trials have reported significant weight loss caused by tirzepatide and semaglutide [2, 6-9].
The current meta-analysis (MA) was planned to compare the effectiveness of tirzepatide versus semaglutide in producing weight loss. The number of clinical trials comparing the two drugs in direct head-to-head comparisons is scarce. Only two randomized controlled trials (RCTs) are available until now [6, 10]. To address their comparative efficacy, we decided to pool results from head-to-head comparisons of clinical trials and real-world studies (retrospective cohorts). Combining observational studies (retrospective cohort) and clinical trials in a single MA is permissible provided that the study design must be considered as a potential source of heterogeneity and subgroup analysis based on the study design must be conducted [11]. Including real-world studies/retrospective cohorts in an MA of RCTs could provide in-depth knowledge. The researchers can assess the consistency between the pooled results of the two study types (retrospective cohorts and RCTs) by looking at the direction and significance of summary effects and their confidence intervals (CIs).
| Materials and Methods | ▴Top |
The study protocol was registered in Open Science Foundation (OSF) registries on January 21, 2025 [12]. Methods and results were reported according to PRISMA guidelines.
Search strategy
Three databases, PubMed, Scopus, and Web of Science were searched with the key concepts and their alternatives (concept 1: tirzepatide; concept 2: semaglutide; concept 3: body weight) (Table 1).
 Click to view | Table 1. Search Strategy and Results |
After removing duplicate results, studies were screened at the title/abstract level first followed by “full text” screening against the following eligibility criteria keeping in view the “PICOS” framework.
Inclusion criteria
RCTs and observational studies (retrospective cohorts) published in English that assessed subcutaneous tirzepatide versus subcutaneous semaglutide at any dose against each other for a minimum duration of 12 weeks fulfilling the following PICOS framework were included in the current systematic review: P (population): any population (regardless of their age group and health status); I (intervention): tirzepatide; C (comparator): semaglutide; O (outcome): % change in body weight from the baseline; S (study design): RCTs and observational studies (retrospective cohorts).
Exclusion criteria
Studies comparing any of these drugs with placebo or any other type of drug, intervention duration less than 12 weeks or animal studies or studies not reporting the “change in body weight from baseline”, or “body weight before and after the intervention” were excluded.
The formula to estimate the weight loss % from the pre- and post-intervention values was: (Pre-intervention weight - Post-intervention weight)/(Pre-intervention weight) × 100.
The quality of retrospective cohort studies was assessed by the “Newcastle-Ottawa Quality Assessment Scale” (NOS) [13]. The scale consists of three domains related to the study methodology (selection of the cohorts, comparability of the cohorts, and assessment of outcome). The maximum possible score achieved by any individual study is 9 points (maximum possible scores of 4, 2, and 3 points in the selection, comparability, and outcome domains, respectively). Following scoring system was used: ≥ 7 points were categorized as “good”, 2 to 6 points as “fair”, and ≤ 1 point as “poor” quality. The quality assessment of RCTs was performed using the Cochrane risk of bias (RoB) version 2 tool [14].
Publication bias was estimated visually by funnel plots and statistically by Egger’s test.
Data synthesis
Data were analyzed statistically through RStudio using meta and metafor packages. Heterogeneity among studies was evaluated with Cochran’s Q and I2 tests. A random-effect model was used for the calculation of the pooled summary statistic. “Mean differences” (MDs) were used as a summary statistic. Egger’s regression asymmetry test and funnel plots were used to detect publication bias. A sensitivity analysis was done to validate the study results.
| Results | ▴Top |
A total of 751 studies were recovered from all three databases. After the deduplication of 83 studies, 668 studies were included in the initial screening. A total of 514 studies were excluded in the initial screening, and 147 studies were excluded in full text screening of the articles. Ultimately, seven articles were included: two RCTs and five retrospective cohort studies [15-21]. The complete search strategy is shown in Figure 1.
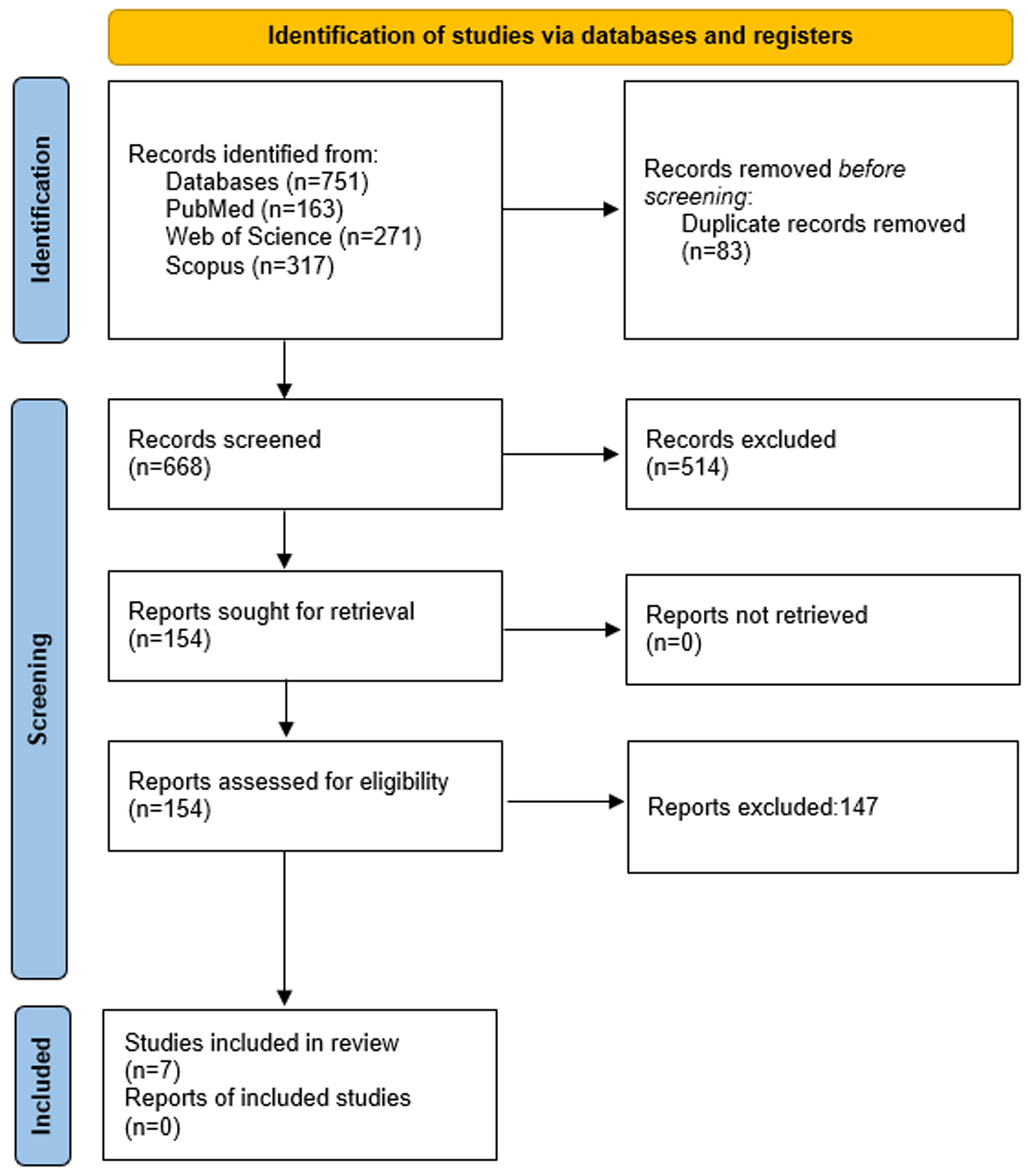 Click for large image | Figure 1. PRISMA flow diagram. |
Study characteristics of the included studies are summarized in Table 2 [15-21].
 Click to view | Table 2. Characteristics of the Included Studies |
Pooled effects
Tirzepatide intervention produced on average a significant reduction in weight loss (MD = 4.23, 95% CI: 3.22 - 5.25, P < 0.05) versus semaglutide intervention (Fig. 2). These results were based on 36,754 and 106,057 participants in tirzepatide and semaglutide arms, respectively. Tirzepatide proved more advantageous and has a better clinical result. However, substantial heterogeneity existed (I2 = 100%; P = 0).
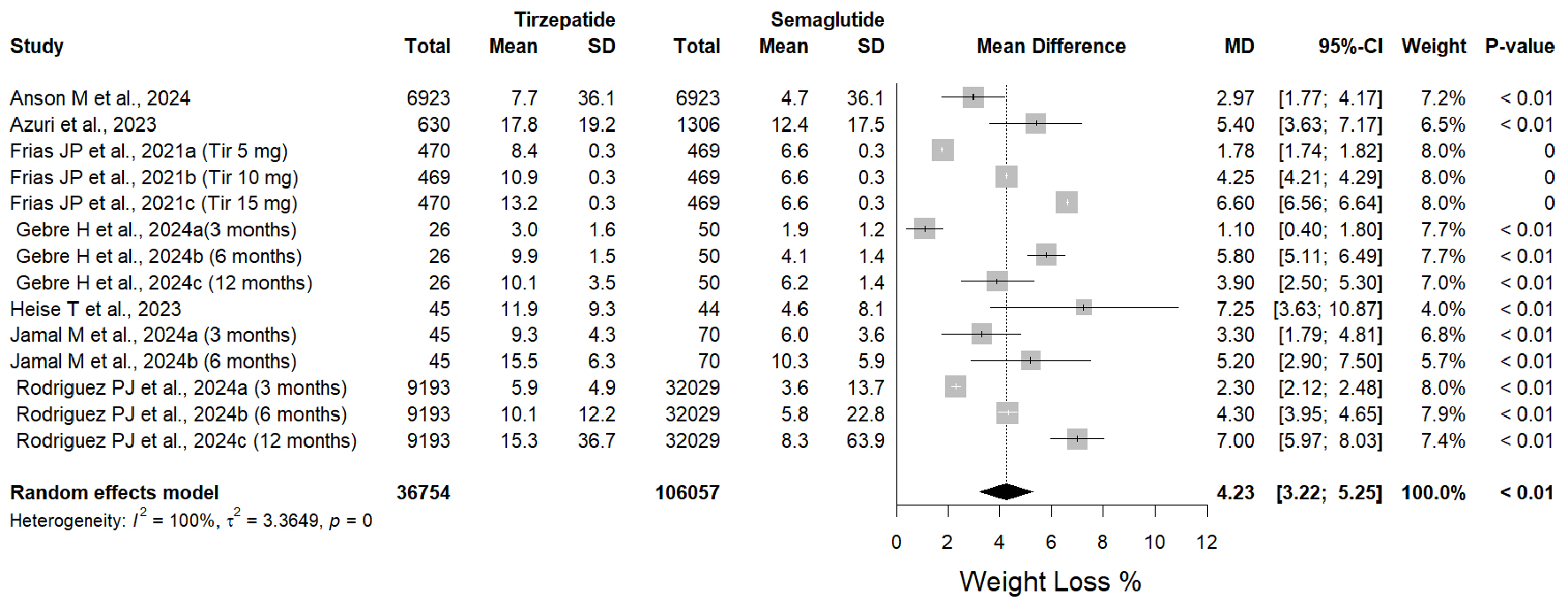 Click for large image | Figure 2. Forest plot of weight loss % mean differences in tirzepatide versus semaglutide interventions. |
Regarding different doses of the tirzepatide, the subgroup analysis revealed a dose-response relationship (Fig. 3). A significant increase in weight loss % of tirzepatide dosage ≤ 10 mg (MD = 3.89, 95% CI: 2.12 - 5.65, P < 0.01; I2 = 0%; P = 0.39), and even a better increase in weight loss % in tirzepatide > 10 mg (MD = 6.50, 95% CI: 5.93 - 7.08, P < 0.01; I2 = 100%; P = 0) vs. semaglutide were observed. Likewise, a dose-response relationship was observed in another subgroup analysis with varying duration of tirzepatide (Fig. 4). A significant increase in weight loss % of tirzepatide dosage ≤ 6 months (MD = 3.50, 95% CI: 2.24 - 4.75, P < 0.01; I2 = 97%; P = 0.39), and an even better increase in weight loss % with tirzepatide > 10 mg (MD = 5.00, 95% CI: 3.48 - 6.52, P < 0.01; I2 = 100%; P = 0) vs. semaglutide were observed. Subgroup analysis based on study design proved superiority of tirzepatide over semaglutide in both study designs by producing significant greater weight loss: RCTs (MD = 4.73, 95% CI: 2.31 - 7.15, P < 0.01; I2 = 100%); retrospective cohort studies (MD = 4.07, 95% CI: 2.92 - 5.22, P < 0.01; I2 = 97%) (Fig. 5).
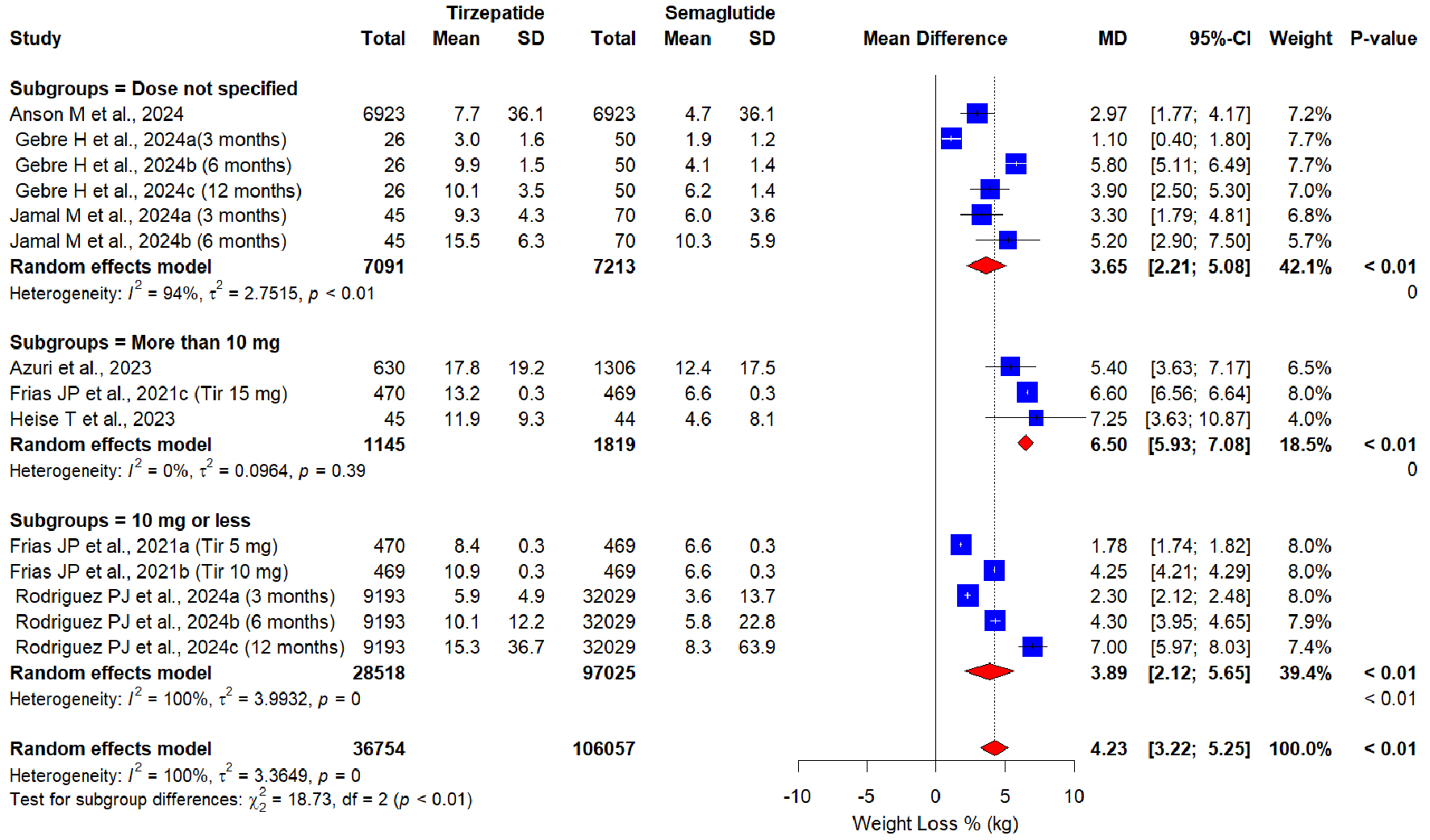 Click for large image | Figure 3. Forest plot of subgroup analysis based on tirzepatide dose. |
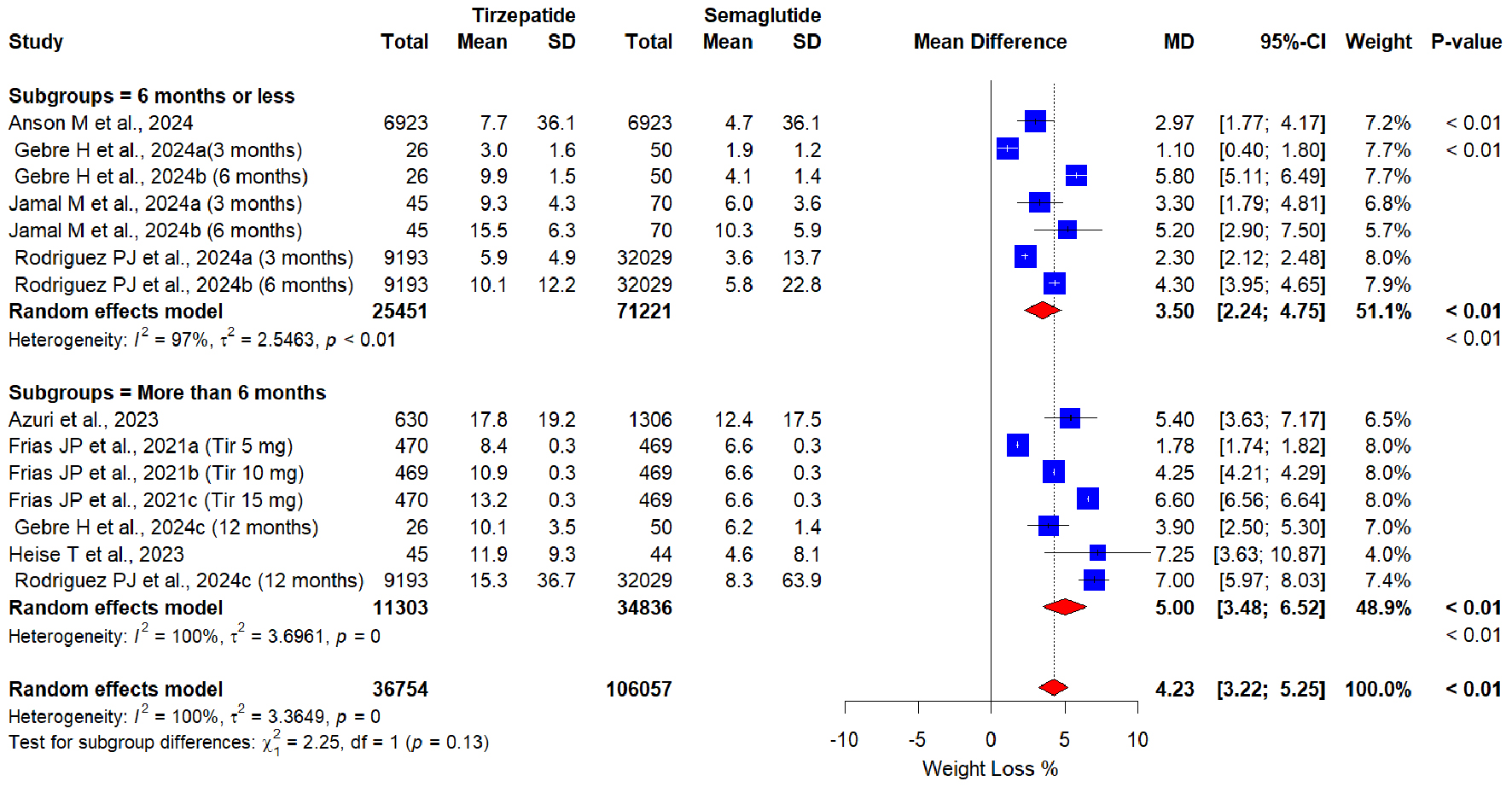 Click for large image | Figure 4. Forest plot of subgroup analysis based on tirzepatide duration. |
 Click for large image | Figure 5. Forest plot of subgroup analysis based on study design. |
Sensitivity analysis
The sequential removal of any study did not depict any change in the results or heterogeneity (Fig. 6). Tirzepatide retained its superiority by producing a higher weight loss % compared to semaglutide (minimum weight loss % MD of 4.00 by omitting Rodriguiez et al, 2024c [21] (12 months duration); maximum weight loss % MD of 4.48 by omitting Gebre et al, 2024a [19] (3 months duration)).
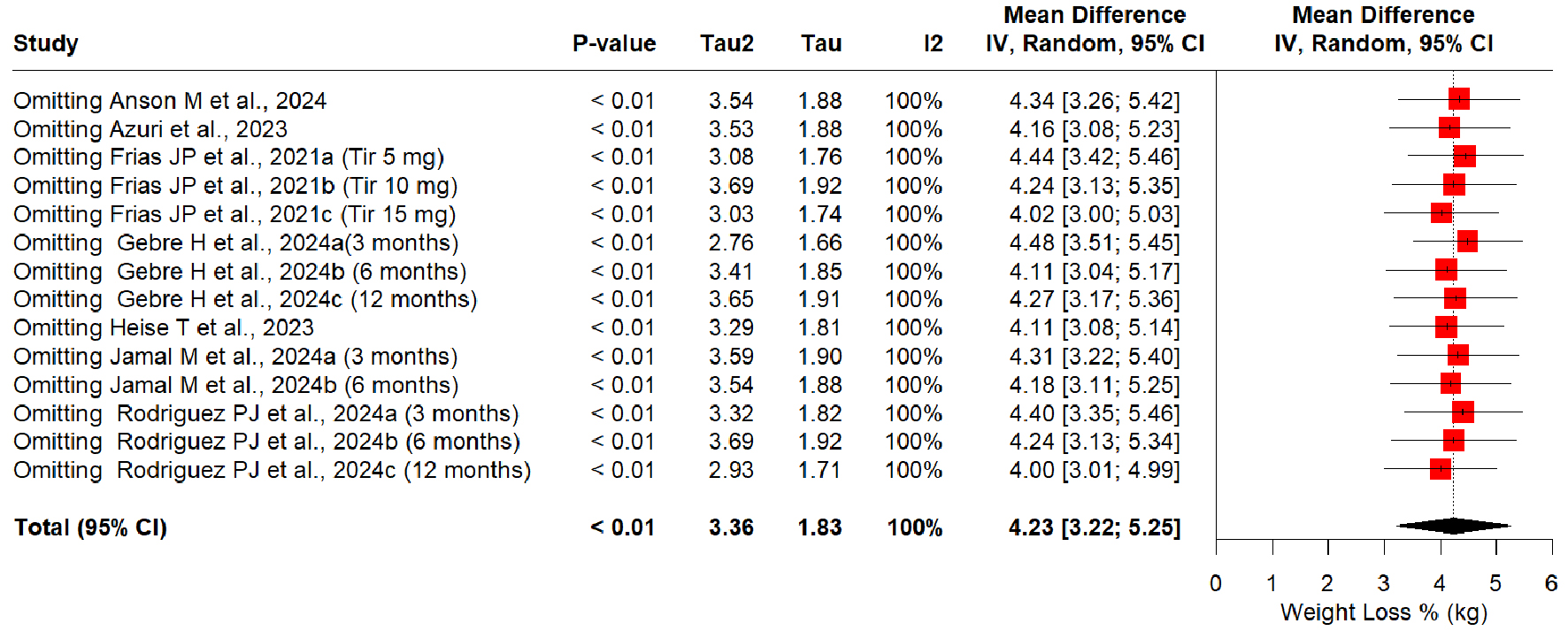 Click for large image | Figure 6. Sensitivity analysis of weight loss % mean differences in tirzepatide versus semaglutide interventions. |
Publication bias
A funnel plot is shown in Figure 7. No publication bias was visible visually as well as through Egger’s test significance levels (Egger’s regression asymmetry test P value 0.94; t = -0.08, df = 12).
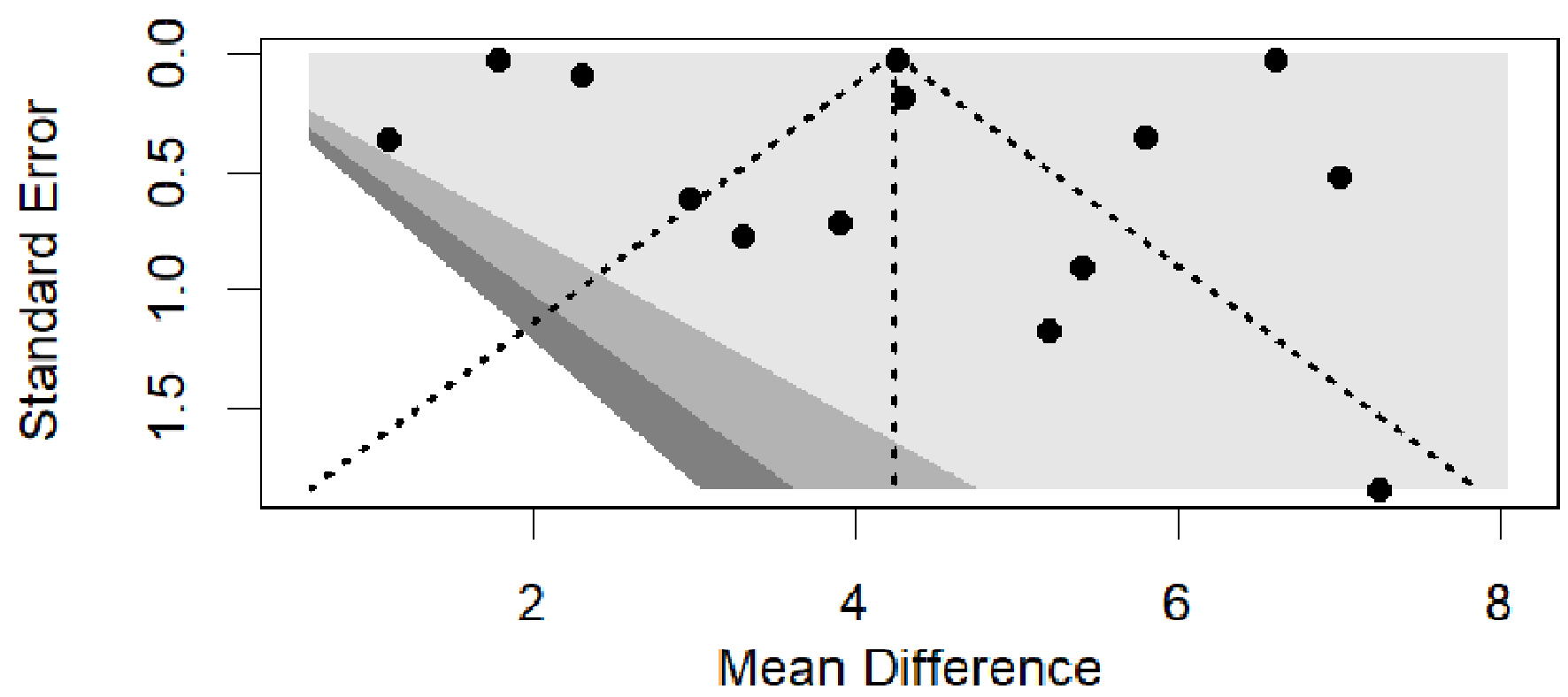 Click for large image | Figure 7. Funnel plots of studies reporting weight loss % induced by tirzepatide or semaglutide. |
Study quality
In retrospective cohort studies, NOS revealed the quality of each study as “good” (≥ 7 points) (Table 3) [17-21]. Both RCTs were found to have a low RoB due to randomization, deviations from intended interventions, missing outcome, measurement of the outcome, and reporting bias as estimated through Cochrane RoB version 2 (Figs. 8 and 9). Hence, overall quality and RoB were estimated as high quality with a low RoB.
 Click to view | Table 3. Newcastle-Ottawa Scale (NOS) Quality Assessment for Retrospective Cohort Studies |
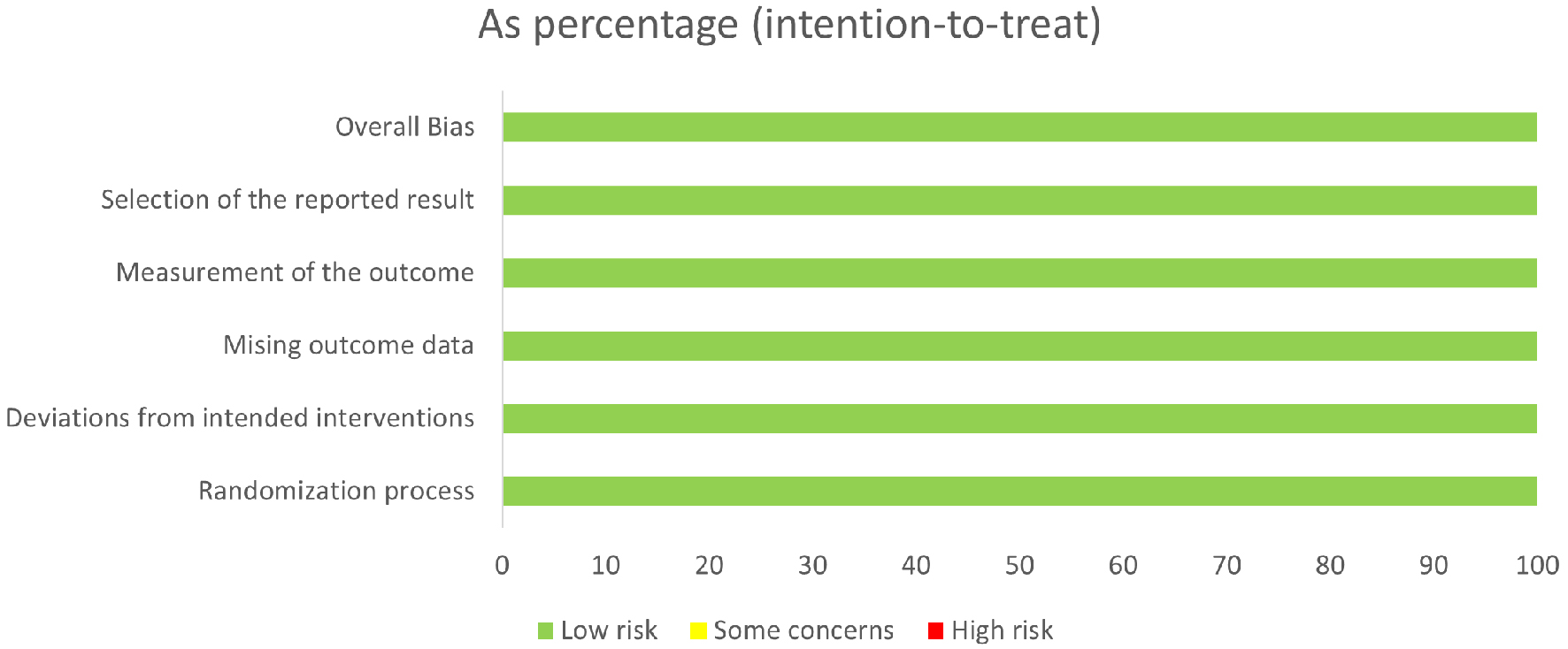 Click for large image | Figure 8. Risk of bias in all studies. |
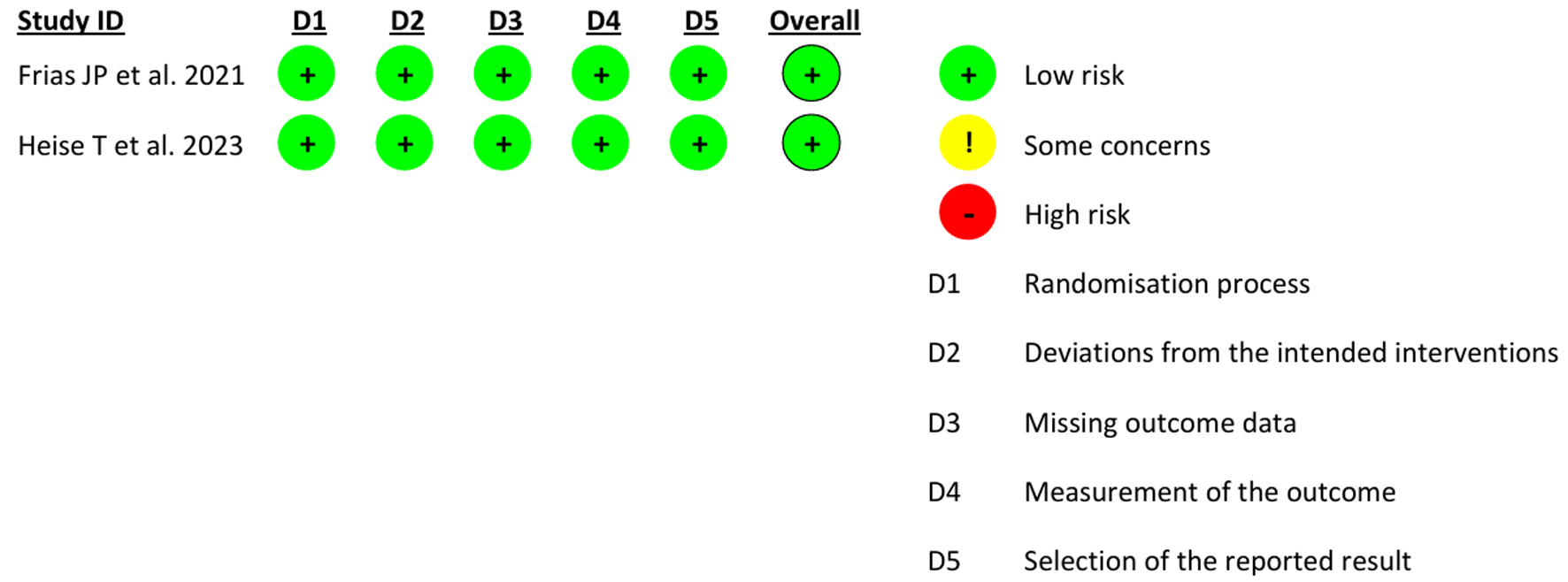 Click for large image | Figure 9. Risk of bias of individual studies. |
| Discussion | ▴Top |
Our pooled estimates indicate that tirzepatide is more advantageous and has better clinical results. Regardless of the study design, tirzepatide produced significantly greater weight loss than semaglutide. A dose-response relationship exists, and the weight loss magnitude increases with the dose or duration of tirzepatide.
Similar results have been demonstrated in other systematic reviews and MA that compared tirzepatide with other drugs such as GLP-1 RAs, placebo, and insulin [22]. Our results agree with another MA by Lv et al [23]. That MA showed that tirzepatide raised the frequency of T2DM patients with > 5% weight loss in a dose-dependent manner. In agreement with our results, Karagiannis et al [24] concluded that tirzepatide was superior to semaglutide for reducing body weight. They reported a weight loss of 5.27 and 9.57 kg with tirzepatide 5 and 15 mg, respectively, and 2.52 and 4.97 kg with semaglutide 0.5 and 2.0 mg, respectively. That MA included two head-to-head comparison RCTs of tirzepatide and semaglutide out of a total of 28 RCTs included. Malecki et al reported that tirzepatide produced weight loss exceeding 15% in individuals with obesity [25].
The mechanism underpinning tirzepatide-induced weight loss may involve concurrent activation of GLP-1R/GIPR and the synergistic effects of the two GLP-1 and GIP at central nervous system (CNS) level [26]. Concurrent intake of GLP-1 and GIP promoted pro-opiomelanocortin (POMC) gene expression in anorexia nervosa, which decreased hunger. There may exist special neurons in the arcuate nucleus of the hypothalamus that are only stimulated when GLP-1 and GIP are administered simultaneously. These neurons contain both GLP-1R and GIPR [27].
While several systematic review (SR) and MA have compared the efficacy of tirzepatide or semaglutide in reducing body weight, the included trials mostly compared either of these drugs with placebo or any other medication [22-24, 28, 29]. Compared to the current study, the MA of Dutta et al [28] included two head-to-head trials of tirzepatide vs. semaglutide. Lv et al [23] included eight RCTs in their MA of which six trials compared tirzepatide with a placebo or dulaglutide or insulin glargine or insulin degludec. In the MA of Cai et al, [22], 10 out of 12 RCTs compared tirzepatide with the placebo or other medications. In the MA of Zhou et al [30], 14 RCTs compared tirzepatide with placebo or other medications.
The present study provides evidence suggesting that tirzepatide is better than semaglutide in reducing body weight. The key strength of the present SR and MA is that it is the first MA that combined RCTs and real-world data to analyze the weight loss potential of tirzepatide vs. semaglutide. We found consistency between the pooled results of the two study types (retrospective cohorts and RCTs) as the results were in the same direction. The superiority of tirzepatide was evident in subgroup analysis based on dose, duration, and study design. Limitations include failure to assess long-term weight loss sustainability, safety profiles, and substantial heterogeneity. High levels of heterogeneity can undermine the validity of pooled results, suggesting that the combined estimate may not accurately represent study outcomes. The heterogeneity persisted in subgroup analysis based on study design, duration, and dose of tirzepatide intervention. This could be due to the diversity of the research subjects in each study. Our inclusion criteria were any population (regardless of age group and health status). The participants with obesity with or without T2DM exhibit a greater degree of weight loss with weight loss therapy [31]. Variability in ethnicity, follow-up times, and different doses may account for constant heterogeneity. Finally, the number of head-to-head comparison RCTs is relatively limited.
Acknowledgments
We would like to thank Muhammad Bin Aamir, School of Business and Computer Science, Caldwell University, USA, for his support in statistical analysis.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Ahmad Bin Aamir: literature search, statistical analysis, figure/table preparation, writing-original draft, and final manuscript approval. Rabia Latif: conceptualization, data analysis, supervision, and final manuscript approval. Jood Faisal Alqoofi: screening of articles against eligibility criteria, data extraction, and final manuscript approval. Fatimah Abdulkarim Almarzoq: screening of articles against eligibility criteria, data extraction, and final manuscript approval. Joory Osamah Fallatah: methodology, data verification, writing-review, and final manuscript approval. Ghala Abdullah Hassan: methodology, data verification, writing-review, and final manuscript approval. Fatimah Abbas Abdullah Al Abu Saab: figure/table preparation, writing-review, and final manuscript approval.
Data Availability
The authors declare that data supporting the results of this study are available within this review.
Abbreviations
CI: confidence interval; FDA: Food and Drug Administration; GIP: glucose-dependent insulinotropic peptide; GIPR: glucose-dependent insulinotropic peptide receptor; GLP-1R: glucagon-like peptide-1 receptor; GLP-1 RA: glucagon-like peptide-1 receptor agonist; MA: meta-analysis; MD: mean difference; NOS: Newcastle-Ottawa Quality Assessment Scale; OSF: Open Science Foundation; RCT: randomized controlled trial; RoB: risk of bias; SR: systematic review
| References | ▴Top |
- https://www.fda.gov/news-events/press-announcements/fda-approves-new-medication-chronic-weight-management.
- Willard FS, Douros JD, Gabe MB, Showalter AD, Wainscott DB, Suter TM, Capozzi ME, et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight. 2020;5(17):e140532.
doi pubmed - Nauck MA, Meier JJ. Incretin hormones: Their role in health and disease. Diabetes Obes Metab. 2018;20(Suppl 1):5-21.
doi pubmed - Jung HN, Jung CH. The Upcoming Weekly Tides (Semaglutide vs. Tirzepatide) against Obesity: STEP or SURPASS? J Obes Metab Syndr. 2022;31(1):28-36.
doi pubmed - https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-first-2014.
- Davies M, Faerch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, Rosenstock J, et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971-984.
doi pubmed - Garvey WT, Batterham RL, Bhatta M, Buscemi S, Christensen LN, Frias JP, Jodar E, et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med. 2022;28(10):2083-2091.
doi pubmed - Garvey WT, Frias JP, Jastreboff AM, le Roux CW, Sattar N, Aizenberg D, Mao H, et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2023;402(10402):613-626.
doi pubmed - Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, Kiyosue A, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205-216.
doi pubmed - Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, et al. Once-weekly Semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002.
doi pubmed - Cheurfa C, Tsokani S, Kontouli KM, Boutron I, Chaimani A. Synthesis methods used to combine observational studies and randomised trials in published meta-analyses. Syst Rev. 2024;13(1):70.
doi pubmed - https://osf.io/mjt9p.
- Wells G, Shea B, O’Connell D,J Peterson, V Welch, M Losos, P Tugwell. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
doi pubmed - Frias JP, Davies MJ, Rosenstock J, Perez Manghi FC, Fernandez Lando L, Bergman BK, Liu B, et al. Tirzepatide versus Semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503-515.
doi pubmed - Heise T, DeVries JH, Urva S, Li J, Pratt EJ, Thomas MK, Mather KJ, et al. Tirzepatide reduces appetite, energy intake, and fat mass in people with type 2 diabetes. Diabetes Care. 2023;46(5):998-1004.
doi pubmed - Anson M, Henney AE, Broadwell N, Zhao SS, Ibarburu GH, Lip GYH, Wilding JPH, et al. Incidence of new onset type 2 diabetes in adults living with obesity treated with tirzepatide or semaglutide: real world evidence from an international retrospective cohort study. EClinicalMedicine. 2024;75:102777.
doi pubmed - Azuri J, Hammerman A, Aboalhasan E, Sluckis B, Arbel R. Tirzepatide versus semaglutide for weight loss in patients with type 2 diabetes mellitus: A value for money analysis. Diabetes Obes Metab. 2023;25(4):961-964.
doi pubmed - Gebre H, Snell-Bergeon JK, Shah VN. 736-P: Comparison of Semaglutide vs. Tirzepatide therapy in adults with type 1 diabetes (T1D). Diabetes. 2024;73 (Supplement_1):736-P.
doi - Jamal M, Alhashemi M, Dsouza C, Al-Hassani S, Qasem W, Almazeedi S, Al-Sabah S. Semaglutide and Tirzepatide for the management of weight recurrence after sleeve gastrectomy: a retrospective cohort study. Obes Surg. 2024;34(4):1324-1332.
doi pubmed - Rodriguez PJ, Goodwin Cartwright BM, Gratzl S, Brar R, Baker C, Gluckman TJ, Stucky NL. Semaglutide vs Tirzepatide for weight loss in adults with overweight or obesity. JAMA Intern Med. 2024;184(9):1056-1064.
doi pubmed - Cai W, Zhang R, Yao Y, Wu Q, Zhang J. Tirzepatide as a novel effective and safe strategy for treating obesity: a systematic review and meta-analysis of randomized controlled trials. Front Public Health. 2024;12:1277113.
doi pubmed - Lv X, Wang H, Chen C, Zhao Y, Li K, Wang Y, Wang L, et al. The effect of Tirzepatide on weight, lipid metabolism and blood pressure in overweight/obese patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Syndr Obes. 2024;17:701-714.
doi pubmed - Karagiannis T, Malandris K, Avgerinos I, Stamati A, Kakotrichi P, Liakos A, Vasilakou D, et al. Subcutaneously administered tirzepatide vs semaglutide for adults with type 2 diabetes: a systematic review and network meta-analysis of randomised controlled trials. Diabetologia. 2024;67(7):1206-1222.
doi pubmed - Malecki MT, Batterham RL, Sattar N, Levine JA, Rodriguez A, Bergman BK, Wang H, et al. Predictors of >/ = 15% weight reduction and associated changes in cardiometabolic risk factors with Tirzepatide in adults with type 2 diabetes in SURPASS 1-4. Diabetes Care. 2023;46(12):2292-2299.
doi pubmed - NamKoong C, Kim MS, Jang BT, Lee YH, Cho YM, Choi HJ. Central administration of GLP-1 and GIP decreases feeding in mice. Biochem Biophys Res Commun. 2017;490(2):247-252.
doi pubmed - Adriaenssens AE, Biggs EK, Darwish T, Tadross J, Sukthankar T, Girish M, Polex-Wolf J, et al. Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food intake. Cell Metab. 2019;30(5):987-996.e986.
doi pubmed - Dutta D, Kamrul-Hasan ABM, Nagendra L, Bhattacharya S. Efficacy and safety of novel Twincretin Tirzepatide, a dual GIP/GLP-1 receptor agonist, as an anti-obesity medicine in individuals without diabetes: a systematic review and meta-analysis. touchREV Endocrinol. 2024;20(2):72-80.
doi pubmed - Rochira V, Greco C, Boni S, Costantino F, Dalla Valentina L, Zanni E, Itani L, et al. The effect of Tirzepatide on body composition in people with overweight and obesity: a systematic review of randomized, controlled studies. Diseases. 2024;12(9):204.
doi pubmed - Zhou Q, Lei X, Fu S, Liu P, Long C, Wang Y, Li Z, et al. Efficacy and safety of tirzepatide, dual GLP-1/GIP receptor agonists, in the management of type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetol Metab Syndr. 2023;15(1):222.
doi pubmed - Papamargaritis D, le Roux CW, Holst JJ, Davies MJ. New therapies for obesity. Cardiovasc Res. 2024;119(18):2825-2842.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.