| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 5, May 2025, pages 247-255
Does Receiving Information on Clinical Trials Affect Patients’ Condition?
Hideaki Shimadaa, e, Keisuke Okamurab, c, Tetsuji Ohyamad, Hidenori Uratac, Osamu Imakyurea
aClinical Research Support Center, Fukuoka University Chikushi Hospital, Chikushino, Fukuoka, Japan
bDepartment of Cardiology and Cardiovascular Center, Imamura Hospital, Tosu, Saga, Japan
cDepartment of Cardiovascular Diseases, Fukuoka University Chikushi Hospital, Chikushino, Fukuoka, Japan
dBiostatistics Center, Kurume University, Kurume, Fukuoka, Japan
eCorresponding Author: Hideaki Shimada, Clinical Research Support Center, Fukuoka University Chikushi Hospital, Chikushino, Fukuoka 818-8502, Japan
Manuscript submitted March 28, 2025, accepted May 15, 2025, published online May 28, 2025
Short title: Effect of Clinical Trial Information on Patients
doi: https://doi.org/10.14740/jocmr6252
| Abstract | ▴Top |
Background: When performing clinical trials on lifestyle-related diseases at our hospital, we have sometimes experienced patients who fulfilled the inclusion criteria at the time of receiving an explanation of the trial but who no longer met the criteria when they arrived to provide their consent to participate 1 month later. In some of these cases, we noticed that the patient’s lifestyle subsequently improved. Therefore, we hypothesized that receiving information on clinical trials may affect lifestyle-related diseases.
Methods: We enrolled patients aged 85 years or younger who received information on a double-blind randomized clinical trial on treatment-resistant hypertension (R-HT) or one on diabetic nephropathy. In these patients, we evaluated whether the trial information affected a range of variables. In addition, we compared the rate of change in variables between two groups, i.e., patients who became ineligible to participate and were not randomized (early dropouts) and patients who decided to participate and were randomized (patients randomized to treatment). We also conducted a questionnaire on changes in patients’ motivation level, health awareness and behavior, and expectations and concerns and evaluated changes from before to after receiving an explanation of the trial.
Results: Seven patients who received an explanation of the R-HT trial and 14 who received an explanation of the diabetic nephropathy trial participated in the present study. The only significant change in any variable was in the R-HT clinical trial, where systolic and diastolic blood pressure significantly decreased in the early dropout group. There were no significant differences between the two groups in the rate of change in variables. After receiving information about one of the studies, patients who became more proactive or involved in changing their health-related behavior, such as their exercise, eating, and drinking habits, increased in both groups.
Conclusions: Receiving information on a clinical trial on hypertension can significantly affect blood pressure. Future research should examine whether providing information on clinical trials on other lifestyle-related diseases motivates patients to improve their lifestyles.
Keywords: Clinical trial; Lifestyle diseases; Health awareness
| Introduction | ▴Top |
In clinical trials of lifestyle-related diseases, some patients drop out before the observation period (i.e., after receiving an explanation of the trial but before providing informed consent) or during the observation period (i.e., after providing informed consent but before being randomized).
After receiving clinical trial information, either while considering participating in the clinical trial or during the pre-randomization observation period, patients may experience changes in various variables as they become more conscious of their health and try to improve their lifestyle, and these changes may result in patients no longer fulfilling the clinical trial participation criteria at the time of obtaining informed consent. Similarly, when performing clinical trials on lifestyle-related diseases at our hospital, we have sometimes experienced patients who were no longer eligible after receiving information about a trial. Furthermore, we noticed that some of these patients subsequently improved their health-related lifestyle.
If the number of patients who no longer fulfill clinical trial inclusion criteria increases, the actual dropout rate will be higher than the expected dropout rate, which will increase the period until the planned number of participants is recruited. Although many patient awareness surveys on clinical trial participation have been conducted to date [1-4], few reports mention whether involvement in clinical trials itself affects diseases. We hypothesized that receiving information on clinical trials may affect lifestyle-related diseases, so while investigating changes in patients’ health awareness, motivation, and behavior due to involvement in clinical trials, we conducted a pilot study to examine whether receiving information on clinical trials affects lifestyle-related diseases.
| Materials and Methods | ▴Top |
Study population
Study participants were patients aged 85 years or younger who received an explanation of one of four clinical trials on lifestyle-related diseases. Two trials evaluated a device for treatment-resistant hypertension (R-HT) [5, 6], and the other two, a drug for diabetic nephropathy. Both trials were ongoing at our hospital at the time of September 2017.
As of July 2017, a total of 35 patients had given informed consent to participate in the current study, so the target was set at 35 cases.
The study protocol was approved by the Fukuoka University Medical Ethics Review Board (approval no. R17-047), and written informed consent was obtained from patients. Informed consent for each patient to participate in this study was provided by a physician and a clinical research coordinator (CRC). This study was conducted in accordance with the Japanese guidelines for clinical research, “Ethical guidelines for medical and biological research involving human subjects,” which are derived from the Declaration of Helsinki.
Variables and evaluations
In the R-HT trial, we evaluated blood pressure (BP), hemoglobin A1c, creatinine (Cre), and estimated glomerular filtration rate (eGFR); and in the diabetic nephropathy trial, BP, hemoglobin A1c, potassium, Cre, eGFR, urinary protein, and the urine albumin-to-creatinine ratio. In the present study, we compared these variables in patients before and after they received the clinical trial explanation (but before randomization) and between early dropouts (EDOs), i.e., patients who became ineligible to participate in the clinical trial after receiving an explanation of the study but before providing informed consent and patients who dropped out during the pre-randomization observation period after providing informed consent, and patients randomized to treatment (PRTs) (Fig. 1). In addition, we conducted a questionnaire survey at two time points (Fig. 1) to determine whether patients who provided informed consent were motivated to improve their health by receiving information about the clinical trial, how they accepted and practiced guidance given during routine medical treatment, and their expectations and concerns about the clinical trials.
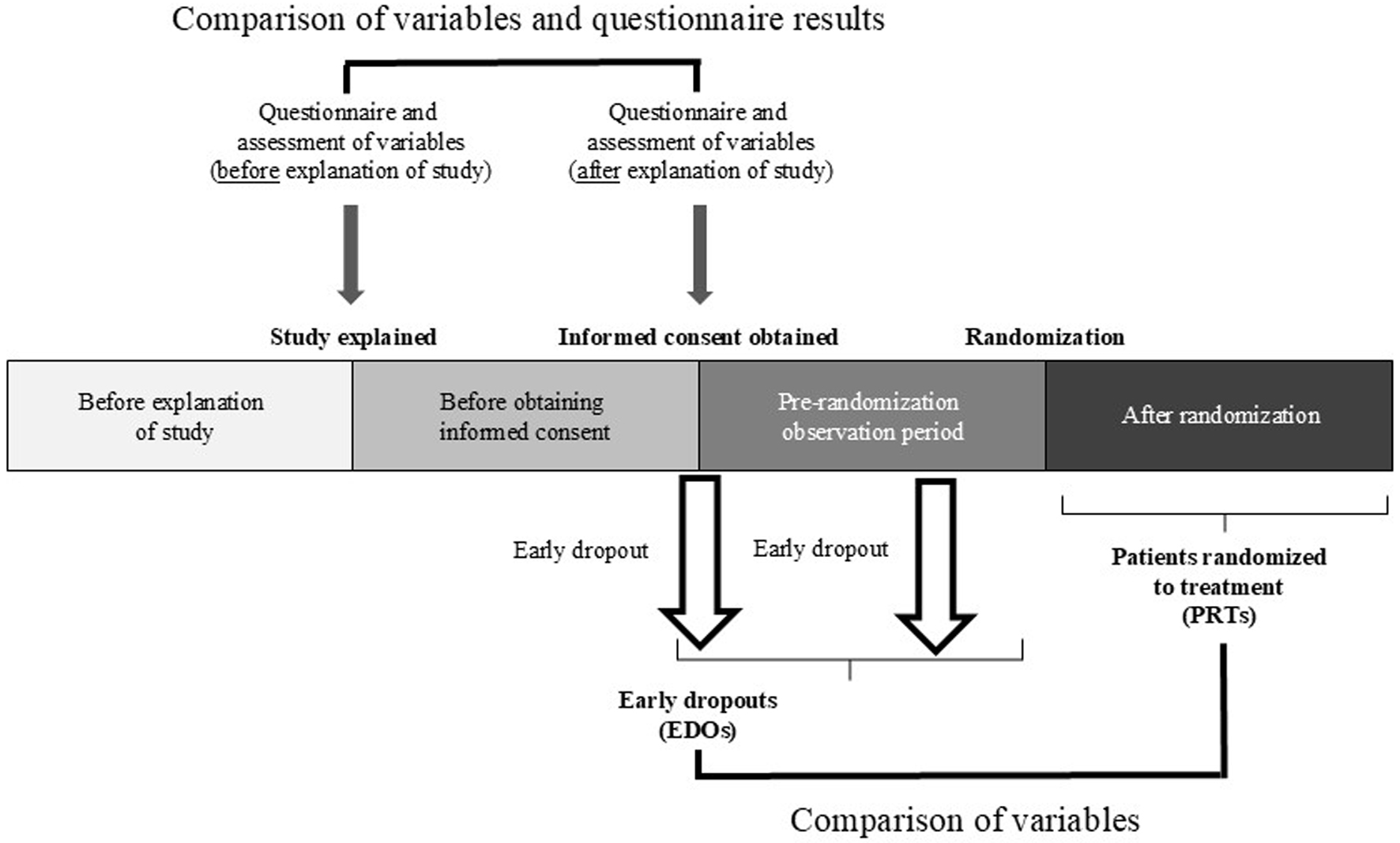 Click for large image | Figure 1. Clinical trial design. |
Endpoints
The main endpoint was the change in variables from before to after receiving the explanation of the clinical trial, and the secondary endpoints were the difference in the rate of change of variables between the EDO and PRT groups and the change in the questionnaire scores from before the trial explanation to after providing informed consent in the EDO patients who provided informed consent and in the PRT group.
Statistical analysis
Data were analyzed at the Biostatistics Center, Kurume University, Kurume, Fukuoka, Japan, with the statistical analysis software R. To evaluate the main endpoint, we transformed the data logarithmically and used a paired t-test to evaluate the changes from before to after the trial explanation. Secondary endpoints were evaluated with a covariance analysis model, with the logarithmically transformed rate of change as the objective variable and the values before and after explanation of the study as covariates. For each endpoint, cases with missing data were excluded from the analysis. The significance level was set at 5% on both sides, and no adjustment was made for multiplicity because this was an exploratory study.
| Results | ▴Top |
Data were collected from September 2017 to March 2019. Table 1 shows the patient characteristics. Informed consent to participate in the present study was obtained from a total of 21 patients: seven who were informed about the R-HT trial and 14 who were informed about diabetic nephropathy trial. Of these 21 patients, six were EDOs and 15 were PRTs. The six EDOs included one patient who withdrew consent because of family objections and five patients in whom the following values were outside those specified for inclusion in the trial: BP (three patients), potassium and urine albumin-to-creatinine ratio (one patient), and eGFR (one patient).
 Click to view | Table 1. Patient Characteristics |
As shown in Table 2, in the overall group, there were no significant changes in variables from before to after the explanation of the clinical trial. However, Table 3 shows that BP changed significantly from before to after the explanation of the R-HT clinical trial. In this trial, systolic blood pressure (SBP) and diastolic blood pressure (DBP) both decreased significantly (SBP, from 173 to 152 mm Hg, P < 0.05; DBP, from 99 to 86 mm Hg, P < 0.05).
 Click to view | Table 2. Changes in Variables From Before to After Receiving an Explanation of a Double-Blind Randomized Clinical Trial on R-HT or Diabetic Nephropathy |
 Click to view | Table 3. Changes in Blood Pressure From Before to After Receiving an Explanation of a Double-Blind Randomized Clinical Trial on R-HT or Diabetic Nephropathy |
Table 4 shows a comparison of the rate of change of variables between the EDO and PRT groups. The geometric mean and coefficient of variation of the rate of change were calculated for variables in both groups, and no significant intergroup difference was found.
 Click to view | Table 4. Rate of Change in Variables Related to Participation Criteria for EDOs and PRTs |
Questionnaire survey results were obtained only in patients in the EDO group who provided informed consent and in the PRT group. Table 5 shows changes in the guidance provided by medical staff from before to after explanation of the clinical trial in these patients. In all cases, the number of patients who voluntarily changed aspects of their lifestyle, such as diet, exercise, and drinking, increased after receiving an explanation of the study; however, few patients made efforts to reduce their salt intake. Table 6 shows the changes in expectations before receiving the explanation to after providing informed consent to participate. Participants mentioned that by joining the clinical trial, they expected to receive detailed medical examinations and tests and to be able to face their illness more positively. Before the clinical trial had been explained, patients were concerned about side effects, but once they had received an explanation of the trial and provided informed consent, the percentage who were concerned decreased significantly (Table 7).
 Click to view | Table 5. Changes From Before to After Receiving the Explanation of the Clinical Trial in the Type of Guidance Given by Medical Staff and the Implementation of Guidance by Patients |
 Click to view | Table 6. Changes in the Number of Patients Ranking Expectations as First, Second, or Third Most Important From Before to After Receiving an Explanation of a Double-Blind Randomized Clinical Trial on Treatment-Resistant Hypertension or Diabetic Nephropathy |
 Click to view | Table 7. Change in the Number of Patients Ranking Anxieties as First, Second, or Third Most Important From Before to After Receiving an Explanation of a Double-Blind Randomized Clinical Trial on Treatment-Resistant Hypertension or Diabetic Nephropathy |
The level of recognition that being involved in a clinical trial provides motivation for working to improve health was similar in the EDO and PRT groups (Table 8).
 Click to view | Table 8. Comparison of Changes in Health-Related Behavior in Patients Who Received an Explanation of a Double-Blind Randomized Clinical Trial on Treatment-Resistant Hypertension or Diabetic Nephropathy |
| Discussion | ▴Top |
After the current Good Clinical Practice (GCP) guidelines came into effect, fewer clinical trials were performed in Japan and drug lag worsened. According to the Pharmaceuticals and Medical Devices Agency in Japan, various efforts were subsequently made to eliminate drug lag, which improved from 3.3 years in 2009 to 1.7 years in 2015 [7, 8]. Drug lag broadly includes review lag and development lag. Although the former was greatly reduced from about 9.5 months to less than 1 month, the latter decreased only from 2.5 to 1.7 years. The factors that influence development lag include the length of the pre-trial and case enrollment periods. Although efforts to shorten the pre-trial period, such as standardizing application formats, have had some success, the length of the enrollment period remains a major issue. Despite educational activities to raise awareness of the benefits of clinical trial participation, enrollment still relies heavily on the efforts of medical institutions.
According to GCP Article 6, when planning a clinical trial, the trial organizer must estimate the dropout rate and confirm that the medical institutions involved can secure sufficient cases within the implementation period.
Although large-scale clinical trials are important for accumulating evidence and advancing medicine, they require large amounts of research funding. Therefore, most trials are funded by pharmaceutical companies, even though this can cause potential bias and conflicts of interest among researchers [9], and companies can incur economic losses when participant enrollment is slower than expected.
The present study found that when patients received an explanation of the R-HT clinical trial, BP subsequently decreased. This decrease led to approximately half of those who consented to the study dropping out, suggesting that the explanation of the trial may have affected their health awareness. Of course, this outcome was beneficial for patients, but it is not desirable when aiming to enroll a certain number of cases within a certain period in a clinical trial. In fact, the enrollment period for the REQUIRE study on R-HT was extended from 0.5 to 3.75 years [10]. Of the 411 patients who provided informed consent to participate in the REQUIRE study, 268 dropped out, and 180 of these dropouts were due to a change in BP. In addition, a large reduction in BP was shown in the control group, which was not treated with the device, suggesting that the trial explanation may have played a large role in the reduction of BP. In the control group of the REQUIRE study, the reduction in BP was affected by extremely poor adherence to antihypertensive medication [6]. BP is susceptible to drug adherence, and provision of sufficient information during the informed consent process may have a large effect on treatment compliance and thus BP. For example, in the HERB-Digital Hypertension 1 study, an application that provided lifestyle guidance lowered BP in hypertensive patients, and the authors suggested that lifestyle guidance interventions appear to be particularly effective in hypertension [11].
In addition, the LIGHT trial, which used smart phones, conducted a lifestyle intervention in which patients entered their daily home BP and received motivational messages, and it was reported that this effectively controlled patients’ BP and reduced their cardiovascular risk [12]. Furthermore, a subanalysis of LIGHT trial revealed that proB-type natriuretic peptide (proBNP) and left ventricular mass index decreased among patients with lower BP [13]. These findings indicated that lifestyle interventions using mobile technology may offer new insights into the management of primary prevention against cardiovascular diseases [12].
When performing clinical trials on lifestyle-related diseases, especially hypertension, it is essential to design a protocol that includes a thorough explanation of the trial and double-blind testing. Factors that influence patient participation in clinical trials include research objectives, schedule, and medical cost burden [14]; patient personal characteristics, enabling factors that involve patient-centered attitudes or circumstances, and aversion [15]; treatment effects and side effects [3]; and knowledge and awareness of exams, religious beliefs, transportation, childcare, and access to medical care [16].
Many patients spontaneously express a positive attitude towards clinical trials, believing that they are necessary for the development of medicine and that participation is a moral obligation [1, 3]. On the other hand, patients feel anxious when participating in clinical trials [14], and they are particularly likely to express discomfort with randomization and the use of placebos [3]. Furthermore, patients do not trust information obtained from the media and appear not to trust the pharmaceutical industry [3]. In this study, patients initially had many concerns about side effects before the clinical trial, but after they had received an explanation of the clinical trial, those concerns tended to subside.
In clinical trials, patients are given detailed explanations by medical professionals, and this process may help them to face their diseases more positively. The use of quantitative information, such as specific numbers and graphs, in the explanatory materials for clinical trials is also believed to help patients understand the clinical trial and the disease better than qualitative information with verbal expressions [17].
Appropriately understanding and resolving patients’ positions eliminates psychological barriers to clinical trials, reduces health inequalities, and improves health [15, 18]. The questionnaire used in the present study showed that an increasing number of patients voluntarily improved their lifestyle after receiving an explanation of the clinical trial, suggesting that receiving information about clinical trials is an opportunity for health management.
In both the EDO and PRT groups in the present study, receiving information about the clinical trials resulted in 76% of patients becoming more committed to improving their health. The effect may not have been large enough to reach statistical significance because the trial period was too short.
Patients can easily improve their lifestyles in terms of diet, exercise, and drinking alcohol, but they may find it difficult to sustain their motivation in the long term. We were concerned that changes in variables resulting from the intervention of the CRC may introduce a bias and affect the results of the trial, but the present results indicate that the intervention did not have this effect.
Conclusion
Although most variables did not change after receiving an explanation of the clinical trials, receiving information on a randomized double-blind trial on R-HT led to a significant change in BP. This finding suggests that information on a clinical trial may help to improve patient health.
Study limitations
This study has some limitations. First, this study had a small sample size because it only surveyed participants at our hospital. In particular, the number of subjects in the R-HT trial was limited to 7 among the two trials. Therefore, the topic needs to be investigated in a larger number of cases. Second, because we observed variations in variables in each disease, in the future, each disease needs to be evaluated separately. Last, we did not take into account the influence of diet and exercise therapy prior to the clinical trial.
Acknowledgments
We thank Ichiro Abe, Kunihisa Kobayashi, Izumi Kukita, Tamaki Maki, and Chika Inoue for their excellent assistance.
Financial Disclosure
The authors have no financial disclosure or funding conflict of interest directly relevant to the content of this article.
Conflict of Interest
The authors have no conflict of interest directly relevant to the content of this article.
Informed Consent
Written informed consent was obtained from patients.
Author Contributions
Hideaki Shimada: study design, data collection, data interpretation, manuscript preparation, and literature search. Keisuke Okamura: study design, data collection, statistical analysis, data interpretation, manuscript preparation, and literature search. Tetsuji Ohyama: statistical analysis and data interpretation. Hidenori Urata: study design. Osamu Imakyure: study design and study supervision.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
BP: blood pressure; CRC: clinical research coordinator; Cre: creatinine; CVD: cardiovascular disease; DBP: diastolic blood pressure; EDOs: early dropouts; eGFR: estimated glomerular filtration rate; GCP: Good Clinical Practice; LVIM: left ventricle mass index; pro-BNP: pro-brain natriuretic peptide; PRTs: patients randomized to treatment; R-HT: treatment-resistant hypertension; SBP: systolic blood pressure
| References | ▴Top |
- Kaoru Miyata, Keiko Sato. Attitude of participants toward clinical trials, when they were approached about entering a clinical traial. Jpn J Clin Pharmacol Ther 2014;45(1):11-15 (in Japanese).
- Asumi Kojima, Shoji Yamazaki Taku Yoshio. Quatitative analysis of perception canges in clivical trial participants during clinical trials. Jpn J Clin Pharmacol Ther 2017;48(2):41-49 (in Japanese, abstract in English).
- Madsen SM, Holm S, Riis P. Attitudes towards clinical research among cancer trial participants and non-participants: an interview study using a Grounded Theory approach. J Med Ethics. 2007;33(4):234-240.
doi pubmed - Weckstein DJ, Thomas CA, Emery IF, Shea BF, Fleury A, White ME, Chase E, et al. Assessment of perceived cost to the patient and other barriers to clinical trial participation. J Oncol Pract. 2011;7(5):330-333.
doi pubmed - Mauri L, Kario K, Basile J, Daemen J, Davies J, Kirtane AJ, Mahfoud F, et al. A multinational clinical approach to assessing the effectiveness of catheter-based ultrasound renal denervation: The RADIANCE-HTN and REQUIRE clinical study designs. Am Heart J. 2018;195:115-129.
doi pubmed - Kario K, Kai H, Nanto S, Yokoi H. Anti-hypertensive medication adherence in the REQUIRE trial: post-hoc exploratory evaluation. Hypertens Res. 2023;46(8):2044-2047.
doi pubmed - Pharmaceuticals and Medical Devices Agency. Estimation of Drug Lag: 2009 to 2013. Accessed [2017.9.15]. https://www.pmda.go.jp/files/000206039.pdf (in Japanese).
- Pharmaceuticals and Medical Devices Agency. Estimation of Drug Lag: 2011 to 2015. Accessed [2017.9.15]. https://www.pmda.go.jp/files/000215837.pdf (in Japanese).
- Sawata H, Tsutani K. How can the evidence from global large-scale clinical trials for cardiovascular diseases be improved? BMC Res Notes. 2011;4:222.
doi pubmed - Kario K, Yokoi Y, Okamura K, Fujihara M, Ogoyama Y, Yamamoto E, Urata H, et al. Catheter-based ultrasound renal denervation in patients with resistant hypertension: the randomized, controlled REQUIRE trial. Hypertens Res. 2022;45(2):221-231.
doi pubmed - Kario K, Harada N, Okura A. The first software as medical device of evidence-based hypertension digital therapeutics for clinical practice. Hypertens Res. 2022;45(12):1899-1905.
doi pubmed - Tekkesin AI, Hayiroglu MI, Cinier G, Ozdemir YS, Inan D, Yuksel G, Pay L, et al. Lifestyle intervention using mobile technology and smart devices in patients with high cardiovascular risk: A pragmatic randomised clinical trial. Atherosclerosis. 2021;319:21-27.
doi pubmed - Hayiroglu MI, Cinier G, Pay L, Yuksel G, Durak F, Palice A, Ayhan G, et al. The relation between average 1-year home blood pressure and the change in pro-BNP and left ventricle mass index. Blood Press Monit. 2022;27(5):327-333.
doi pubmed - Natsume O, Kabashima S, Nakazato J, Yamamoto-Hanada K, Narita M, Kondo M, Saito M, et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;389(10066):276-286.
doi pubmed - McMahon VA, Matthews S, Capper H, Chudleigh JB, McLachlan CS. Understanding decision and enabling factors influencing clinical trial participation in Australia: a view point. Asian Pac J Cancer Prev. 2011;12(11):3153-3156.
pubmed - Rivers D, August EM, Sehovic I, Lee Green B, Quinn GP. A systematic review of the factors influencing African Americans' participation in cancer clinical trials. Contemp Clin Trials. 2013;35(2):13-32.
doi pubmed - Man-Son-Hing M, O'Connor AM, Drake E, Biggs J, Hum V, Laupacis A. The effect of qualitative vs. quantitative presentation of probability estimates on patient decision-making: a randomized trial. Health Expect. 2002;5(3):246-255.
doi pubmed - Kirkpatrick CE, Hu S, Lee N, Hong Y, Lee S, Hinnant A. Overcoming black Americans' psychological and cognitive barriers to clinical trial participation: effects of news framing and exemplars. Health Commun. 2023;38(12):2663-2675.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.