| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 000, Number 000, May 2025, pages 000-000
Left Ventricular Diastolic Dysfunction and Its Predictive Factors Among Saudi Patients With Type 2 Diabetes Mellitus
Reem A. Aldhahia, Mazen M. Barhoushb, Dina S. Almunifa, Mohammed Jamal M. Anabic, Khaled H. Aburishehb, d
aDepartment of Family and Community Medicine, College of Medicine, King Saud University, Riyadh, Saudi Arabia
bUniversity Diabetes Center, King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia
cKing Abdulaziz University Hospital, King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia
dCorresponding Author: Khaled Hani Aburisheh, University Diabetes Center, King Saud University Medical City, King Saud University, PO Box 11472, Riyadh 7805, Saudi Arabia
Manuscript submitted March 12, 2025, accepted May 5, 2025, published online May 13, 2025
Short title: LVDD Predictors in Saudi T2DM Patients
doi: https://doi.org/10.14740/jocmr6233
| Abstract | ▴Top |
Background: Diabetes mellitus places a significant burden on society in terms of healthcare expenditures and poor health outcomes and complications. Heart failure is one of its complications which increases morbidity and mortality for patients with diabetes. The purpose of this study was to assess the prevalence of left ventricular diastolic dysfunction (LVDD) and its predictors among Saudi patients with type 2 diabetes mellitus (T2DM).
Methods: This retrospective cross-sectional study was conducted between May 2021 and May 2022 at King Saud University Medical City in Riyadh, Saudi Arabia. Medical records of adult patients with T2DM without prior cardiovascular disease who underwent echocardiographic examination were reviewed, and data were extracted. Echocardiographic findings were reviewed for the diagnosis of LVDD.
Results: A total of 251 participants were included in the study. LVDD was diagnosed in 66.9% of the participants. The majority (89.9%) had grade I. The mean age was 59 ± 9.1 years and the mean diabetes duration was 20 ± 8.5 years. Of the patients, 76.9% had hypertension and 81.2% had dyslipidemia. The mean body mass index was 32.9 ± 6.6 kg/m2 and the mean glycated hemoglobin level was 7.7±2.3%. LVDD correlated with older age, longer duration of diabetes, obesity, poor glycemic control, higher systolic blood pressure, the presence of hypertension, and the usage of antihypertensive and lipid-lowering medications. In logistic regression analysis, older age and higher body mass index were the only independent risk factors of LVDD.
Conclusion: The prevalence of LVDD among Saudi patients with T2DM was high. It was associated significantly with age and obesity. These findings highlight the need for early monitoring, and treatment to prevent its progression and reduce morbidity and mortality.
Keywords: Type 2 diabetes mellitus; Left ventricular diastolic dysfunction; Glycated hemoglobin
| Introduction | ▴Top |
The prevalence of diabetes mellitus (DM) is increasing worldwide. In 2019, the global prevalence of DM was estimated to be 9.3% (463 million people), and it is expected to rise to 10.9% (700 million) by 2045 [1]. In Saudi Arabia, the prevalence of DM is 25.5% among people aged 30 years and over [2]. Diabetes imposes a significant burden on society in terms of healthcare costs and health complications [1]. In Saudi Arabia, healthcare costs for patients with diabetes are 10 times higher than those without diabetes [3]. Diabetes is also a well-known risk factor for heart failure (HF), independent of age or the presence of other illnesses increasing morbidity and mortality [4, 5].
HF is classified according to ejection fraction (EF) percentage into HF with reduced EF (HFrEF, ≤ 40%), HF with mildly reduced EF (HFmrEF, 41-49%), and HF with preserved EF (HFpEF, ≥ 50%). Patients with current or prior HF, regardless of EF, should be considered for guideline-directed medical therapy [6]. Left ventricular diastolic dysfunction (LVDD) is considered the preclinical stage of HFpEF and is most prevalent in patients with diabetes [7]. It is defined as an inability to fill the left ventricular (LV) normally during rest or exercise without an abnormal increase in LV end-diastole or mean left atrial (LA) pressure (LAP) or an inability to increase LV volume and cardiac output during exercise [8].
Diabetes can cause myocardial damage, affecting the heart’s diastolic function before its systolic function [9]. The mechanism of cardiac muscle toxicity in diabetes is believed to be related to chronic glucotoxicity, insulin resistance, oxidative stress, inflammation, and fibrosis, which contribute to cardiac injury through complex mechanisms [4, 9]. LVDD is often clinically silent but can present with LV failure symptoms [10]. Asymptomatic patients with LVDD are at risk of developing HF and structural heart disease. Early screening for this abnormality of cardiac function is crucial to improving the prognosis of these patients, as it can lead to early intervention and potentially prevent the progression of heart disease [11].
Although there are many reports on LVDD in patients with diabetes [7, 12-20], no documented studies have been conducted among Saudi patients with diabetes. Therefore, this study aimed to highlight this complication and its predictive factors in Saudi patients with diabetes by applying updated echocardiographic criteria for diastolic dysfunction. This will align with past findings and contribute to understanding LVDD in this population.
| Materials and Methods | ▴Top |
Study design and setting
This retrospective cross-sectional study was conducted between May 2021 and May 2022 at King Saud University Medical City in Riyadh, Saudi Arabia. The study was approved by the Institutional Review Board, College of Medicine, King Saud University (No. E-21-6020). This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Participants and survey instrument
The medical records of all adult patients with type 2 diabetes mellitus (T2DM) aged 18 - 75 years who were followed at King Saud University Medical City’s diabetes clinics and underwent echocardiographic examination from 2018 to 2020 were reviewed. The exclusion criteria were structural heart disease (cardiomyopathy, valvular heart disease, HF, cardiovascular disease (CVD)), chronic pulmonary disease, estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2, age more than 75 years, and patients with a poor transthoracic echocardiograph window, gestational diabetes, and type 1 DM.
Data were meticulously retrieved from the electronic medical records using a structured data form. This comprehensive approach ensured that all relevant demographic and clinical variables, including age, gender, DM duration, presence of DM comorbidities and complications, height, weight, fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglyceride (TG), total cholesterol, creatinine, blood pressure, the medication used to control diabetes and other comorbidities, and 3D echocardiographic data, were accurately captured.
The diagnosis of DM was based on the diagnostic criteria of the American Diabetes Association as FPG ≥ 126 mg/dL (7.0 mmol/L), oral glucose tolerance test ≥ 200 mg/dL (11.1 mmol/L), HbA1c ≥ 6.5% or classic diabetes symptoms with random plasma glucose ≥ 200 mg/dL (11.1 mmol/L) [21].
Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2). The eGFR was assessed using the Chronic Kidney Disease Epidemiology Collaboration equation.
Transthoracic echocardiography was performed using the PHILIPS EPIQ 7 ultrasound system. A cardiology consultant conducted a comprehensive assessment of specific measures of LV filling pressure and diastolic function grade, such as mean pulmonary capillary wedge pressure (PCWP), mean LAP, LV pre-atrial filling (A) pressure, mean LV diastolic pressure, and LV end-diastolic pressure (LVEDP). These measures were meticulously correlated to different Doppler signals, including mitral peak A velocity at the tips level, A-wave duration at the annulus, A velocity deceleration time (DT), pulmonary vein peak atrial reversal (Ar) velocity, Ar velocity duration, Ar-A duration, tissue Doppler-derived mitral annular a′ velocity, mitral peak early filling E-wave velocity, E/A ratio, E velocity DT, E/early diastolic (e′) ratio, pulmonary vein systolic-to-diastolic velocity ratio, and peak velocity of tricuspid regurgitation (TR) by continuous-wave (CW) Doppler. The diagnosis of LVDD was made based on the American Society of Echocardiography and European Association of Cardiovascular Imaging diagnostic criteria (annular e′ velocity: septal e′ < 7 cm/s, lateral e′ < 10 cm/s, average E/e′ ratio > 14, LA volume index > 34 mL/m2 , and peak TR velocity > 2.8 m/s). LVDD is present if three or more parameters meet these cutoff values [22].
LVDD was graded as grade I diastolic dysfunction (E/A ratio ≤ 0.8 along with a peak E velocity of ≤ 50 cm/s, mean LAP is either normal or low), grade II diastolic dysfunction (E/A ≤ 0.8 along with a peak E velocity of > 50 cm/s, or an E/A ratio > 0.8 but < 2, then additionally more than two criteria of peak velocity of TR jet > 2.8 m/s, average E/e′ ratio > 14, and LA maximum volume index > 34 mL/m2), and grade III diastolic dysfunction (E/A ratio ≥ 2) [22].
Using an alpha of 0.05, an effect size of 0.2, and a power of 0.9, the calculated sample size was 255 participants.
Statistical analysis
The data were analyzed using IBM SPSS version 25 (IBM Corp., Armonk, NY, USA). Descriptive statistics were calculated using frequency, percentage, mean, and standard deviation. The normality of the data was tested using the Shapiro-Wilk test, and the data were found to be not normally distributed, which is common in medical research. Hence, non-parametric tests were used for inferential analysis. The Mann-Whitney U test was used to find the effect of categorical variables on the continuous variables. Spearman correlation was used to evaluate the correlation between the continuous variables. Multivariate analysis was carried out to identify independent variables associated with the outcome. We included variables found to be correlated with the presence of LVDD in our analysis. The multivariate analysis provided adjusted odds ratio (aOR) for having the outcome of interest (LVDD), given that a particular exposure is present while adjusting for the effect of all other included predictor factors. A P-value of ≤ 0.05 and 95% confidence intervals were used to report statistical significance and precision of the results.
| Results | ▴Top |
During the study period, 390 patients’ electronic medical files were screened, out of which 251 were included. The mean age of participants was 59 ± 9.1 years, and 61.8% of them were women. The mean diabetes duration among study participants was 20 ± 8.5 years. There was a high prevalence of hypertension (HTN) (76.9%) and dyslipidemia (81.2%) among them. However, few participants experienced diabetes-associated complications including diabetic nephropathy (8.8%), neuropathy (4.4%), retinopathy (9.2%), cerebrovascular accident (13.1%), and peripheral vascular disease (4.8%). On average, the participants were obese, with a mean BMI of 32.9 ± 6.6 kg/m2. Mean blood pressure was 134/72 ± 23/12 mm Hg. Diabetes was modestly controlled among the participants, with a mean HbA1c level of 7.7±2.3%. Lipid parameters were almost in the recommended range, with a mean total cholesterol of 4.1 ± 1.06 mmol/L, mean HDL of 1.27 ± 0.35 mmol/L, mean LDL of 2.3 ± 0.8 mmol/L, and mean TG of 1.6 ± 0.79 mmol/L. The mean eGFR was 88 mL/min/1.73 m2 (Table 1).
 Click to view | Table 1. Demographic and Clinical Characteristics |
The prevalence of LVDD among the study’s participants was 66.9%. The majority of LVDD cases were grade I (89.8%), followed by grade II (9.6%), with only one participant experiencing grade III LVDD (0.6%) (Fig. 1).
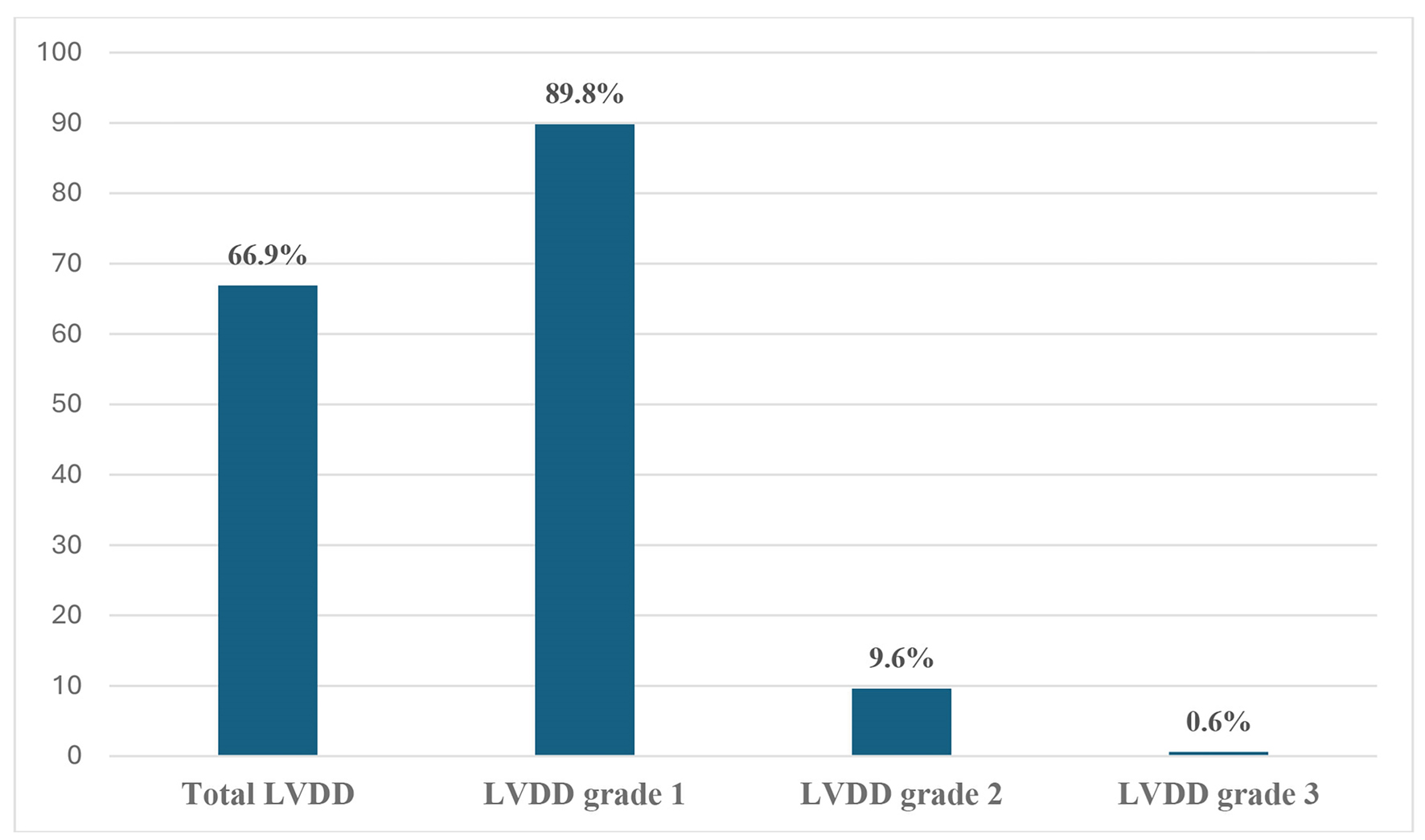 Click for large image | Figure 1. Prevalence of left ventricular diastolic dysfunction (LVDD) and its grades. |
Regarding diabetes medications, metformin was the most commonly prescribed glucose-lowering agent (82.5%), followed by insulin (46.6%) and dipeptidyl peptidase 4 (DPP-4) inhibitors (41.8%). Thiazolidinedione, glucagon-like peptide 1 (GLP-1) receptor agonist, and alpha-glucosidase inhibitors were the least prescribed medications (6.8%, 5.2%, and 0.4%, respectively).
Around two-thirds of the participants used angiotensin II receptor blockers (ARBs) or angiotensin-converting enzyme inhibitors (ACEi). Diuretics were prescribed to 44.6% of participants, beta-blockers were used by 35.1%, and calcium channel blockers were used by 36.7%. Additionally, most participants took antiplatelet agents (69.2%) and lipid-lowering agents (88%) (Table 1).
Compared with participants without LVDD, those with LVDD had a higher prevalence of HTN (85.1% vs. 60.2%, P = 0.000) and were more likely to use ARBs or ACEi, diuretics, and lipid-lowering agents (69% vs. 48.2%, P = 0.001; 53% vs. 27.7%, P = 0.000; 69.2% vs. 50%, P = 0.036, respectively). There were no significant differences in using different glucose-lowering agents according to LVDD status.
Participants with LVDD were significantly older and had longer DM duration than those without LVDD. Regarding metabolic parameters, BMI, systolic blood pressure (SBP), FPG, and HbA1c were higher in those with LVDD (P = 0.006, 0.000, 0.034, and 0.017, respectively). Furthermore, eGFR was lesser among them (P = 0.000) (Tables 2 and 3).
 Click to view | Table 2. Comparison of Patients’ Categorial Characteristics According to the Presence of LVDD |
 Click to view | Table 3. Correlation Between Presence of LVDD and Clinical Parameters |
In a multiple regression analysis, older age and higher BMI were identified as the only significant independent risk factors for LVDD in our study population adjusted for the effect of all other included predictor factors in the analysis. It was found that each unit increase in age significantly increased the risk of LVDD by 10% (P = 0.003). Additionally, each unit increase in BMI increased the risk of LVDD by 14% (P = 0.002) (Table 4).
 Click to view | Table 4. Multiple Logistic Regression Analysis for the Predictors of LVDD |
| Discussion | ▴Top |
In this study, our aim was to evaluate the prevalence of LVDD in Saudi patients with T2DM and its predictive factors. Our findings contribute significantly to the existing body of evidence, shedding light on the high prevalence of LVDD and identifying key demographic and clinical factors associated with LVDD. These factors include age, duration of DM, presence of comorbidities, BMI, metabolic control, and usage of HTN and lipid medications. Our study underscores the crucial role of early screening in diabetic populations and emphasizes the need for a comprehensive, multifactorial approach to management, considering cardiovascular and systemic comorbidities, to reduce morbidity and mortality.
Our study supports evidence of the high prevalence of LVDD among patients with T2DM [7, 12, 13, 16, 17, 19]. More than two-thirds of the patients in our study had LVDD, with most being grade I (89.9%). This is consistent with other studies, such as one in Malaysia, which showed that 70.1% of T2DM patients without prior CVD had LVDD. In more than 90%, it was mild [17]. Additionally, a study in India found a 66% prevalence of LVDD in participants with T2DM. The mean age in that study was 54.1 years, and the mean BMI was 31.3 kg/m2, comparable to that in our study (59.4 years and 32.9 kg/m2, respectively). Their participants had poorer glycemic control (the mean HbA1c 9.93% vs. 7.6%) and shorter DM duration (6.33 vs. 20.28 years) than ours [12]. However, a systematic review and meta-analysis showed a pooled prevalence of LVDD at 46%, with a wide range and high heterogenicity [7]. Furthermore, another study observed an increase in the prevalence of LVDD from 49% to 67% over 3 years among patients with T2DM [15]. The variability in LVDD prevalence can be due to the differences in demographic and clinical characteristics between studies, such as age, DM duration, medications used by the participants, diabetes control, and the presence of DM comorbidities and complications.
LVDD typically affects patients for many years before progressing to overt HF. At this early stage, LVDD may represent a reversible stage of HF, and patients can benefit significantly if LVDD is recognized and treated [9]. Our study showed that most patients with LVDD had grade 1/mild severity (89.8%), a finding consistent with previous reports [17, 19, 20]. The predominant absence of higher grades of LVDD in our study population can be attributed to the use of lipid-lowering agents, antiplatelet medications, and ARBs by most patients, targeting cardiovascular risk and reducing HF progression [11].
Our study found a significant correlation between LVDD in patients with T2DM and older age, presence of HTN, longer DM duration, higher BMI, SBP, FPG and HbA1c levels, and lower eGFR. This observation is consistent with findings from other studies [13, 16, 17, 19, 20, 23]. These variables, which are considered cardiovascular risk factors, can cause heart injury through complex mechanisms, leading to LVDD and progression to overt HF [11]. This underscores the importance of addressing these factors in the management strategies to reduce the prognosis of LVDD.
Furthermore, our study showed a correlation between antihypertensive (mainly ARBs or ACEi, diuretics, and calcium channel blockers) and lipid-lowering medications and LVDD, as we found patients with LVDD used these medications more than the other group. This can be explained by the high prevalence of HTN in LVDD patients, and they have a higher risk of CVD requiring medication intensification. Few studies included these medications in their analysis, and they showed no correlation with LVDD in patients with T2DM [15, 17, 19, 23, 24]. However, Faden et al showed a correlation between the average number of antihypertensive medications used and LVDD. They found no difference regarding the type of medications used [19]. Additionally, Bergerot et al observed no association between ARBs, ACEi, and statins with LVDD deterioration over 3 years [15]. On the other hand, a study conducted on patients with long-term HTN showed that both the average number and different types of antihypertensive medications used were associated with the presence of LVDD [25].
A multivariant regression analysis confirmed that only age and BMI were positively associated with the risk of LVDD. There is a discrepancy in the literature about the predictors of LVDD in patients with diabetes; however, most of them confirmed that older age is an independent risk factor [15, 17, 19, 26, 27]. Lumori et al’s observation was similar to our findings, as they found that age ≥ 50 years with BMI ≥ 25 kg/m2 significantly increased the risk of LVDD by 13.82 times compared by comparator groups [26].
This study, while providing valuable insights into the high prevalence of LVDD and its predictors in Saudi patients with T2DM, has several limitations that warrant consideration. Firstly, the sample size was relatively small from a single hospital, so we could not generalize the findings. Secondly, the study’s retrospective nature can lead to missing some data retrieved from medical records. Thirdly, we excluded CVD from documented medical records; however, a detailed investigation, such as a coronary angiogram, was needed to confirm the exclusion as it could be the cause of LVDD rather than diabetic cardiomyopathy. Furthermore, this cross-sectional study could not conclude the causality of diabetes and predictor factors with LVDD. Finally, our analysis did not involve the measurement of myocardial strain parameters by speckle tracking echocardiography, a method for identifying LV longitudinal myocardial dysfunction. This dysfunction is considered in recent evidence as the primary marker of preclinical cardiac dysfunction in patients with diabetes, surpassing LVDD [28]. Therefore, further prospective longitudinal studies with myocardial strain parameters measurement of larger sample sizes, including a non-diabetic control group, are needed to confirm our findings and provide a deeper understanding of the pathophysiological mechanisms underpinning LVDD and subclinical LV systolic dysfunction in patients with diabetes.
Conclusion
DM is now a well-known risk for LVDD and, ultimately, HF. In the present study, the prevalence of LVDD among patients with T2DM and no prior known CVD was 66.9%, which was mostly in mild severity. Older age and obesity were the only independent risk factors of LVDD. These findings highlight the need for implementing strategies for early screening, monitoring, and treatment of LVDD in its early stages to prevent progression and reduce mortality. A multifactorial approach in management plans, such as controlling comorbidities, including HTN, hyperlipidemia, and obesity, is crucial. Exercise, nutritional programs, and blood sugar control may also benefit patients.
Further studies in Saudi Arabia are crucial to establishing a clearer picture of the impact of LVDD on the Saudi population. These studies will provide the necessary data to implement appropriate preventive and treatment strategies, thereby improving the overall management of LVDD in the country.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Informed Consent
Since this was a retrospective data collection study, the Institutional Review Board, College of Medicine, King Saud University, Riyadh, waived informed consent.
Author Contributions
RAA, MMB, DSA, MJA, and KHA conceived and designed the research; RAA, DSA, and MJA collected the data; KHA analyzed the data; RAA, MMB, DSA, and MJA prepared the manuscript. RAA, MMB, and KHA finalized the manuscript. MMB and KHA supervised the project. All the authors read and approved the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ACEi: angiotensin-converting enzyme inhibitor; ARBs: angiotensin II receptor blockers; BMI: body mass index; CVD: cardiovascular disease; DM: diabetes mellitus; DPP-4: dipeptidyl peptidase 4; EF: ejection fraction; eGFR: estimated glomerular filtration rate; FPG: fasting plasma glucose; GLP-1: glucagon-like peptide 1; HbA1c: glycated hemoglobin; HDL: high-density lipoprotein; HF: heart failure; HFmrEF: HF with mildly reduced EF; HFpEF: HF with preserved EF; HFrEF: HF with reduced EF; HTN: hypertension; LA: left atrial; LAP: left atrial pressure; LDL: low-density lipoprotein; LV: left ventricular; LVDD: left ventricular diastolic dysfunction; LVEDP: LV end-diastolic pressure; PCWP: pulmonary capillary wedge pressure; SBP: systolic blood pressure; T2DM: type 2 diabetes mellitus; TG: triglyceride; TR: tricuspid regurgitation
| References | ▴Top |
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843.
doi pubmed - Al-Rubeaan K, Al-Manaa H, Khoja T, Ahmad N, Al-Sharqawi A, Siddiqui K, AlNaqeb D, et al. The Saudi abnormal glucose metabolism and diabetes impact study (SAUDI-DM). Ann Saudi Med. 2014;34(6):465-475.
doi pubmed - Alhowaish AK. Economic costs of diabetes in Saudi Arabia. J Family Community Med. 2013;20(1):1-7.
doi pubmed - Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34(1):29-34.
doi pubmed - Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC, Jr. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27(3):699-703.
doi pubmed - Gibson G, Blumer V, Mentz RJ, Lala A. Universal definition and classification of heart failure: a step in the right direction from failure to function. American College of Cardiology. 2021;13.
- Bouthoorn S, Valstar GB, Gohar A, den Ruijter HM, Reitsma HB, Hoes AW, Rutten FH. The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: A systematic review and meta-analysis. Diab Vasc Dis Res. 2018;15(6):477-493.
doi pubmed - Appleton CP, Firstenberg MS, Garcia MJ, Thomas JD. The echo-Doppler evaluation of left ventricular diastolic function. A current perspective. Cardiol Clin. 2000;18(3):513-546.
doi pubmed - Ritchie RH, Abel ED. Basic mechanisms of diabetic heart disease. Circ Res. 2020;126(11):1501-1525.
doi pubmed - Wenzel JP, Kellen RB, Magnussen C, Blankenberg S, Schrage B, Schnabel R, Nikorowitsch J. Diastolic dysfunction in individuals with and without heart failure with preserved ejection fraction. Clin Res Cardiol. 2022;111(4):416-427.
doi pubmed - Kosmala W, Marwick TH. Asymptomatic left ventricular diastolic dysfunction: predicting progression to symptomatic heart failure. JACC Cardiovasc Imaging. 2020;13(1 Pt 2):215-227.
doi pubmed - Vittal DS, Babu MV. Prevalence of left ventricular diastolic dysfunction among patients with asymptomatic diabetes mellitus type 2. 2019.
- Patil VC, Patil HV, Shah KB, Vasani JD, Shetty P. Diastolic dysfunction in asymptomatic type 2 diabetes mellitus with normal systolic function. J Cardiovasc Dis Res. 2011;2(4):213-222.
doi pubmed - Vinayagan A, Verma GC, Wahid A. The prevalence of diastolic dysfunction in diabetic mellitus in the age group of 20-40 years and its correlation with duration of diabetes, HBA1C level and diabetic retinopathy. J Assoc Physicians India. 2020;68(1):51.
pubmed - Bergerot C, Davidsen ES, Amaz C, Thibault H, Altman M, Bellaton A, Moulin P, et al. Diastolic function deterioration in type 2 diabetes mellitus: predictive factors over a 3-year follow-up. Eur Heart J Cardiovasc Imaging. 2018;19(1):67-73.
doi pubmed - Ashour KJJHCR. Early detection of diastolic dysfunction in diabetic patients (single center cross sectional study). J Heart Cardiovasc Res. 2018;2(1):114.
- Chee KH, Tan KL, Luqman I, Saiful SS, Chew YY, Chinna K, Tan ATB. Prevalence and predictors of left ventricular diastolic dysfunction in Malaysian patients with type 2 diabetes mellitus without prior known cardiovascular disease. Front Cardiovasc Med. 2021;8:676862.
doi pubmed - Kumar VS, Sreelatha M, Ramesh K, Shekar GC. Study of left ventricular diastolic dysfunction in type 2 diabetes mellitus patients. International Journal of Scientific Study. 2017;5(4):219-224.
- Faden G, Faganello G, De Feo S, Berlinghieri N, Tarantini L, Di Lenarda A, Faggiano P, et al. The increasing detection of asymptomatic left ventricular dysfunction in patients with type 2 diabetes mellitus without overt cardiac disease: data from the SHORTWAVE study. Diabetes Res Clin Pract. 2013;101(3):309-316.
doi pubmed - Chandey M, Kamboj R, Sikri T, Kaur N. Left ventricular dysfunction in patients with type 2 diabetes mellitus. 2020.
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and classification of diabetes: standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S20-S42.
doi pubmed - Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321-1360.
doi pubmed - Venskutonyte L, Jarnert C, Ryden L, Kjellstrom B. Longitudinal development of left ventricular diastolic function in patients with type 2 diabetes. Diabetes Care. 2014;37(11):3092-3097.
doi pubmed - Hassan Ayman KM, Abdallah Mahmoud A, Abdel-Mageed Eman A, Marwa S, Soliman Mona M, Kishk Yehia T. Correlation between left ventricular diastolic dysfunction and dyslipidaemia in asymptomatic patients with new-onset type 2 diabetes mellitus. The Egyptian Journal of Internal Medicine. 2021;33:1-11.
- Dabrowska E, Harazny JM, Miszkowska-Nagorna E, Stefanski A, Graff B, Kunicka K, Swierblewska E, et al. Lumen narrowing and increased wall to lumen ratio of retinal microcirculation are valuable biomarkers of hypertension-mediated cardiac damage. Blood Press. 2019;29(2):70-79.
doi pubmed - Lumori BAE, Nuwagira E, Abeya FC, Araye AA, Masette G, Mondo CK, Okello S, et al. Association of body mass index with left ventricular diastolic dysfunction among ambulatory individuals with diabetes mellitus in rural Uganda: a cross-sectional study. BMC Cardiovasc Disord. 2022;22(1):279.
doi pubmed - Porel R, Shyama S, Ahmad S, Kumar N, Ahmad S, Biswas R, Ojha VS. Can glycated haemoglobin (HbA1c) be used as a predictor of left ventricular diastolic dysfunction in non-hypertensive patients with newly diagnosed type 2 diabetes mellitus: a cross-sectional study at a tertiary care centre in Eastern India. BMJ Open. 2024;14(3):e081269.
doi pubmed - Silverii GA, Toncelli L, Casatori L, Bossini R, Nannelli F, Pala L, Mannucci E. Assessment of left ventricular global longitudinal strain in patients with type 2 diabetes: Relationship with microvascular damage and glycemic control. Nutr Metab Cardiovasc Dis. 2022;32(4):994-1000.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.