| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 6, June 2025, pages 334-343
Survival After Partial Cystectomy Versus Radical Cystectomy for Non-Urothelial Carcinoma of the Bladder: A Population-Based Study
Shuang Liua, d, Tai Song Wangb, Ren Bin Yuana, c, d, e
aCollege of Medicine, Southwest Jiaotong University, Chengdu 610031, Sichuan, China
bDepartment of Urology, the Third People’s Hospital of Chengdu, Chengdu 610031, Sichuan, China
cDepartment of Urology, Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu 610031, Sichuan, China
dThese authors contributed equally to this work.
eCorresponding Author: Ren Bin Yuan, Department of Urology, Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan 610031, China
Manuscript submitted April 24, 2025, accepted June 25, 2025, published online June 30, 2025
Short title: PC vs. RC Survival in Stage T2N0M0 Bladder NUCB
doi: https://doi.org/10.14740/jocmr6263
| Abstract | ▴Top |
Background: The aim of this study was to compare cancer-specific survival (CSS) and overall survival (OS) after partial cystectomy (PC) versus radical cystectomy (RC) in patients with stage T2N0M0 non-urothelial carcinoma of the bladder (NUCB).
Methods: Data on patients with stage T2N0M0 NUCB treated with PC or RC were retrospectively retrieved from the Surveillance, Epidemiology, and End Results (SEER) database from 2007 to 2015. Propensity score matching (PSM) was used to create matched cohorts, which were used to calculate OS and CSS.
Results: Among 999 histologically confirmed NUCB patients (752 in PC group and 247 in RC group), significant differences were found in age, marital status, tumor-related features, and treatment modalities. After 1:1 PSM, 169 pairs were obtained. In the matched cohort, the RC group had significantly higher 1-year, 3-year, and 5-year OS and CSS rates than the PC group (OS: P = 0.002; CSS: P = 0.004). Cox regression analysis showed that older age, unmarried status, and PC were independent risk factors for poor prognosis, while RC was associated with improved survival (OS: hazard ratio (HR) = 0.34, 95% confidence interval (CI): 0.26 - 0.44, P < 0.001; CSS: HR = 0.47, 95% CI: 0.31 - 0.72, P < 0.001). T2b-stage patients had lower cancer-specific mortality than T2a-stage patients (P = 0.01). Subgroup analysis indicated that RC generally led to better survival, except in the neuroendocrine carcinoma subgroup for OS (P = 0.085) and the other carcinoma subgroup for CSS (P = 0.132).
Conclusions: This study reveals that RC is associated with superior CSS and OS compared to PC in patients with NUCB. Patient-related factors (age and marital status) and histological subtype significantly influence prognosis, highlighting the need for personalized treatment strategies.
Keywords: Blader cancer; Partial cystectomy; Radical cystectomy; Histological subtypes
| Introduction | ▴Top |
Bladder cancer (BC) is ranked as the 10th most frequently diagnosed malignancy globally, with an estimated 573,000 new cases and 213,000 deaths reported in 2020 according to GLOBOCAN data. As the second most prevalent urological tumor, BC exhibits significant geographic disparities, with higher incidence rates observed in Southern and Western Europe and elevated mortality risks in Northern Africa [1]. Urothelial carcinoma (UC), which accounts for approximately 90% of all bladder malignancies, originates from urothelial epithelium [2]. In contrast, non-urothelial carcinoma of the bladder (NUCB) represents a heterogeneous subgroup, including squamous cell carcinoma (SCC), adenocarcinoma, neuroendocrine carcinoma (NEC), and sarcomatoid variants, collectively accounting for less than 10% of cases. While UC has well-characterized molecular pathways and established treatment algorithms, the rarity of NUCB has led to limited understanding of its tumor biology and evidence-based management strategies [3]. For non-muscle-invasive bladder cancer (NMIBC), categorized into low- and intermediate-risk groups, standard management involves endoscopic resection followed by risk-adapted intravesical adjuvant therapy. Muscle-invasive bladder cancer (MIBC) and high-risk NMIBC require aggressive interventions, such as neoadjuvant/adjuvant systemic chemotherapy combined with radical cystectomy (RC) or bladder-preserving trimodal therapy (maximal transurethral resection and chemoradiation) [4]. Compared to UC, the pathogenesis and biological characteristics of NUCB remain poorly understood [5, 6]. The rarity of NUCB results in its exclusion or underrepresentation in clinical trials, leading to a lack of robust evidence for therapeutic strategies and the absence of specific diagnostic and treatment guidelines. Consequently, clinicians often rely on individual case analyses and expert consensus to develop treatment strategies for this BC subtype.
To address this knowledge gap, we investigated the survival outcomes of NUCB patients undergoing partial cystectomy (PC) and RC, comparing cancer-specific survival (CSS) and overall survival (OS) across different NUCB subtypes under varying surgical approaches.
| Materials and Methods | ▴Top |
Data source and patient population
All patients in this study were obtained from the National Cancer Institute’s the Surveillance, Epidemiology, and End Results (SEER) database registry [7]. Data extraction and screening were performed using SEER*Stat software (version 8.4.5). SEER*Stat database: Incidence-SEER Study Data, 17 registries, November 2023 Sub (2000 - 2021) was used as the most recent database. All data in the SEER database are freely available for research use when access is granted.
Inclusion criteria included: 1) diagnosis based on International Classification of Diseases (ICD) Oncology-3 (ICD-O-3) topology codes C67.0-67.6, C67.8-67.9; 2) identification of the four major non-urothelial histological variants of BC according to the 2016 World Health Organization (WHO) classification, including SCC, adenocarcinoma, other epithelial tumors, and neurologic endocrine carcinomas; and 3) patient age over 18 years. Exclusion criteria included: 1) patients with lymph node or visceral metastases; 2) patients with insufficient clinical and pathologic information; 3) patients with urachal carcinoma; and 4) patients with only death certificates and autopsy cases.
Based on inclusion and exclusion criteria, the crude dataset included all patients enrolled with: patient ID, gender, year of diagnosis, race, primary site, grading, ethnicity, type of reporting source, marital status at diagnosis, AYA lesion site recoded 2020 revision, histologic type of primary site ICD-O-3, grading recoded (through 2017), diagnosis confirmed, Derivative AJCC T 6th Edition (2004 - 2015), Derived AJCC N 6th Edition (2004 - 2015), Derived AJCC M 6th Edition (2004 - 2015), RX Summ-Surg Prim Site (1998+), RX Summ-Scope Reg LN. Sur (2003+), Radiation Therapy Recoding, Chemotherapy recode (yes, no/unknown), recoding for radiation therapy, and recoding for chemotherapy (yes, no), COD to site recode, SEER cause-specific death classification, SEER other cause of death classification, survival months, COD to site rec KM, vital status recode (study cutoff used). The study ultimately included 999 patients diagnosed with primary NUCB between 2007 and 2015 (Fig. 1).
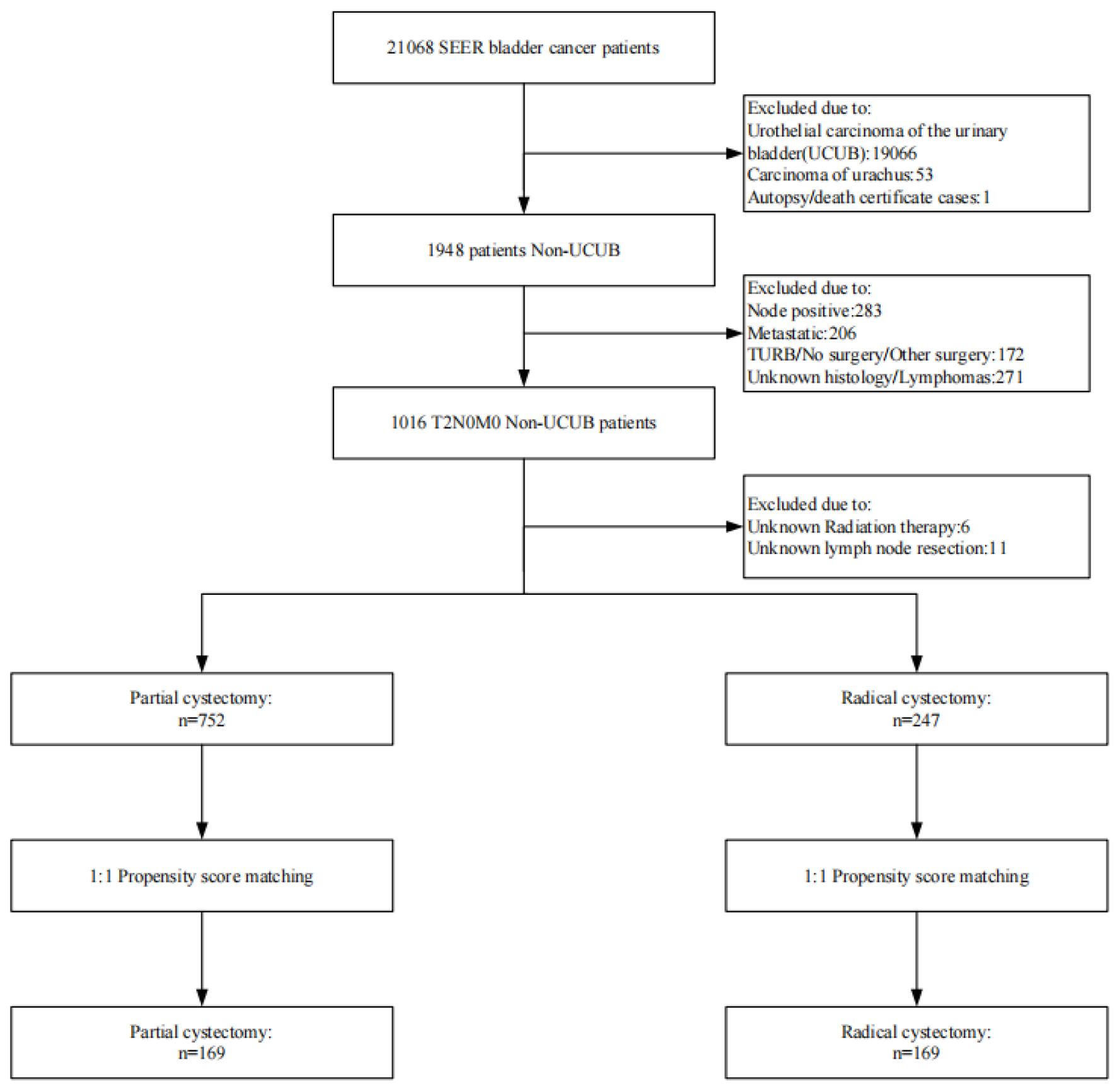 Click for large image | Figure 1. Flowchart of study cohort. |
Ethical statement
Since all data in the SEER program are fully anonymized and publicly accessible, the clinical information utilized in this study was obtained from open-source repositories and did not require direct subject involvement. Consequently, this study did not require ethical review and approval from an institutional review board (IRB). In addition, informed consent was not required for this retrospective analysis because the data were obtained from the publicly available SEER database, which adheres to stringent de-identification protocols to ensure participant confidentiality.
Statistical analysis
All statistical analyses in this study were carried out using R software version 4.4.2 [8]. The χ2 test or Fisher’s exact test was used to examine the relationships between surgery and categorical variables. Next, the propensity score matching (PSM) method was then used, and the RC group was matched to the PC group using the MatchIt package in the R project, with a 1:1 nearest-neighbor PSM algorithm that took all potential confounders into account (caliper width = 0.01SD).
Kaplan-Meier curves with log-rank tests were used to analyze unadjusted OS and CSS. OS was defined as the time between surgery and the date of death or last follow-up, whereas CSS was defined as the time between surgery and the date of cancer-related death or last contact. The independent prognostic indicators were identified using univariate and multivariate Cox proportional hazards regression models. All P values less than 0.05 were considered statistically significant.
| Results | ▴Top |
Demographic and clinicopathological characteristics
This study analyzed 999 histologically confirmed NUCB patients who met the inclusion and exclusion criteria, comprising 752 in the PC group and 247 in the RC group. With respect to patient characteristics, the PC group included 16.0% of patients younger than 65 years old, 40.0% female, 9.6% Black, and 48.3% married; in contrast, the RC group consisted of 36.4% of patients younger than 65 years old, 33.6% female, 6.9% Black, and 65.2% married. Regarding tumor features, in the PC group, 2.9% had grade I tumors, 18.5% had adenocarcinoma, and 25.1% were at T2a stage; in the RC group, 4.5% had grade I tumors, 21.9% had adenocarcinoma, and 27.5% were at T2a stage. In terms of treatment, only 0.5% of patients in the PC group underwent lymph node dissection, 75.0% received no/unknown radiotherapy, and 67.2% received no/unknown chemotherapy; in the RC group, 92.3% underwent lymph node dissection, 98.0% received no/unknown radiotherapy, and 62.8% received no/unknown chemotherapy. Correlation analysis revealed that age (P < 0.001), marital status (P < 0.001), tumor grade (P = 0.002), T stage (P < 0.001), lymph node dissection (P < 0.001), and radiation therapy (P < 0.001) were significantly associated with PC.
To minimize confounding factors and better reflect the inherent differences between the two strategies, a PSM model was employed. After 1:1 PSM adjustment for all potential confounders, the distribution of all included variables was adequately balanced (Table 1). Ultimately, 169 PC patients and 169 RC patients were perfectly matched. Supplementary Material 1 (jocmr.elmerjournals.com) illustrates the distribution and histograms of propensity scores before and after matching.
 Click to view | Table 1. Demographic and Clinicopathological Characteristics of With Histologically Confirmed Bladder Cancer, Treated With PC or RC and Identified Within the Surveillance, Epidemiology, and End Results Database |
Relationship between RC with survival in the matched data
In the matched population, the 1-year, 3-year, and 5-year OS rates were 64.3%, 46.5%, and 39.9%, respectively, while the corresponding CSS rates were 74.3%, 58.5%, and 55.0%. Further analysis showed that the median OS in the RC group was significantly higher than that in the PC group (P = 0.002). For instance, the 1-year, 3-year, and 5-year OS rates in the RC group were 85.1%, 66.7%, and 58.3% respectively, contrasting with 43.5%, 26.2%, and 21.4% in the PC group. In terms of CSS, the RC group had a significantly longer median survival time (P = 0.004), with 1-year, 3-year, and 5-year CSS rates of 90.3%, 77.0%, and 73.5% respectively, compared to 56.5%, 37.3%, and 33.6% in the PC group (Fig. 2).
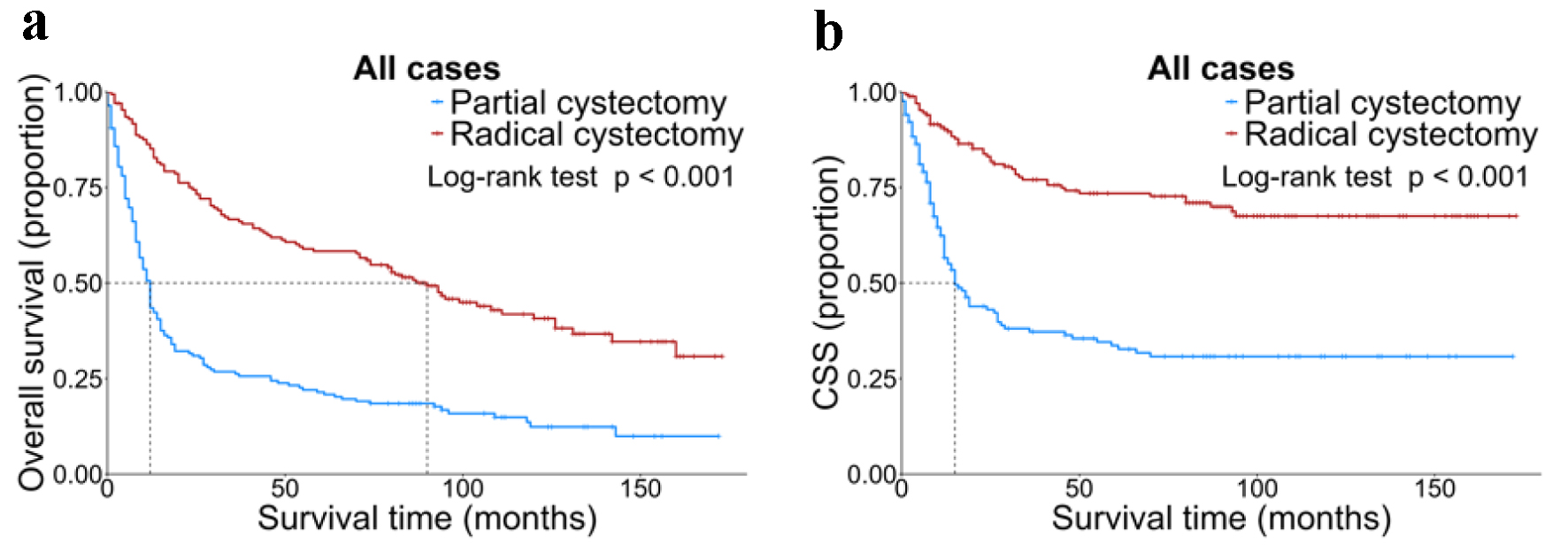 Click for large image | Figure 2. OS (a) and CSS (b) stratified by surgery in all matched patients. CSS: cancer-specific survival; OS: overall survival. |
Univariate and multivariable Cox regression analyses were performed to evaluate the prognostic factors for OS and CSS in BC patients (Table 2). The results indicated that older age and unmarried status were independently associated with poorer OS and CSS. Compared with PC, RC was significantly correlated with improved survival outcomes (P < 0.001, HR = 0.34; 95% CI: 0.26 - 0.44 for OS; P < 0.001, HR = 0.47; 95% CI: 0.31 - 0.72 for CSS). Additionally, higher mortality was independently related to age over 65 years, unmarried status, and the extent of muscularis propria invasion of the bladder wall.
 Click to view | Table 2. Univariate and Multivariate Cox Regression Analysis for Overall Survival and Cancer-Specific Survival in the Matched Data |
In the matched data, advanced age (≥ 65 years) and unmarried status were independent prognostic factors that had a negative impact on both OS and CSS. Compared with PC, RC significantly reduced both overall mortality (HR = 0.33, 95% CI: 0.25 - 0.43, P < 0.001) and cancer-specific mortality (HR = 0.47, 95% CI: 0.31 - 0.72, P < 0.001). In addition, precise T-stage stratification is crucial for the formulation of treatment plans, as patients with T2b-stage disease had a significantly lower cancer-specific mortality risk compared to those with T2a-stage disease (P = 0.01).
Subgroup interaction analysis
The subgroup analysis was carried out to identify subgroups that might benefit differently from RC. RC was generally associated with better OS and CSS than PC in most subgroups, but there were no significant differences in the NEC subgroup for OS (P = 0.085) and in the other carcinoma subgroup for CSS (P = 0.132) (Figs. 3 and 4).
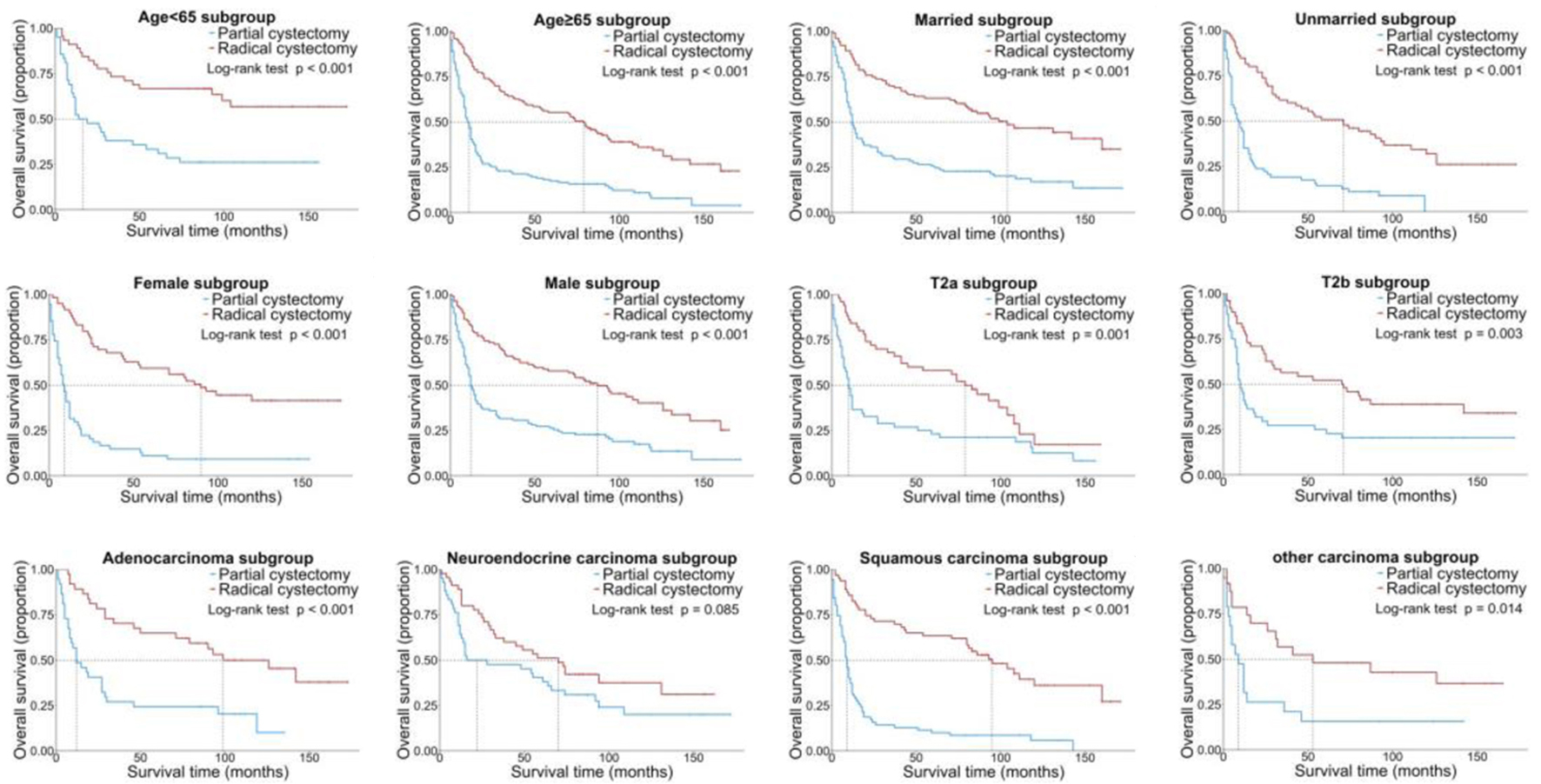 Click for large image | Figure 3. Kaplan-Meier OS curves of the patients with NUCB according to different clinical characteristics. NUCB: non-urothelial carcinoma of the bladder; OS: overall survival. |
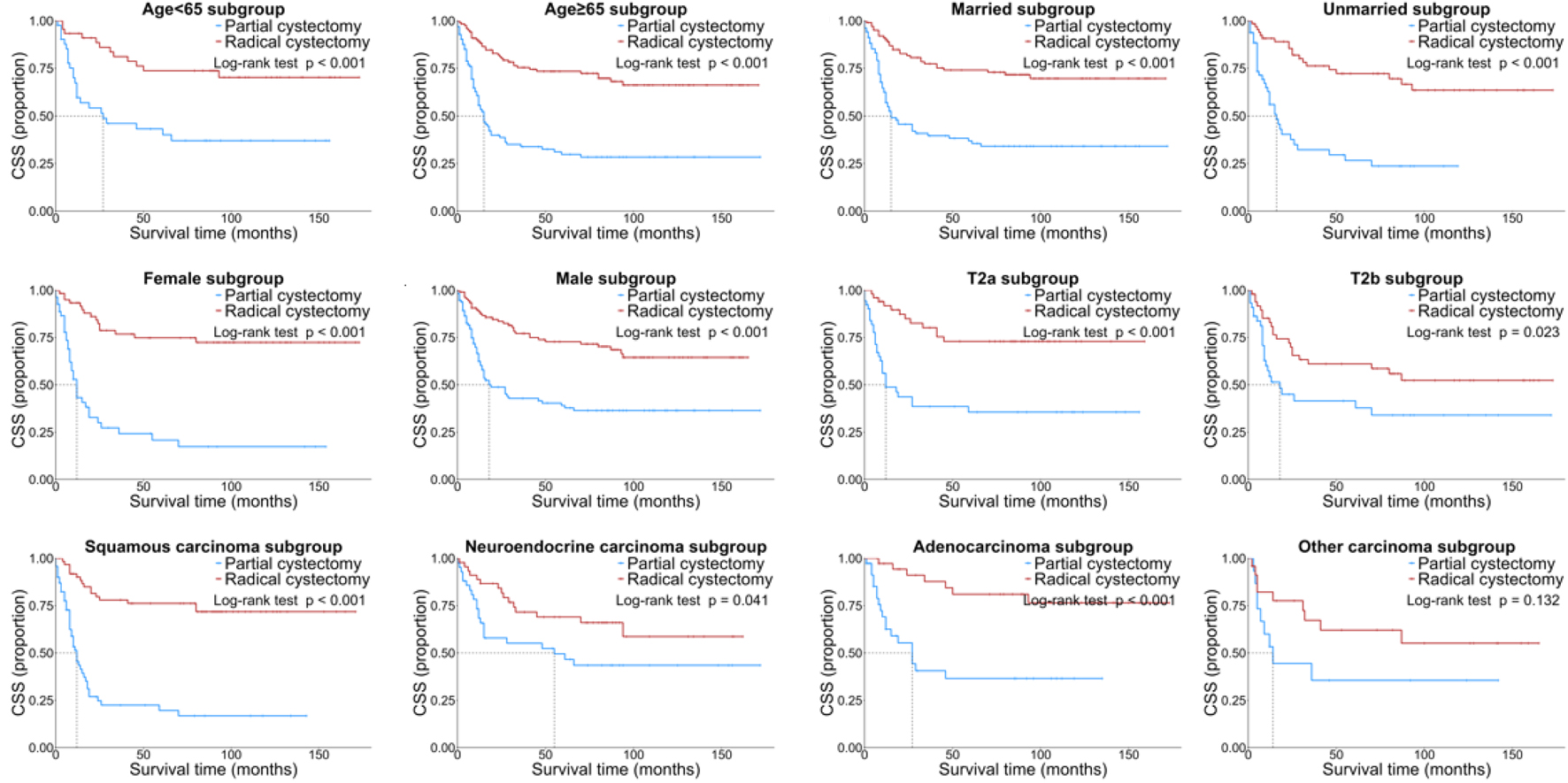 Click for large image | Figure 4. Kaplan-Meier CSS curves of the patients with NUCB according to different clinical characteristics. CSS: cancer-specific survival; NUCB: non-urothelial carcinoma of the bladder. |
In general, the forest plot shows the hazard ratio (HR) and interaction of different subgroups in the analysis of OS and CSS, and the results show that the factor has a significant effect on survival in most subgroups, but the interaction between different subgroups is mostly not significant, and the interaction between sex (P = 0.042) and histological subtype (P = 0.023) in OS is significant (Fig. 5).
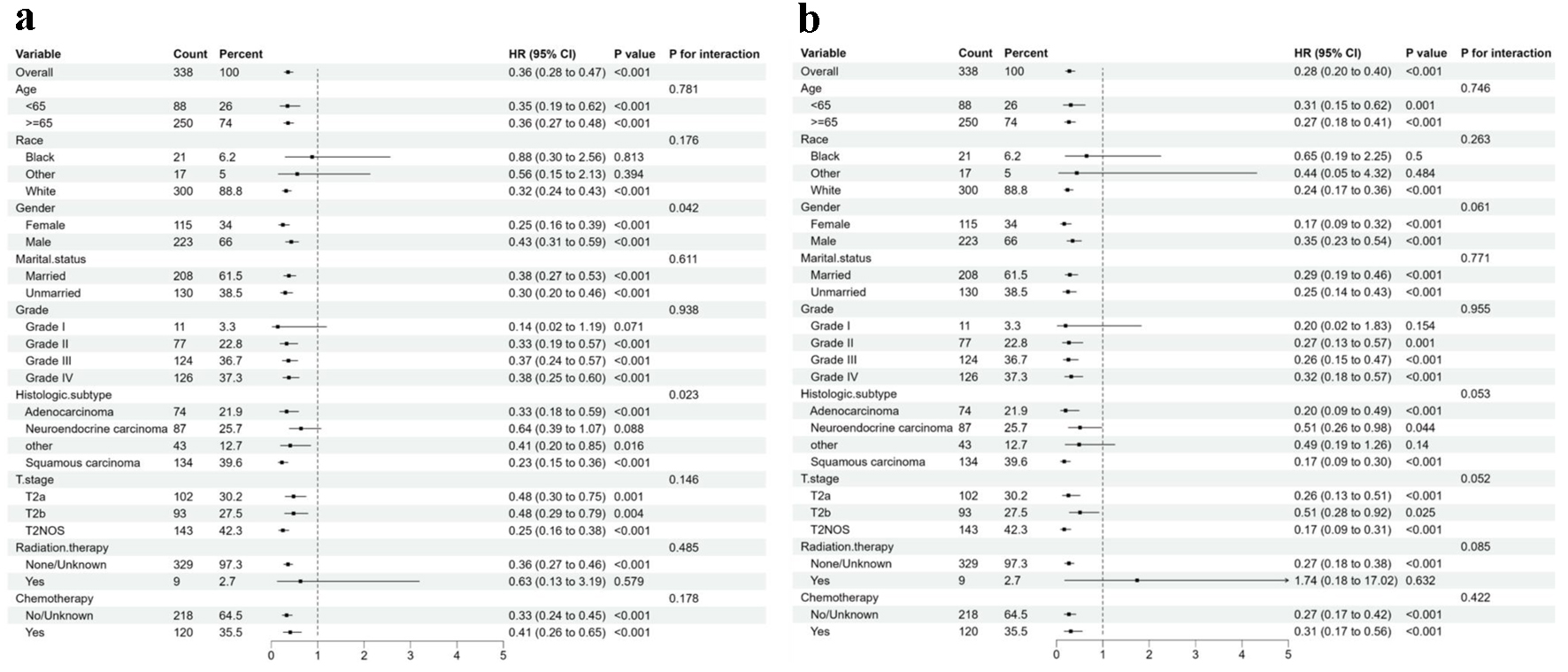 Click for large image | Figure 5. Forest plot of the association of surgery with total mortality and cancer-specific mortality in subgroup analyses. (a) Overall survival. (b) Cancer-specific survival. |
| Discussion | ▴Top |
The findings of our study provide novel insights into the management of NUCB, a rare and clinically challenging subtype of BC. Our comprehensive analysis of 999 NUCB patients from the SEER database demonstrated that RC was associated with significantly improved CSS and OS compared to PC in the propensity score-matched cohort. These results underscore the potential benefits of more aggressive surgical interventions for this patient population.
One of the key strengths of our study is the robust statistical methodology employed to address confounding factors. By utilizing PSM, we successfully balanced the distribution of potential confounders between the PC and RC groups, thereby minimizing selection bias and enhancing the accuracy of survival outcome comparisons. This approach is particularly critical in observational studies such as ours, where randomization of treatment groups is not feasible. The significant differences in survival outcomes observed after PSM adjustment strongly indicate that RC confers a survival advantage over PC in NUCB patients, with important implications for clinical decision-making.
Our identification of independent prognostic factors provides critical insights into the biology of NUCB and can inform the development of treatment strategies. Specifically, older age and unmarried status were independently associated with poorer OS and CSS, underscoring the importance of incorporating these patient-related factors during counseling and treatment planning. Age and gender are well-established independent risk factors for BC incidence and progression [9, 10]. In our study, age was stratified using a threshold of 65 years, reflecting the eligibility criteria for Medicare, a US government-sponsored health insurance program. Notably, 80.0% of our cohort comprised patients aged ≥ 65 years, and survival rates declined progressively with advancing age. Furthermore, married patients generally demonstrated superior survival outcomes, potentially attributable to enhanced social support, which may influence treatment adherence and psychological well-being [11, 12]. Consistent with these findings, our analysis revealed that unmarried patients had significantly poorer prognoses compared to their married counterparts. Marital status may serve as a surrogate marker for the complex interplay of social support mechanisms, although further investigation is warranted to elucidate these associations.
The extent of muscularis propria invasion, as indicated by the T stage, emerged as a crucial determinant of survival [13]. Notably, patients with T2b-stage disease exhibited a significantly lower cancer-specific mortality risk compared to those with T2a-stage disease, challenging the conventional assumption that more advanced T stages uniformly predict worse prognosis. This discrepancy may be attributed to variations in tumor biology or treatment response within the heterogeneous group of NUCB, warranting further research into the underlying mechanisms.
The results of our subgroup analysis introduce additional complexity to the understanding of NUCB treatment. While RC generally correlated with better survival outcomes than PC across most subtypes, notable exceptions were observed. For instance, in the NEC subgroup, the difference in OS between RC and PC was not statistically significant (P = 0.085), and in the other carcinoma subgroup, there was no significant difference in CSS (P = 0.132). These findings suggest that the optimal treatment approach may vary depending on the histological subtype of NUCB, highlighting the need for more subtype-specific research to develop personalized treatment strategies. Additionally, the significant interaction between sex and histological subtype in the analysis of OS further emphasizes the importance of integrating multiple factors when predicting survival and tailoring treatment for NUCB patients.
NUCB, accounting for 5-10% of BC cases, are highly aggressive malignancies. Their origin is commonly attributed to the differentiation of non-urothelial stem cells or the progression of urothelial metaplasia [14]. The European Association of Urology categorizes non-urothelial histological variants as the highest-risk subgroup, with these patients typically exhibiting poor prognoses [15]. Retrospective studies consistently demonstrate that these variants are associated with adverse clinical outcomes. NUCB encompasses diverse histological subtypes, including SCC, adenocarcinoma, and NEC [5, 16]. Other variants include sarcomatoid, micropapillary, and plasmacytoid subtype [17]. RC remains the standard treatment for locally advanced NUCB, involving en bloc removal of the bladder, adjacent organs (prostate/seminal vesicles in males; uterus/cervix in females), and pelvic lymph nodes [18]. The research of Grilo et al [19] indicates that surgery is the primary treatment method for SCC, with platinum-based chemotherapy potentially offering therapeutic benefits. The treatment of adenocarcinoma shares similarities with colorectal cancer management, while small cell carcinoma treatment largely follows regimens used for small cell lung cancer. A study by Mistretta et al [20] examined adherence and survival outcomes of multimodality therapy (radical cystectomy plus chemotherapy and/or radiotherapy) in patients with pT2-3 M0 stage NUCB. The findings revealed an increased rate of multimodality therapy and survival benefit in patients with NECs, whereas patients with adenocarcinomas and SCCs did not show a significant difference.
The prognosis for non-UC is usually worse than pure UC [21]. For patients with MIBC, RC is recommended. For NMIBC, RC is also recommended unless the patient has a contraindication to surgery or refuses surgery. Our findings suggest that RC in NUCB patients with limited T2N0M0 improves patients’ OS and CSS. In the SEER database, 99% of patients who underwent PC did not undergo lymph node dissection, in contrast to only 4% of those who underwent RC. Therefore, the pathological lymph node status of the vast majority of patients was not recorded. This may be associated with a higher recurrence rate, as well as poorer OS and CSS in PC patients. It should be emphasized that the SEER database does not record important pathological data, such as the status of surgical margins and lymphovascular invasion status. Both of these factors have previously been associated with a higher recurrence rate after UC surgery [22].
However, our study is not without limitations. First, the SEER database lacks well-established risk factors such as smoking history, occupational carcinogen exposure, and environmental exposures, which could have provided deeper insights into our analysis. Second, the rarity of NUCB limits the availability of external datasets for independent validation of our findings. Consequently, further external validation using multi-institutional cohorts is warranted to corroborate our conclusions. Third, the absence of molecular or genetic information in the SEER database represents a critical limitation, as such data are increasingly recognized as pivotal for prognostic stratification and therapeutic decision-making in oncology. We strongly advocate for future investigations utilizing datasets integrating molecular profiling. Additionally, the retrospective nature of this study introduces inherent biases associated with secondary data analysis. Although PSM reduces selection bias by matching baseline variables, residual confounding may still exist in tumor clinical stage and grade. For example, patients in the PC group might have been preferentially selected for bladder preservation due to more localized tumor location. Larger prospective studies with standardized data collection protocols are needed to establish definitive conclusions. Despite these limitations, our study provides clinically relevant insights into prognostic assessment and personalized treatment strategies for NUCB patients. We maintain that our findings constitute a meaningful contribution to this understudied field, particularly given the scarcity of population-level evidence on NUCB outcomes. The methodological framework established here may inform future research designs while awaiting validation through molecularly enriched prospective cohorts.
Conclusion
In conclusion, our study provides critical insights into the survival outcomes of NUCB patients treated with PC and RC. The results demonstrate that RC is associated with improved survival compared to PC and highlight the importance of patient-related factors and histological subtype in determining prognosis. These findings have significant implications for the clinical management of NUCB and emphasize the need for further research to enhance understanding and treatment of this rare and challenging disease.
| Supplementary Material | ▴Top |
Suppl 1. Distribution and Histograms of Propensity Scores Before and After Matching.
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant.
Conflict of Interest
The authors have no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Ren Bin Yuan had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Shuang Liu: data acquisition, data analysis, statistical analysis, manuscript writing, and manuscript review; Tai Song Wang: data acquisition and manuscript review.
Data Availability
The data that support the findings of this study are openly available in the SEER database.
Abbreviations
BC: bladder cancer; CSS: cancer-specific survival; MIBC: muscle-invasive bladder cancer; NMIBC: non-muscle-invasive bladder cancer; NUCB: non-urothelial carcinoma of the bladder; OS: overall survival; PC: partial cystectomy; PSM: propensity score matching; RC: radical cystectomy; SEER: the Surveillance, Epidemiology, and End Results; UC: urothelial carcinoma
| References | ▴Top |
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Dyrskjot L, Hansel DE, Efstathiou JA, Knowles MA, Galsky MD, Teoh J, Theodorescu D. Bladder cancer. Nat Rev Dis Primers. 2023;9(1):58.
doi pubmed - Lopez-Beltran A, Cookson MS, Guercio BJ, Cheng L. Advances in diagnosis and treatment of bladder cancer. BMJ. 2024;384:e076743.
doi pubmed - Flaig TW, Spiess PE, Abern M, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, et al. NCCN Guidelines(R) insights: bladder cancer, Version 2.2022. J Natl Compr Canc Netw. 2022;20(8):866-878.
doi pubmed - Park S, Reuter VE, Hansel DE. Non-urothelial carcinomas of the bladder. Histopathology. 2019;74(1):97-111.
doi pubmed - Moschini M, D'Andrea D, Korn S, Irmak Y, Soria F, Comperat E, Shariat SF. Characteristics and clinical significance of histological variants of bladder cancer. Nat Rev Urol. 2017;14(11):651-668.
doi pubmed - https://seer.cancer.gov/.
- http://www.r-project.org/.
- Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder cancer: a review. JAMA. 2020;324(19):1980-1991.
doi pubmed - Li Z, Xu Z, Wang J, Wang M. Nomograms for predicting the overall and cancer-specific survival in patients with non-urothelial carcinoma of the bladder: A population-based study. Biomol Biomed. 2024;24(3):633-646.
doi pubmed - Pan S, Yan N, Zhao Y, Li Z. Marital status as an independent prognostic factor for patients of malignant pleural mesothelioma. Front Med (Lausanne). 2022;9:955619.
doi pubmed - Li Y, Wu G, Zhang Y, Yang W, Wang X, Duan L, Niu L, et al. Effects of marital status on survival of retroperitoneal liposarcomas stratified by age and sex: A population-based study. Cancer Med. 2023;12(2):1779-1790.
doi pubmed - Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48.
doi pubmed - Deuker M, Martin T, Stolzenbach F, Rosiello G, Colla Ruvolo C, Nocera L, Tian Z, et al. Bladder cancer: a comparison between non-urothelial variant histology and urothelial carcinoma across all stages and treatment modalities. Clin Genitourin Cancer. 2021;19(1):60-68.e61.
doi pubmed - Turker P, Bostrom PJ, Wroclawski ML, van Rhijn B, Kortekangas H, Kuk C, Mirtti T, et al. Upstaging of urothelial cancer at the time of radical cystectomy: factors associated with upstaging and its effect on outcome. BJU Int. 2012;110(6):804-811.
doi pubmed - Chalasani V, Chin JL, Izawa JI. Histologic variants of urothelial bladder cancer and nonurothelial histology in bladder cancer. Can Urol Assoc J. 2009;3(6 Suppl 4):S193-198.
doi pubmed - Erdem GU, Dogan M, Sakin A, Oruc Z, Yaman E, Yesil Cinkir H, Uysal M, et al. Non-urothelial bladder cancer: comparison of clinicopathological and prognostic characteristics in pure adenocarcinoma and non-bilharzial squamous cell carcinoma of the bladder. Oncol Res Treat. 2018;41(4):220-225.
doi pubmed - Comperat E, Amin MB, Cathomas R, Choudhury A, De Santis M, Kamat A, Stenzl A, et al. Current best practice for bladder cancer: a narrative review of diagnostics and treatments. Lancet. 2022;400(10364):1712-1721.
doi pubmed - Grilo I, Rodrigues C, Soares A, Grande E. Facing treatment of non-urothelial bladder cancers in the immunotherapy era. Crit Rev Oncol Hematol. 2020;153:103034.
doi pubmed - Mistretta FA, Negrean-Dzyuba C, Palumbo C, Pecoraro A, Knipper S, Tian Z, Musi G, et al. Adherence to guideline recommendations for multimodality treatment of patients with pT2-3 M0 non-urothelial carcinoma of the urinary bladder: Temporal trends and survival outcomes. Int J Urol. 2020;27(5):402-407.
doi pubmed - Willis DL, Porten SP, Kamat AM. Should histologic variants alter definitive treatment of bladder cancer? Curr Opin Urol. 2013;23(5):435-443.
doi pubmed - Mari A, Campi R, Tellini R, Gandaglia G, Albisinni S, Abufaraj M, Hatzichristodoulou G, et al. Patterns and predictors of recurrence after open radical cystectomy for bladder cancer: a comprehensive review of the literature. World J Urol. 2018;36(2):157-170.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.