| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Case Report
Volume 17, Number 10, October 2025, pages 595-600
A Challenging Case of Immune-Related Organizing Pneumonitis Following Programmed Cell Death 1 Inhibitor Therapy in Non-Small Cell Lung Cancer
Giovanni Paolozzia, Roberta Gualtierottia, b, Raffaella Rossiob, Barbara Ferrarib, Niccolo Bittob, Flora Peyvandia, b, c
aDepartment of Pathophysiology and Transplantation, Universita degli Studi di Milano, Milan, Italy
bFondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy
cCorresponding Author: Flora Peyvandi, Department of Pathophysiology and Transplantation, Universita degli Studi di Milano, Milan, Italy
Manuscript submitted April 13, 2025, accepted August 6, 2025, published online October 29, 2025
Short title: Immune-Related Organizing Pneumonitis in NSCLC
doi: https://doi.org/10.14740/jocmr6255
| Abstract | ▴Top |
The immune system plays a vital role in defending the body against infections and tumors, inspiring the development of innovative therapies like immune checkpoint inhibitors (ICIs) that have transformed the treatment of advanced cancers. Pembrolizumab, a monoclonal antibody targeting the programmed cell death 1 (PD-1) receptor, is a powerful ICI effective against various malignancies but frequently associated with immune-related adverse events (irAEs). In this report, we present a case of organizing pneumonitis that developed 3 months after initiation of pembrolizumab treatment for non-small cell lung cancer (NSCLC). A 64-year-old woman with NSCLC, undergoing maintenance therapy with pembrolizumab, presented with multiple lung consolidations. Her medical history included thalassemia minor, a pre-pyloric ulcer, hiatal hernia, and a history of smoking. Extensive microbiological testing, including bronchoalveolar lavage, was negative, and her condition did not improve with broad-spectrum antibiotics. This led to a suspected diagnosis of pembrolizumab-induced pneumonitis. Treatment with high-dose corticosteroids resulted in full clinical and radiological resolution. This case underscores the importance of monitoring for irAEs during ICI therapy, as differential diagnosis between immunotherapy-induced organizing pneumonia and tumor progression is challenging in patients with advanced lung cancer.
Keywords: Pneumonitis; Pembrolizumab; Immune checkpoint inhibitors; Tumor immunology; Cancer treatment
| Introduction | ▴Top |
The immune system plays a central role in protecting the body against infections and tumors. The ability to distinguish between “self” and “non-self” is the foundation of both innate and adaptive immunity, which work together to identify and eliminate abnormal or infected cells [1]. Based on the evidence that tumors can evade immune surveillance through various mechanisms, the immune system has become a crucial target in oncological research. This has led to the development of novel therapeutic strategies, such as immune checkpoint inhibitors (ICIs), a groundbreaking innovation in cancer treatment in patients with advanced tumors [2].
Tumor cells evade immune surveillance and advance through different mechanisms, including the activation of immune checkpoint pathways that suppress antitumor immune responses [3]. ICIs revitalize host antitumor immune responses by disrupting co-inhibitory signaling pathways and fostering immune-mediated elimination of tumor cells [4].
Programmed cell death 1 (PD-1) is expressed on activated T cells (both CD8+ and CD4+), B cells, monocytes, dendritic cells, natural killer and T regulatory (Treg) cells [5, 6]. Inflammatory cytokines associated with tumor growth induce the expression of programmed cell death ligand 1 (PD-L1) on various cell types and PD-L2 mainly on antigen-presenting cells [7]. The interaction between PD-1 and PD-L1 or PD-L2 negatively impacts the function of T and B cells, resulting in reduced function, impaired cytokine production and antibody formation [8, 9]. This inhibition contributes to the regulation of autoimmunity, anti-tumor responses, and anti-infectious immunity [10].
ICIs have shown significant efficacy in tumors such as melanoma and lung cancer. However, their mechanism of action has an intrinsic risk of unrestrained T-cell activation, which is the hallmark of the ICI immune-related adverse events (irAEs) [11]. ICI-associated irAEs are various and may range from mild dermatological and gastrointestinal involvement to severe endocrine, pulmonary and cardiac complications [12].
Although ICI-related pneumonitis has been previously described in the literature, this report aims to provide insights particularly relevant to the internal medicine setting, where the case was observed and where community- or hospital-acquired pneumonias are common and often considered in differential diagnosis. We describe a case of grade 3 pneumonitis related to pembrolizumab, a rare but potentially life-threatening adverse event, characterized by heterogeneous clinical and radiological features. Our experience highlights the diagnostic challenges encountered by clinicians outside oncology-specialized units and emphasizes the importance of a structured diagnostic approach and adherence to current guidelines for the appropriate management of irAEs.
| Case Report | ▴Top |
A 64-year-old woman with stage IV non-small cell lung cancer (NSCLC) was admitted to our hospital with worsening dyspnea, a persistent productive cough with whitish sputum, and left scapular pain lasting for 2 days, unrelieved by paracetamol. She was a former hospital healthcare assistant. Her body mass index (BMI) was 25.7, with a weight of 70 kg and a height of 165 cm.
Her medical history was notable for thalassemia minor, a pre-pyloric ulcer, hiatal hernia, and a past history of tobacco smoking (20 cigarettes per day from age 20, totaling 43 pack-years).
The patient had been diagnosed 1 year earlier with NSCLC, exhibiting a K-Ras (KRAS) gene c.34G>T mutation in exon 2 and a PD-L1 expression level of 30%. Radiological staging revealed metastases in the spine, iliac wing, ribs, femur, and adrenal glands. Three months earlier, she had completed four cycles of chemotherapy with carboplatin, pemetrexed, and pembrolizumab, along with palliative radiotherapy targeting spinal metastases. Following remission confirmed by positron emission tomography (PET), she received maintenance therapy with pemetrexed and pembrolizumab for an additional four cycles, with the last cycle administered 1 month prior to hospitalization (Fig. 1).
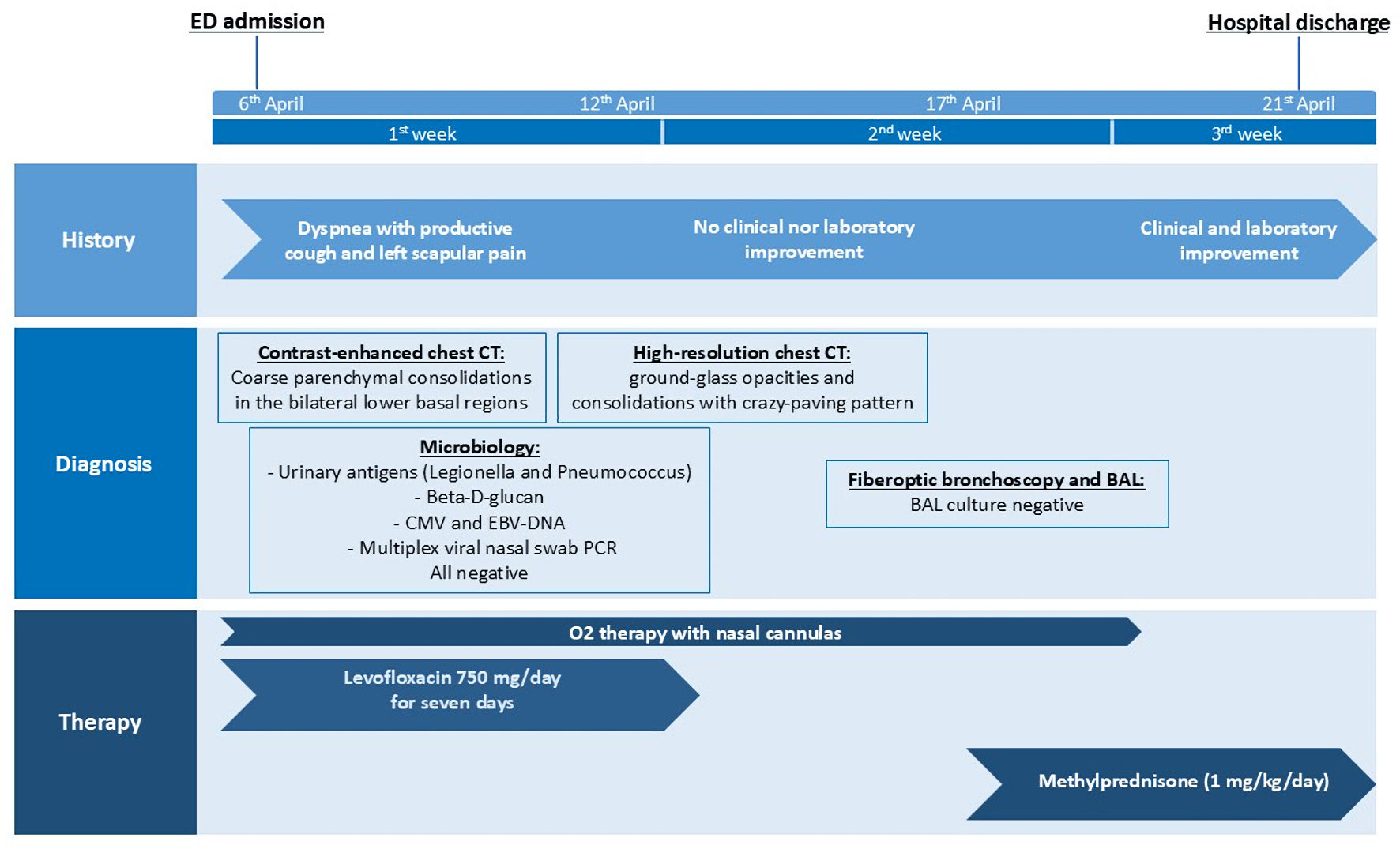 Click for large image | Figure 1. Timeline of the clinical case. CT: computed tomography; BAL: bronchoalveolar lavage; CMV: cytomegalovirus; EBV: Ebstein-Barr virus; ED: emergency department; PCR: polymerase chain reaction. |
Physical examination
Upon referral, the patient presented with worsening dyspnea on mild exertion, a blood pressure of 150/80 mm Hg, heart rate of 100 beats per minute (bpm), and peripheral oxygen saturation at 90%. The axillary temperature was 37.5 °C. Low-flow oxygen therapy was initiated at 2 L/min. Cardiac examination revealed regular, rhythmic heart tones within normal frequency and no heart murmurs. Pulmonary assessment showed diminished vesicular breath sounds at the lower right base and bilateral basal crepitations.
Diagnostic assessment
The nasopharyngeal swab test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen was negative. Blood levels of electrolytes and glucose, along with liver and kidney function tests, were within normal limits. Laboratory tests showed a white cell count of 6,800/µL (neutrophils 5,800/µL, lymphocytes 710/µL), hemoglobin level of 7.4 g/dL, and platelet count of 121,000/µL. C-reactive protein (CRP) was 0.97 mg/dL, with a prothrombin time (PT) of 1.37 and activated partial thromboplastin time (aPTT) of 1.23.
A contrast-enhanced chest computed tomography (CT) scan ruled out pulmonary embolism, revealing multiple inflammatory consolidations in the basal segments of the bilateral lower lobes and the apical segment of the left upper lobe, consistent with an inflammatory process [13]. Coarse fibrotic changes were noted in both apices, with thickened intralobular septa showing a fibrotic appearance. A small subpleural micronodule of nonspecific significance was also observed in the anterior segment of the left upper lobe.
The patient was subsequently transferred to our Internal Medicine Department. Despite a 7-day course of levofloxacin, she continued to experience persistent high fever and unresolved respiratory insufficiency. Further nasopharyngeal swabs were tested for metapneumovirus, respiratory syncytial virus A and B, influenza virus A (H1, A H3), parainfluenza virus (1, 2, 3, 4), adenovirus, coronavirus (229E, NL63, OC43), rhinovirus (A/B/C), enterovirus, and bocavirus, all yielding negative results. Urinary antigen tests for Legionella spp. and Streptococcus pneumoniae were also negative. To further investigate, a bronchoalveolar lavage (BAL) was performed, with microbiological tests for cytomegalovirus, Aspergillus, Pneumocystis jirovecii, fungi, and galactomannan all yielding negative results. Despite appropriate antibiotic therapy, inflammatory markers remained elevated (CRP 8 mg/dL).
Therefore, we revised the potential differential diagnosis of tumor progression. The most recent PET-CT, conducted about 4 months before admission, showed a significant reduction in metabolic activity of the known left lung lesion and skeletal lesions, along with near-complete resolution of spinal and right adrenal lesions. This response to the initial four cycles of immunochemotherapy, which led to maintenance therapy, made the findings from the high-resolution chest CT inconsistent with likely tumor progression.
After a multidisciplinary discussion with our oncology team, who played a crucial role in the diagnostic and therapeutic process, we suspected an ICI-associated pneumonitis.
Supporting this hypothesis, the initial CT scan in the emergency department revealed fibrotic streaks in the middle lobe and lingula, along with thickened intralobular septa with a fibrotic appearance at the apices, in addition to the previously noted consolidations. A subsequent high-resolution computed tomography (HRCT) demonstrated the expansion of the previous consolidations in the apical and posterior regions of the upper lobes and the basal regions of the lower lobes, predominantly on the right. New ground-glass opacities and a crazy-paving pattern in the upper and lower lobes were also evident (Fig. 2).
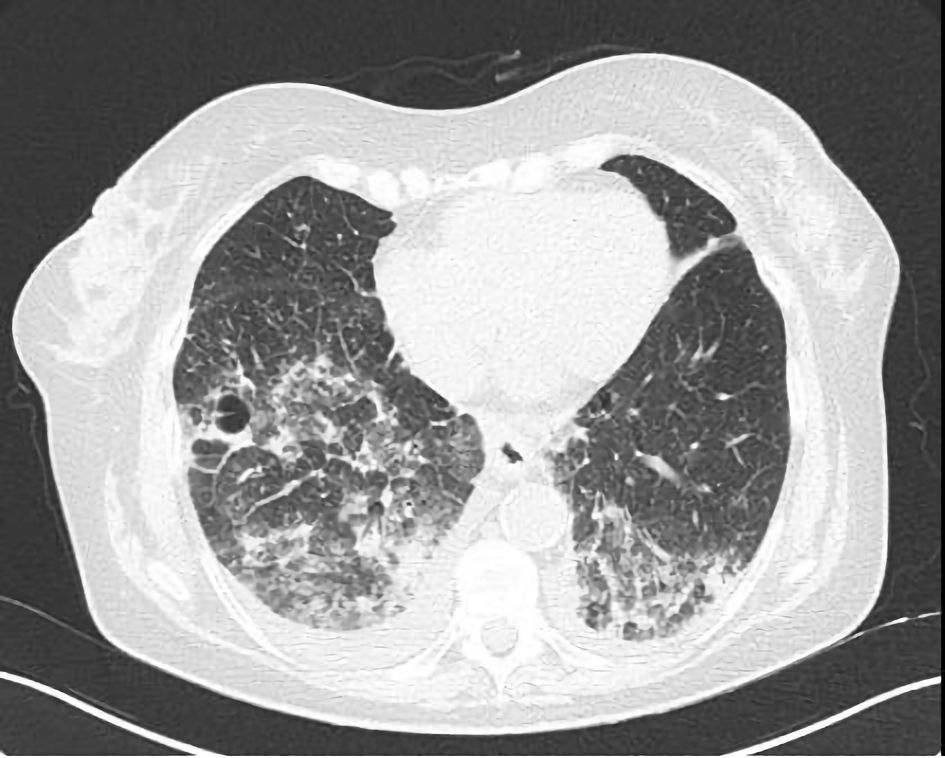 Click for large image | Figure 2. Chest computed tomography scan showing ground-glass opacities, pleural effusion and a crazy-paving pattern in both the lower lobes. |
Therapeutic interventions
After a final consultation with oncology and infectious disease specialists, a diagnosis of pembrolizumab-induced pneumonitis was made. We started an intravenous immunosuppressive therapy with methylprednisolone at 1 mg/kg/day, which led to a marked clinical improvement, including weaning off oxygen therapy within 48 - 72 h and resolution of fever. After a rapid recovery, the patient was discharged at home with instructions to continue tapered oral corticosteroid therapy based on clinical progress during subsequent outpatient follow-ups.
Follow-up and outcomes
Following hospital discharge, a staging PET-CT revealed persistent disease in the lower lobe of the lung with reduced metabolic activity. Based on oncological reassessment, systemic therapy with pemetrexed and pembrolizumab was resumed. Exertional and nocturnal respiratory insufficiency persisted. Treatment was later continued with pemetrexed alone, but complications including anemia, recurrent pneumonias, sepsis, acute-on-chronic kidney injury, and deep vein thrombosis led to repeated hospitalizations and clinical deterioration. Given the patient’s declining performance status and limited treatment benefit, oncologic therapy was discontinued, and palliative care was initiated; unfortunately, the patient eventually died a few months later.
| Discussion | ▴Top |
The introduction of ICIs has revolutionized the therapeutic landscape of oncology, offering durable responses in a variety of advanced malignancies. Cancer growth and progression are linked with immune suppression. The concept of immune surveillance was first proposed by Paul Ehrlich in 1909 and later developed by Frank Macfarlane Burnet in the 1950s [14]. They both hypothesized the immune system ability to recognize and destroy emerging tumor cells before they can form clinically evident tumors. However, some tumors can escape immune surveillance through a process known as immunoediting, which occurs in three stages: elimination, equilibrium, and escape [15]. Tumors adopt various strategies to evade immune elimination, such as the loss of immunogenic antigens or major histocompatibility complex molecules, the activation of Tregs, or expression of inhibitory ligands such as PD-L1, which interacts with the PD-1 receptor on T cells, inducing a state of functional impairment known as “T cell exhaustion” [16].
The blockade of PD-1 and cytotoxic T-lymphocyte antigen-4 (CTLA-4), two key immune checkpoints, has been shown to restore T-cell functionality and enhance the immune response against tumors [3]. These drugs act by releasing the “brakes” of the immune system, allowing T cells to recognize and destroy tumor cells more effectively [3].
However, their distinct mechanism of action, unlike cytotoxic chemotherapy, can lead to the uncontrolled reaction of T cells against self-antigens, manifesting as a broad spectrum of irAEs. These may affect any organ, most commonly the gastrointestinal tract, skin, liver, endocrine glands, and lungs. A high index of suspicion is required to promptly identify treatment-related toxicity. This warrants careful personalized management of these patients, with specific guidelines to monitor and treat these side effects [17]. The incidence, severity, and timing of irAEs depend on the ICI type, dosage, type of cancer, and patient-specific factors. Anti-PD-1/PD-L1 agents are generally associated with fewer and later-onset irAEs than anti-CTLA-4 therapies, while combination regimens significantly increase both risk and severity [17].
Pneumonitis is a rare but serious and potentially life-threatening adverse event associated with ICIs. Pembrolizumab-induced pneumonitis has a reported incidence of around 5% (range 1-7%) in monotherapy and slightly higher with combination therapy [18]. Nishino et al reported an overall incidence of 2.7% for pembrolizumab for all-grade pneumonitis and 0.8% for grade 3 or higher pneumonitis in the monotherapy group and less than 1% for CTLA-4 agents [19, 20].
The reported case aligns with previous reports of pembrolizumab-induced pneumonitis regarding the timing, clinical presentation, and favorable steroid response of immune-related pneumonitis induced by PD-1 inhibitors.
Patients with NSCLC, such as our patient, face higher odds of all-grade pneumonitis with anti-PD1 than melanoma patients, and a higher pneumonitis-related mortality [20, 21]. Prior thoracic radiotherapy does not significantly affect pneumonitis risk [22], whereas the impact of smoking status remains unclear [23, 24]. Pneumonitis can occur at any time during ICI therapy, with a highly variable onset ranging from a few days to 26 weeks and a median of 1 - 2 months after treatment initiation, but typically presents later than other irAEs [21, 25]. In our case, onset occurred 3 months after treatment with pembrolizumab, and symptoms included dyspnea, cough, and fever [13, 18, 21].
The diagnostic workup in this case was comprehensive and consistent with American Society of Clinical Oncology (ASCO) guidelines, with infection and tumor progression systematically excluded via microbiological testing and PET-CT [17]. A thorough diagnostic workup was conducted to rule out other causes, such as infections and tumor progression, following current guidelines for G2 or higher-grade pneumonitis [17]. Following the suggested workup, a CT scan with contrast enhancement was performed to exclude pulmonary embolism showing instead bilateral heterogeneous parenchymal consolidations and fibrotic changes [17].
Radiologically, the most frequently reported findings of ICI-related pneumonitis are that of cryptogenic organizing pneumonia, with ground-glass or consolidative opacities in peripheral or peribronchial distribution, followed by nonspecific interstitial pneumonia, with ground-glass opacities and reticular opacities primarily in the peripheral and lower lungs, with or without pleural effusion [13, 21, 24]. In our patient, after contrast-enhanced CT, a confirmation HRCT showed bilateral consolidations, ground glass opacities, and a crazy-paving pattern, consistent with pembrolizumab-induced organizing pneumonia. The radiological appearance of coronavirus disease 2019 (COVID-19) and ICI-induced pneumonitis may be similar [26]. In our case, COVID-19 was excluded immediately by PCR testing.
According to the Common Terminology Criteria for Adverse Events (CTCAE), our clinical case qualified as grade 3 pneumonitis (Table 1) [17]. Initial treatment with corticosteroids (2 - 4 mg/kg/day) is recommended for symptomatic ICI pneumonitis, as shown in Table 1. If symptoms do not improve or worsen after 48 h, additional immunosuppressive agents, such as infliximab, cyclophosphamide, intravenous immunoglobulin, or mycophenolate mofetil, should be considered [17]. In case of clinical improvement, steroids should be tapered over a period of at least 6 weeks.
 Click to view | Table 1. Recommended Management of Immune-Related Pneumonitis |
In our case, the rapid response to a high-dose corticosteroid therapy, with complete weaning from oxygen within 48 h, was consistent with reported positive outcomes within few days in > 80% of cases and confirmed the reversibility of grade 3 irAE with timely intervention [17, 21, 23]. When the clinical presentation is consistent with pneumonitis and the response to treatment is rapid, transbronchial biopsy is generally not deemed necessary, as reflected in ASCO guidelines [17]. In line with this and given the prompt clinical improvement, lung biopsy was not performed in our case.
Unlike steroid-refractory cases requiring second-line immunosuppression [16], no relapse or escalation of therapy was needed. While re-challenge with ICIs is controversial and patient-dependent, permanent discontinuation remains standard for grade 3 or higher events.
Overall, this case reinforces the clinical variability of ICI-related pneumonitis and the importance of early recognition, multidisciplinary evaluation, and guideline-driven management to prevent morbidity.
Conclusions
ICIs are powerful tools to stimulate antitumor immunity but require careful, personalized use.
The present case report highlights the importance of closely monitoring irAEs from the start of ICI treatment and emphasizes the clinical challenge to differentiate immunotherapy-induced organizing pneumonitis from tumor progression, particularly in patients with advanced lung cancer.
In the coming years, we advocate for the discovery of biomarkers - such as tumor genetic mutations, microenvironment factors, and patient genetics - to help predict or monitor ICI-related pneumonitis and other irAEs.
Acknowledgments
We thank the patient for agreeing to publish her anonymized data.
Financial Disclosure
This paper was partially supported by the Italian Ministry of Health - Bando Ricerca Corrente.
Conflict of Interest
R. Gualtierotti is on the advisory boards of Bayer, Roche, Sanofi, SOBI, and Novo Nordisk, and has participated in speaker bureau/educational meetings for Pfizer, SOBI, Takeda, and Novo Nordisk. F. Peyvandi is on the advisory boards of CSL Behring, Biomarin, Roche, Sanofi, Sobi, Pfizer and has participated in speaker bureau/educational meetings for Takeda, Spark TX, Sanofi. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Other authors declare no conflicts of interest.
Informed Consent
Informed consent was obtained.
Author Contributions
GP: conception of the work; acquisition, analysis and interpretation of data; drafting of the paper. RG: conception of the work; analysis and interpretation of data; drafting of the paper. RR, BF and NB: acquisition, analysis and interpretation of data; critical revision of the manuscript for important intellectual content. FP: analysis and interpretation of data; critical revision of the manuscript for important intellectual content. All authors contributed final approval of the version to be published.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
aPTT: activated partial thromboplastin time; ASCO: American Society of Clinical Oncology; BAL: bronchoalveolar lavage; CMV: cytomegalovirus; CRP: C-reactive protein; CT: computed tomography; CTCAE: common terminology criteria for adverse events; CTLA-4: cytotoxic T-lymphocyte antigen-4; DLCO: diffusing capacity of lung for carbon monoxide; EBV: Ebstein-Barr virus; ED: emergency department; HRCT high-resolution computed tomography: ; ICI: immune checkpoint inhibitor; irAEs: immune-related adverse events; IV: intravenous; IVIG: intravenous immunoglobulin; KRAS: K-Ras protein; NK: natural killer; NSCLC: non-small cell lung cancer; PCR: polymerase chain reaction; PD-1: programmed cell death 1; PET: positron emission tomography; PT: prothrombin time; Treg: T regulatory
| References | ▴Top |
- Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol. 2016;34:539-573.
doi pubmed - Buchbinder EI, Hodi FS. Impact of precision medicine in oncology: immuno-oncology. Cancer J. 2023;29(1):15-19.
doi pubmed - Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264.
doi pubmed - Ghorani E, Swanton C, Quezada SA. Cancer cell-intrinsic mechanisms driving acquired immune tolerance. Immunity. 2023;56(10):2270-2295.
doi pubmed - Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887-3895.
doi pubmed - Gutic B, Bozanovic T, Mandic A, Dugalic S, Todorovic J, Stanisavljevic D, Dugalic MG, et al. Programmed cell death-1 and its ligands: Current knowledge and possibilities in immunotherapy. Clinics (Sao Paulo). 2023;78:100177.
doi pubmed - Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219-242.
doi pubmed - Khoja L, Butler MO, Kang SP, Ebbinghaus S, Joshua AM. Pembrolizumab. J Immunother Cancer. 2015;3:36.
doi pubmed - Yearley JH, Gibson C, Yu N, Moon C, Murphy E, Juco J, Lunceford J, et al. PD-L2 Expression in human tumors: relevance to anti-PD-1 therapy in cancer. Clin Cancer Res. 2017;23(12):3158-3167.
doi pubmed - van der Vlist M, Kuball J, Radstake TR, Meyaard L. Immune checkpoints and rheumatic diseases: what can cancer immunotherapy teach us? Nat Rev Rheumatol. 2016;12(10):593-604.
doi pubmed - Kumar SS, McNeil CM. Pembrolizumab for the treatment of melanoma. Expert Rev Clin Pharmacol. 2015;8(5):515-527.
doi pubmed - Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chavez A, Keegan N, Khamashta MA, Lambotte O, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6(1):38.
doi pubmed - Johkoh T, Lee KS, Nishino M, Travis WD, Ryu JH, Lee HY, Ryerson CJ, et al. Chest CT diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: a position paper from the fleischner society. Radiology. 2021;298(3):550-566.
doi pubmed - Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337-365.
doi pubmed - Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565-1570.
doi pubmed - Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158-168.
doi pubmed - Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, Atkins MB, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39(36):4073-4126.
doi pubmed - Leroy V, Templier C, Faivre JB, Scherpereel A, Fournier C, Mortier L, Wemeau-Stervinou L. Pembrolizumab-induced pneumonitis. ERJ Open Res. 2017;3(2):00081-2016.
doi pubmed - Habib T, Abu-Abaa M, Kolman-Taddeo D. Nivolumab-induced organizing pneumonia in management of non-small cell lung carcinoma: a case report. Cureus. 2023;15(5):e39217.
doi pubmed - Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2(12):1607-1616.
doi pubmed - Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2(10):1346-1353.
doi pubmed - Hwang WL, Niemierko A, Hwang KL, Hubbeling H, Schapira E, Gainor JF, Keane FK. Clinical outcomes in patients with metastatic lung cancer treated with PD-1/PD-L1 inhibitors and thoracic radiotherapy. JAMA Oncol. 2018;4(2):253-255.
doi pubmed - Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709-717.
doi pubmed - Chuzi S, Tavora F, Cruz M, Costa R, Chae YK, Carneiro BA, Giles FJ. Clinical features, diagnostic challenges, and management strategies in checkpoint inhibitor-related pneumonitis. Cancer Manag Res. 2017;9:207-213.
doi pubmed - Raschi E, Gatti M, Gelsomino F, Ardizzoni A, Poluzzi E, De Ponti F. Lessons to be learnt from real-world studies on immune-related adverse events with checkpoint inhibitors: a clinical perspective from pharmacovigilance. Target Oncol. 2020;15(4):449-466.
doi pubmed - Chang HL, Wei PJ, Wu KL, Huang HL, Yang CJ. Checkpoint inhibitor pneumonitis mimicking COVID-19 infection during the COVID-19 pandemic. Lung Cancer. 2020;146:376-377.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.