| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Review
Volume 17, Number 7, July 2025, pages 375-385
Prevalence and Pathogenetic Mechanisms of Chronic Kidney Disease in Autoimmune-Mediated Systemic Diseases
Daniel Patschana, b, Benedikt Marahrensa, Igor Matyukhina, Henning Hansen-Nootbaara, Wajima Safia, Oliver Rittera, Susann Patschana
aDepartment of Internal Medicine I - Cardiology, Nephrology and Internal Intensive Medicine, Brandenburg University Hospital, Brandenburg Medical School (Theodor Fontane), 14770 Brandenburg an der Havel, Germany
bCorresponding Author: Daniel Patschan, Department of Internal Medicine I - Cardiology, Nephrology and Internal Intensive Medicine, Brandenburg University Hospital, Brandenburg Medical School (Theodor Fontane), 14770 Brandenburg an der Havel, Germany
Manuscript submitted May 5, 2025, accepted July 23, 2025, published online July 31, 2025
Short title: CKD in Autoimmune-Mediated Systemic Diseases
doi: https://doi.org/10.14740/jocmr6271
- Abstract
- Introduction
- Methods
- CKD - Definition, Epidemiology and Outcomes
- Autoimmune-Mediated Inflammatory Rheumatic Diseases - Outline
- Disease-Related CKD Risk
- Additional Factors of Increased CKD Risk in Autoimmune-Mediated Inflammatory Rheumatic Diseases
- Conclusions
- References
| Abstract | ▴Top |
Chronic kidney disease (CKD) affects an estimated 15% of all adults in Central Europe. Those affected are at high risk of cardiovascular disease and death. Inflammatory rheumatic systemic diseases manifest themselves extra-articularly with varying frequency. This article summarized the prevalence and pathogenetic mechanisms of CKD in rheumatic systemic diseases. The following databases were searched for references: PubMed, Web of Science, Cochrane Library, Scopus. The search period spanned from 1975 to 2025. Kidney involvement is almost always present in systemic lupus erythematosus and certain types of systemic vasculitis. In the context of rheumatic diseases, there are additional mechanisms that can contribute to enhancing the functional and structural integrity of the kidneys. These mechanisms include inflammation and an increase in cardiovascular risk. The prevalence of CKD is disproportionately high in certain entities of the rheumatic form. Given the disproportionately high prevalence of CKD in relevant entities of the inflammatory rheumatic group and the associated increase in the risk of cardiovascular disease and death, CKD screening should be an integral part of the care of affected patients.
Keywords: CKD; Rheumatic diseases; DMARD therapy; Cardiovascular risk; Inflammation
| Introduction | ▴Top |
Approximately 10-15% of all adults in Germany are affected by chronic kidney disease (CKD), with the risk increasing with age [1]. The main causes are diabetes mellitus and arterial hypertension, but numerous other specific kidney diseases may also be involved. CKD patients are at the highest risk of cardiovascular complications and premature death [2], a correlation that was already very convincingly demonstrated in 2004 [3]. Autoimmune-mediated inflammatory rheumatic diseases are further subdivided into four major categories: rheumatoid arthritis (RA), seronegative spondyloarthropathies, autoimmune connective tissue diseases (previously known for many years as collagenoses), and vasculitides. Metabolic arthropathies can also be categorized as rheumatic disorders, with the most notable examples being gout and joint manifestations of hemochromatosis.
Certain diseases are characterized by systemic manifestations that extend beyond the joints. While direct involvement of the heart, kidneys, central or peripheral nervous system is rare or even uncommon in RA [4-6] or seronegative spondyloarthropathies [7], such manifestations significantly complicate the course of the disease in other cases. Patients diagnosed with systemic lupus erythematosus (SLE) [8] or antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) [9] face a heightened risk of developing renal, neurological, and cardiac complications as a direct result of the autoimmune inflammatory process. Kidney involvement can lead to a wide range of complications, including asymptomatic manifestations and mild functional disorders, such as those affecting the renal tubules. Furthermore, two major syndromes of nephrology medicine can develop: acute kidney injury (AKI) [10] and CKD [2]. It has been demonstrated that both syndromes may also result from medications used to control disease activity and progression. Finally, inflammatory autoimmune disorders have been associated with an increased risk of AKI and CKD via additive mechanisms, which refers to processes beyond the disease-associated tissue damage or treatment. Approximately 18% of patients attending rheumatology clinics were found to have a glomerular filtration rate (GFR) of 60 mL/min or less [11], compared to the known prevalence of 10-15% in the general population in central Europe [1].
This review article will provide a comprehensive summary of the epidemiology of CKD in autoimmune-mediated inflammatory rheumatic diseases. The discussion will include the underlying mechanisms responsible for the development of CKD, both in relation to the diseases themselves and in terms of additive mechanisms. However, it is important to note that the renal side effects of therapeutic agents, particularly nonsteroidal anti-inflammatory drugs (NSAIDs), will not be covered in this article.
| Methods | ▴Top |
The following databases were searched for references: PubMed, Web of Science, Cochrane Library, and Scopus. The period spanned from 1975 until 2025. The following search terms were applied in variable combinations: “CKD”, “chronic kidney disease”, “chronic kidney failure, “rheumatoid arthritis”, “RA”, “spondylarthritis”, “seronegative spondylarthritis”, “SpA”, “ankylosing spondylitis”, “AS”, “psoriatic arthritis”, “PsA”, “collagenoses”, “systemic lupus erythematosus”, “SLE”, “Sjogren’s syndrome”, “SS”, “idiopathic inflammatory myopathy”, “IIM”, “systemic sclerosis”, “SSc”, “mixed connective tissue disease”, “MCTD”, “vasculitides”, “AAV”, “ANCA-associated vasculitis”, “IgA-vasculitis”, “cryoglobulinemia”, “glomerulonephritis”, “inflammatory activity”, “systemic inflammation”, “cardiovascular risk”, “cardiovascular morbidity”, “cardiovascular mortality”.
| CKD - Definition, Epidemiology and Outcomes | ▴Top |
In 2024, the updated version of the KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease was published [2]. Compared to the previous edition of 2012 [12], the definition of the syndrome has not been significantly changed. It focuses on two main categories: 1) a sustained (more than 3 months) reduction in the estimated glomerular filtration rate (eGFR); and/or 2) any other indicator of renal structural damage, provided that such an indicator must also be present for at least 3 months. The structural damage can be indicated by increased protein loss in the urine, pathological sediment findings, or histological findings of chronic organ damage, or other factors. The two parameters eGFR (according to CKD-EPI [13]) and albuminuria are of fundamental prognostic importance. The risk for significant clinical endpoints increases disproportionately with increasing eGFR reduction and increasing albumin loss. Endpoints include categories such as heart attack, stroke, cardiovascular death, new dialysis requirement and others. The correlation also explains the high medical relevance of the topic: CKD is one of the most potent cardiovascular risk factors of all [3]. A primary reason is that other major cardiovascular risk factors, including diabetes mellitus and arterial hypertension, act as causal factors in CKD and are often present before a CKD diagnosis. CKD-related water and sodium retention can contribute to elevated blood pressure. Independent of these hemodynamic effects, the uremic environment resulting from reduced renal excretory function has been identified as an inducer of endothelial dysfunction (ED), which is an early step in atherogenesis. ED refers to widespread impairment of the endothelium and occurs in CKD due to the accumulation of various endothelium-toxic metabolites and generally increased inflammation. Additionally, CKD-mineral bone disorder (CKD-MBD) is associated with proatherogenic changes, partially due to rising concentrations of fibroblast growth factor-23 (FGF-23) and higher levels of calcium phosphate products in the blood. The mechanistic complexity of the heightened cardiovascular risk observed in CKD can only be briefly outlined here.
Worldwide, diabetes mellitus is the main cause of CKD, followed by arterial hypertension [14]. In Central Europe, CKD affects approximately 10-15% of adults [1], with the average loss of life years due to the syndrome increasing with age. The Global Burden of Disease Study provides very detailed data on the epidemiological situation of CKD globally. This survey was initiated more than 30 years ago with the aim of collecting data on the frequency and outcome of a variety of diseases across the planet that are as accurate as possible [15]). In 2020, Bikbov et al [16] published an extremely comprehensive article on the burden of CKD, with data from 1990 to 2017. The article documented global prevalence rates of CKD, ranging from 5,243 (Andorra) to 14,399 per 100,000 inhabitants annually (Fiji Islands) [16]. The authors also demonstrated the substantial loss of life years due to CKD across various age groups. As age increases, the loss of years initially rises disproportionately. However, in the highest age categories (85 and older), the loss curve flattens out to a certain extent, which is understandable. In summary, CKD remains one of the two major nephrological problem areas worldwide.
| Autoimmune-Mediated Inflammatory Rheumatic Diseases - Outline | ▴Top |
The two most prevalent illnesses are RA (average onset age (aoa): 30-60 years [17, 18]) and seronegative spondyloarthritis (SpA) [19], the latter represented by several entities such as ankylosing spondylitis (AS) (aoa: 26 years [20]), psoriatic arthritis (PsA) (aoa: 30 - 60 years, gender-dependent [21]), reactive arthritis (aoa: 20 - 40 years [22]), and others. The musculoskeletal system is clinically affected in each case, but with different patterns of manifestation (RA typically involves the small joints in the hands and feet, while SpA preferentially affects axial skeleton and/or oligoarticular involvement of large joints). Extra-articular manifestations are possible, including those affecting the kidney. Autoimmune connective tissue diseases are classified into five distinct entities: SLE (aoa: third decade of life [23]), systemic sclerosis (SSc) (aoa: 20 - 50 years [24]), idiopathic inflammatory myopathies (information about aoa is difficult to provide, since seven distinct entities are identified), Sjogren’s disease (aoa: 30 - 60 years [25]), and mixed connective tissue disease (MCTD) (aoa: 15 - 25 years [26]), which can be either undifferentiated or differentiated. In particular, SLE is disproportionately often associated with renal manifestations (50%). The same applies to AAV including granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and Churg-Strauss syndrome (CSS) (aoa of AAV: 50 - 70 years [27]). Other forms, such as ANCA-negative small vessel vasculitis or vasculitis of medium and large vessels, rarely manifest renally, although possible involvement may also be associated with CKD.
This article will not address each of the named entities, for example ANCA-associated forms and immunoglobulin A (IgA) vasculitis (IgAV) will be discussed from the group of vasculitides. The references will be discussed according to the date of publication (older > newer).
| Disease-Related CKD Risk | ▴Top |
RA
In a 2018 review, Kapoor et al [28] reported that historical prevalence rates of CKD in RA exceeded 50%. Prior to the availability of methotrexate and biologics - methotrexate was first reported for use in RA in 1951 [29], etanercept was the first biologic approved by the US Food and Drug Administration (FDA) for RA in 1998 [30] - many patients experienced inadequate control of disease activity. Consequently, more nephrotoxic NSAIDs were often prescribed. Additionally, the use of traditional disease-modifying antirheumatic drugs (DMARDs), such as gold or D-penicillamine, frequently led to direct drug-induced kidney damage, such as membranous glomerulonephritis [31].
In 2015, Chiu et al [32] published a population-based cohort study, in which over 12,000 RA patients were compared with around 38,000 control individuals without RA. All data were extracted from the Taiwan National Health Insurance Research Database, and all patients were followed up for 5 years. Using a regression model, RA was identified as an independent predictor of CKD, even when adjusted for traditional cardiovascular risk factors. The increase in risk of CKD was also more pronounced with increasing cumulative morbidity (adjusted hazard ratio for RA per se 1.31, adjusted hazard ratio for RA + age ≥ 70 years 2.82, adjusted hazard ratio for RA + diabetes mellitus 2.04). Although the effects of medication are discussed in detail elsewhere, it should be mentioned that the study identified various types of drugs as risk factors for CKD: NSAIDs, cyclosporine, glucocorticoids, cyclophosphamide and mycophenolic acid. A subsequent cohort study [33], also retrospective, encompassed over 1,000 RA patients over a 10-year period (summer 2004 to 2014). The KDIGO criteria, published in 2012 [12], were utilized to diagnose CKD, with a prevalence recorded at the time of enrollment of 24.5%. The initial diagnosis of CKD in previously enrolled individuals was also documented. During the observation period, 59.5% of the individuals developed new-onset CKD. The mortality risk was found to be significantly higher in the CKD group, with the most pronounced effect observed in the “very high-risk” CKD group (defined as stages G3aA3, G3bA2, G3bA3, G4, and G5 according to KDIGO [12]). The hazard ratio increased to 4.76.
As already mentioned, direct renal manifestations of RA are uncommon [28]. Specific glomerular disease patterns have been described in isolated cases (membranous, IgA mesangioproliferative and extracapillary proliferative glomerulonephritis), e.g., in autopsy studies [34]. However, the clinical relevance of these manifestations is usually low. In 1987, Wegelius et al [35] reported on two RA patients with a pathological-anatomical pattern of interstitial edema formation in the kidneys (presumably a consequence of hyaluronic acid accumulation); clinically, however, the patients suffered from AKI. Before the advent of more potent DMARDs such as methotrexate or biological agents, RA was associated with secondary amyloidosis in a relevant percentage of cases. Prior to 2010, the prevalence of AA amyloidosis (AAA) in patients with RA ranged from 16.7% to 25.2%. After 2010, this figure decreased markedly to 0.7%, indicating a potential beneficial impact of biologic therapies [36]. However, in a review published in 1994, the disease was named as the most common cause of (secondary) AAA [37]. The possible renal involvement of AAA is clinically characterized by variable proteinuria and, depending on the intrarenal deposition of amyloid fibrils, by a gradual decline in renal excretory function [38]. Both variables define CKD.
SpA
SpA is typically characterized by inflammation of the axial skeleton, encompassing the sacroiliac joints, the intervertebral joints, and potentially the vertebrae themselves [19]. Peripheral joints may also be affected. Clinically, axial inflammation is characterized by inflammatory back pain, for which specific diagnostic criteria have been established [39]. A notable example within this axial SpA group [40] is AS. Conversely, if the clinical picture primarily affects peripheral, large joints, it is classified as peripheral SpA [41]. PsA often manifests in this manner, although not invariably. Taken together, the prevalence of all types of SpA combined is roughly equivalent to that of RA [42]. In this article, only the two entities, AS and PsA, will be discussed. The prevalence of AS is estimated to be between 0.7% and 1.4% of the adult population [43], while the prevalence of PsA is estimated to be between 0.1% and 0.2% of the population [44].
AS
The most prevalent renal manifestation of AS is IgA nephropathy (IgAN). In 2020, Champtiaux et al [45] published a retrospective study of SpA patients with biopsy-confirmed IgAN, 32 cases were included. The mean baseline eGFR was 84 ± 26 mL/min. Two-thirds of the patients suffered from AS. After a median follow-up of 5.9 years, four individuals required dialysis. In more than 40% of the patients, the eGFR had decreased by > 50%. The risk of reaching CKD stages IV and V was associated with arterial hypertension, proteinuria, and the variables interstitial fibrosis and segmental sclerosis according to the Oxford classification [46]. A recent analysis (2024) considered a cohort of 12,000 AS patients from the Korean National Health Insurance Service, with the objective of investigating the association between regular use of NSAIDs and the incidence of CKD [47]. The analysis revealed that, among the entire cohort, 150 individuals (1.2%) were diagnosed with CKD, with an incidence rate of 4.65 per 10,000 patient-years over the observation period (1 year). Notably, the long-term use of NSAIDs was associated with a reduced risk of CKD. While these findings require further interpretation, the data suggest a relatively low prevalence of CKD in AS compared to the average prevalence of CKD in Central Europe (10-15% of all adults [1]. In addition, a few aspects regarding NSAID dosage and frequency of use in AS should be mentioned. A 2-year randomized controlled trial [48] investigated the effects of continuous versus on-demand NSAID treatment on radiographic progression in patients with AS. Out of 215 patients, those receiving continuous NSAID treatment showed significantly less radiographic progression (mean score of 0.4) compared to the on-demand group (mean score of 1.5, P = 0.002). There were no significant differences in signs and symptoms between the two groups, and the radiographic benefit persisted even after adjusting for various factors. Although adverse events were more common in the continuous treatment group, the differences were not statistically significant. The study concluded that continuous NSAID use reduces radiographic progression in AS patients without substantially increasing toxicity. The update to the ASAS-EULAR recommendations for the management of axial SpA published in 2022 recommends NSAIDs as first-line therapies, to be used regularly where possible [49].
PsA
Limited systematic data are available on the prevalence of CKD in PsA. In 2024, Kharouf et al [50] published a prospective cohort study of 1,336 PsA patients, with a relatively long median follow-up duration (8.2 years). During the entire observation period, 9.2% were diagnosed with CKD, based only on a reduction of eGFR to below 60 mL/min over 3 months. About 20% of CKD patients were already diagnosed when they were enrolled in the study. The risk factors identified for CKD included diabetes mellitus (hazard ratio 2.58), uric acid (hazard ratio 1.21) and daily use of NSAIDs (hazard ratio 1.77). Methotrexate, on the other hand, was associated with a reduced risk (hazard ratio 0.51). Comparable to AS, the prevalence of CKD in PsA appears to be at least no higher than in the general adult population. Specific renal disease patterns that could result in CKD in PsA patients are rarely mentioned in the literature. In 1998, the case of a PsA patient with gold-induced membranous glomerulonephritis was published, who developed myeloperoxidase-positive vasculitis [51]. These are certainly rare cases.
Collagenoses
SLE
The prognosis of SLE depends substantially on possible kidney involvement and its extent. Without question, possible involvement of the nervous system and cardiovascular manifestations are of comparable prognostic significance in SLE [52]. Experience shows that 50% of all patients are affected by this manifestation [53]. The term lupus nephritis (LN) stands for six possible pathological-anatomical findings or classes of glomerular inflammation. The most prognostically unfavorable classes are III and IV, each characterized by proliferative glomerular damage with or without spread into the Bowman capsule space. The classification of LN has been repeatedly adapted over the years. In 2004, the “2003 International Society of Nephrology (ISN)/Renal Pathology Society (RPS) Classification of Lupus Nephritis (LN)” was published simultaneously in “Kidney International” [54] and the “Journal of the American Society of Nephrology” [55). The classification is largely valid, although a revision or extension was published in 2018 [56]. The clinical spectrum of LN is extremely variable, all conceivable clinical correlates of glomerular inflammation can occur, more frequently in some classes, less frequently in others: isolated mild proteinuria/hematuria, nephrotic syndrome, nephritic syndrome, renoparenchymatous arterial hypertension and progressive CKD [57]. Interestingly, certain autoantibody specificities are associated with a distinct risk of kidney involvement. Notably, antibodies against monomeric C-reactive protein (anti-mCRP) are prevalent in the serum of SLE patients with kidney involvement, in contrast to antibodies against pentameric CRP (anti-pCRP). These antibodies may also be pathogenetically relevant [58]. It is presumed that these antibodies are also involved in the pathogenesis of TINU syndrome [58]. A review article published in 2016 [59] reported epidemiological data on various clinical correlates of LN. Proteinuria was identified as the most common manifestation, occurring in 100% of cases, followed by hematuria at 80%. The prevalence of rapid deterioration of renal function, or AKI, was reported at 15%, while renal insufficiency was noted at 60%. The latter likely corresponds to CKD. However, the actual prevalence of CKD is probably higher, as prolonged proteinuria is also a defining criterion for CKD. Strictly speaking, it can be assumed that nearly all patients with LN have CKD, particularly if the KDIGO criteria from 2024 [2] are consistently applied. These criteria state that a loss of kidney function (eGFR decreases below 60 mL/min) and/or any other indicators of renal structural damage (such as proteinuria, hematuria, or histological findings of organ damage) define CKD, provided the time frame exceeds 3 months. For the sake of completeness, it should be noted that SLE can also be associated with various renal pathologies beyond glomerulonephritis, including interstitial nephritis and thrombotic microangiopathy, with or without the presence of antiphospholipid antibody syndrome [57].
SSc
SSc is characterized by progressive induration (sclerosing) of the subcutis and possibly also of the interstitium of various organs, some of which are vital (myocardium, lungs). Depending on the spread of skin infestation, a distinction is generally made between limited forms (leaving out the trunk of the body) and generalized or diffuse forms [60]. The most well-known renal manifestation and a prognosis-critical complication of SSc is renal crisis, which is clinically characterized by a rapid decline in renal function, often presenting as AKI. Histologically, renal crisis is associated with proliferative endarteritis, resembling the changes observed in thrombotic microangiopathies [61]. Since the 1970s, the implementation of angiotensin-converting enzyme (ACE) inhibitors has significantly reduced mortality rates associated with scleroderma renal crisis (SRC), declining from 76% to below 10% [62]. Iliopoulos et al [63] published a paper on the prevalence of persistent disorders related to excretory renal function, as well as persistent proteinuria and hematuria in SSc. Almost 800 patients with SSc were analyzed from a total of five selected studies; in over one-third of patients (31.5%), the eGFR was below 90 mL/min, in 19.5% of cases even below 60 mL/min. Thus, assuming that these were indeed prolonged cases of renal insufficiency, the prevalence of CKD in SSc would be slightly above the Central European average of 10-15% [1]. As early as 2016, it was suggested that the disease could result in impaired renal function or CKD through the mechanism of latent renal ischemia, independent of renal crises. Gigante et al identified an inverse correlation between increases in acral hypoperfusion and decreases in eGFR [64].
Sjogren’s syndrome (SS)
Although patients with SS suffer primarily from the consequences of exocrine insufficiency of the salivary and lacrimal glands and from variable arthralgias, extraglandular and extra-articular manifestations are not uncommon (central nervous system, lungs, lymph nodes, etc.). In 2015, Ramos-Casals et al [65] published a systematic review that utilized the EULAR Sjogren’s Syndrome Disease Activity Index (ESSDAI). The ESSDAI was designed to accurately document the potential organ manifestations of SS [66]. The review evaluated over 270 articles, highlighting the diverse cutaneous, pulmonary, and renal manifestations associated with the condition. With regard to renal manifestations, two main disease patterns were reported: renal tubular acidosis (RTA) and glomerulonephritis. The heterogeneity of the possible forms of glomerulonephritis (membranous, membranoproliferative, focal segmental glomerulonephritis, etc.) was surprising, although the prevalence was reported to be low (4%). RTA, on the other hand, affected up to 9% of patients. RTA can result from interstitial nephritis, for example. Both interstitial nephritis and glomerulonephritis are potential causes of CKD. In 2019, Goules et al [67] reported on 20 patients with SS who exhibited renal involvement due to the underlying disease. At the beginning of the study, 14 individuals were diagnosed with interstitial nephritis, three of whom had CKD. During the observation period, CKD developed for the first time in six patients with interstitial nephritis. Additionally, six patients were initially diagnosed with glomerulonephritis. Of these, two presented with CKD, while the remaining four were classified as either nephrotic or simply proteinuric. According to the KDIGO criteria for CKD [2], these four patients should also be considered to have CKD. It can be concluded that renal manifestations of SS are commonly associated with CKD, particularly in cases of glomerulonephritis and predominantly in cases of interstitial nephritis. If RTA were interpreted as a correlate of CKD, and the KDIGO guidelines from 2024 permitted such a conclusion [2], the prevalence of CKD in SS would be at least 13% (glomerulonephritis: 4%, RTA: 9%).
Idiopathic inflammatory myopathy (IIM)
IIM is represented by the following diseases: idiopathic dermatomyositis (IDM), juvenile dermatomyositis (JDM), idiopathic amyopathic dermatomyositis, idiopathic polymyositis (PM), immune-mediated necrotizing myopathy (IMNM), anti-synthetase syndrome (ASys), paraneoplastic idiopathic poly-/dermatomyositis, inclusion body myositis, and overlap myositis [68]. Certain forms are associated with rarer autoantibody findings, such as anti-MDA5 in idiopathic amyopathic dermatomyositis.
In an article published in 2014, Couvrat-Desvergnes et al [69] reported on renal disease manifestations in IIM. However, in this older article, three entities were distinguished: dermatomyositis, polymyositis, and the antisynthetase syndrome. One hundred fifty patients were evaluated, the CKD prevalence was 20.7%. Renal biopsy results were available for 14 patients and showed predominantly immune complex-induced glomerulonephritis. No further references on this specific topic were available as of January 2025.
MCTD
The diagnosis of MCTD is made when clinical features of collagenosis are present (e.g. Raynaud’s syndrome in combination with arthralgia/arthritis), but no specific entity (SLE, SSc) can be determined based on existing classification criteria. Anti-U1-RNP antibodies are detectable in a proportion of cases.
As of January 2025, there were exactly 10 references available on the topic of “MCTD” and “CKD”. A notable paper published in 2021 by Hanaoka et al [70] included a study with 38 patients who had pulmonary hypertension (PH) due to MCTD. The study observed the effects of vasodilator monotherapy and combination therapy, finding that earlier initiation of combination therapy was associated with less CKD progression. However, precise information on CKD prevalence in MCTD is currently unavailable.
Vasculitides
The following conditions from the diverse group of vasculitides will be addressed: AAV and IgAV. Other forms were excluded due to their lower prevalence.
AAV
AAV (antineutrophil cytoplasmic antibody-associated vasculitis) encompasses the most common small vessel vasculitides of primary origin [71]. Three main entities are recognized: GPA, MPA, and CSS. Among these, CSS has the lowest association rate with anti-neutrophil cytoplasmic antibodies, at approximately 40% [72]. Patients with GPA and MPA are particularly at high risk for renal complications, with extracapillary diffuse-proliferative glomerulonephritis being especially concerning. The absence of distinct findings in immunofluorescence histology is why these conditions are referred to as “pauci-immune” [73]. This article will not focus on acute glomerulonephritis, which typically leads to AKI. Given the prognostic significance of kidney involvement in AAV, it is surprising that a systematic literature search using the terms “AAV” or “ANCA-associated vasculitis” in conjunction with “chronic kidney disease” (CKD) yielded virtually no results as of January 2025. Therefore, it is important to highlight that AKI is a known risk factor for the development of CKD. This unique aspect of nephrology is revisited at the conclusion of the article. Consequently, it is essential to evaluate how often CKD develops in the later stages following acute glomerulonephritis. Sachez-Alamo et al [74] published a summarized analysis in 2024 that examined seven controlled randomized trials involving nearly 850 patients with AAV. These studies were conducted between 1995 and 2012. In the overall cohort, the prevalence of CKD requiring dialysis (stage 5D according to CKD-EPI) was found to be 22% after 10 years. However, the article did not provide specific information on the prevalence of other, milder stages of CKD after approximately 5 or 10 years. It can be inferred that the prevalence of milder stages is likely significantly higher than that of a non-vasculitic comparison group with CKD. Notably, the prevalence of dialysis-requiring CKD was already higher than the overall prevalence of CKD in Central Europe. A study conducted by Kramer et al in 2024 [75] examined 358 patients with AAV who were treated in Germany and Switzerland between 1999 and 2022. The authors analyzed patients with renal involvement at the time treatment was initiated separately. During the follow-up, renal events were classified as either a persistent requirement for dialysis (CKD stage 5D) or an eGFR of less than 15 mL/min without the need for dialysis. The study found that renal events occurred more frequently in MPA as compared to GPA patients and were also more common after treatment with rituximab compared to cyclophosphamide therapy. In summary, the prevalence of severe CKD, specifically stages 5 non-dialysis and dialysis, as a late complication of renal involvement in AAV, is notably high. It nearly matches the overall prevalence of CKD observed in adult Central Europeans without AAV.
IgAV
IgAV (previously known as Schoenlein Henoch purpura) is more commonly observed in children than in adults. Renal involvement can manifest as IgA-positive mesangioproliferative glomerulonephritis. IgAN may be considered an isolated renal manifestation of IgAV [76]. Similar challenges arise in determining the prevalence of CKD in patients with IgAV as seen in those with AAV. There is a lack of systematic data in the literature regarding the prevalence of CKD within this specific condition. Therefore, assessing the long-term outcomes of patients with significant renal involvement remains the primary approach. A retrospective study conducted in 2023 [77] evaluated the long-term outcomes of both pediatric (n = 60) and adult patients (n = 60) with IgAV, compared to adults with IgAN (n = 45). At 1 year post-diagnosis, the rates of dialysis did not differ significantly between adults with IgAV and those with IgAN. However, in the adult IgAV cohort, the mean serum creatinine level was 1.29 mg/dL after 1 year. Hematuria was observed in nearly 50% of cases (46.4%), and dipstick proteinuria was present in almost 32% of patients. These findings suggest that adult IgAV is frequently associated with CKD or may lead to CKD.
| Additional Factors of Increased CKD Risk in Autoimmune-Mediated Inflammatory Rheumatic Diseases | ▴Top |
Two aspects need to be addressed: first, the potential effects of inflammatory activity associated with underlying diseases on renal function; and second, the multifactorial increase in the risk of cardiovascular disease in autoimmune conditions. As this risk escalates, so too does renal morbidity and the likelihood of developing CKD.
In 2016, Kochi et al [78] published a retrospective observational cohort study of 345 RA patients with the aim of identifying associations between the extent of systemic inflammation and the risk of new manifestations of CKD. This KDIGO-adapted risk was defined by a reduction in eGFR to below 60 mL/min and/or a positive proteinuria finding in the urine test strip (time window > 3 months duration). Three categories of CRP levels were defined as correlates of systemic inflammation: no elevated CRP, intermittently elevated CRP and permanently elevated CRP. During the observation period of 89 months, 14% of the patients developed CKD according to the definition, with increasing incidences in the three CRP categories. Permanently elevated CRP levels (category 3) were identified as independently predictive of incident CKD. An older study from 2011 [79] found no clear correlation between CKD prevalence and inflammatory activity of RA, but the number of patients included was not very high, with barely 190 individuals (divided into three groups). In addition, it was not a follow-up study that would have allowed the identification of incident CKD cases.
The fact that patients with RA suffer from a higher risk of cardiovascular disease and death should now be considered certain. The literature is clear in this regard. The increase in cardiovascular risk has two main causes: the almost regular use of drugs that have a proven proatherogenic effect, such as NSAIDs and glucocorticoids. Although dedicated DMARDs even have antiatherogenic or cardioprotective properties (methotrexate [80]), the deleterious effects of the other two groups mentioned are usually in the foreground. Another proatherogenic factor is likely to be the inflammatory activity of the underlying disease. The association between inflammatory rheumatic disease and increased cardiovascular disease burden is so evident that official EULAR recommendations on cardiovascular risk management in RA and other entities have already been published [81]. Premature or aggravated atherosclerosis is associated with the manifestation of hypertensive atherosclerotic nephropathy, which is now the second most common cause of CKD [82].
A final aspect that is worth mentioning concerns the possible transition of AKI into CKD [83-85]. We recently published a separate review article on the topic of AKI in rheumatic diseases [86]. Certain entities of the inflammatory rheumatic spectrum are associated with a disproportionately high risk of AKI, namely SLE and AAV. NSAIDs and other therapeutic agents can also induce AKI. Each individual episode of AKI increases the lifetime risk of CKD, and life expectancy is reduced overall [87]. This aspect must always be considered if individuals with an underlying rheumatic disease of autoimmune origin suffer from AKI.
Although the article primarily does not address potential drug effects on kidney function and structure, some concluding information is essential. The question arises as to which substances from the relatively extensive group of antirheumatic medications either increase or reduce the risk of CKD. Here, only the direct effects of the medications will be considered, excluding, for example, the therapeutic effects of immunosuppressants on potential glomerulonephritis. The successful treatment of glomerulonephritis in SLE, for instance, would inherently reduce the CKD risk. It is well established that NSAIDs can be nephrotoxic. The most well-known example is analgesic nephropathy, a severe and often chronic kidney disorder often resulting from the use of phenacetin [88]. This substance, which is no longer available, generated strong vasoconstrictive effects after being metabolized into phenetidine and paracetamol. The morphological correlate was ischemic papillary necrosis. Contemporary NSAIDs (such as ibuprofen, diclofenac, and naproxen) also reduce renal blood flow (due to decreased intrarenal prostaglandin production), and the risk of significant blood flow disturbances is particularly concerning in patients with inherently reduced renal perfusion, such as those with heart failure. In such cases, renal blood flow is stabilized by increased prostaglandin production in the kidney tissue. Clinically, this often manifests as AKI [89], which is a known risk factor for subsequent CKD [85]. Regardless of hemodynamic effects, acute or chronic interstitial nephritis can develop with the use of nearly any NSAID, leading to implications for CKD risk. The exact prevalence of chronic interstitial nephritis associated with NSAID therapy is likely not well established, as many cases may be oligo- or asymptomatic. Literature indicates that approximately 10% of medication-induced cases of acute interstitial nephritis are attributed to the use of NSAIDs [90]. Glucocorticoids do not exhibit direct nephrotoxic effects, nor do the substances azathioprine (case reports of interstitial nephritis [91]) and mycophenolic acid. Sporadically, sulfasalazine may also trigger interstitial nephritis [92]. Calcineurin inhibitors are significantly nephrotoxic, causing acute damage due to vasoconstrictive effects and (possibly) subacute to chronic damage due to pro-mesenchymal effects. The latter effects have been questioned in the past [93]. Acute toxicity can be somewhat managed with regular monitoring of drug levels. Methotrexate does not harm the renal parenchyma at rheumatological dosages; however, in oncology, much higher doses are used, which can lead to tubular precipitation and impaired function (crystalline nephropathy) [94]. Among the increasingly extensive group of biologics, no substances are known to have nephrotoxic effects, and the same applies to JAK inhibitors. No rheumatic medication, whether short- or long-term, exhibits direct nephroprotective effects. However, it is known that methotrexate can influence the cardiovascular risk of patients with RA [95]. As cardiovascular risk decreases, so does the risk of hypertensive-atherosclerotic kidney damage, thereby reducing the risk of CKD.
Figure 1 summarizes pathogenetic processes of CKD manifestation in inflammatory rheumatic diseases, both with regard to direct organ involvement of the underlying diseases and with regard to additive processes (inflammation, cardiovascular disease risk).
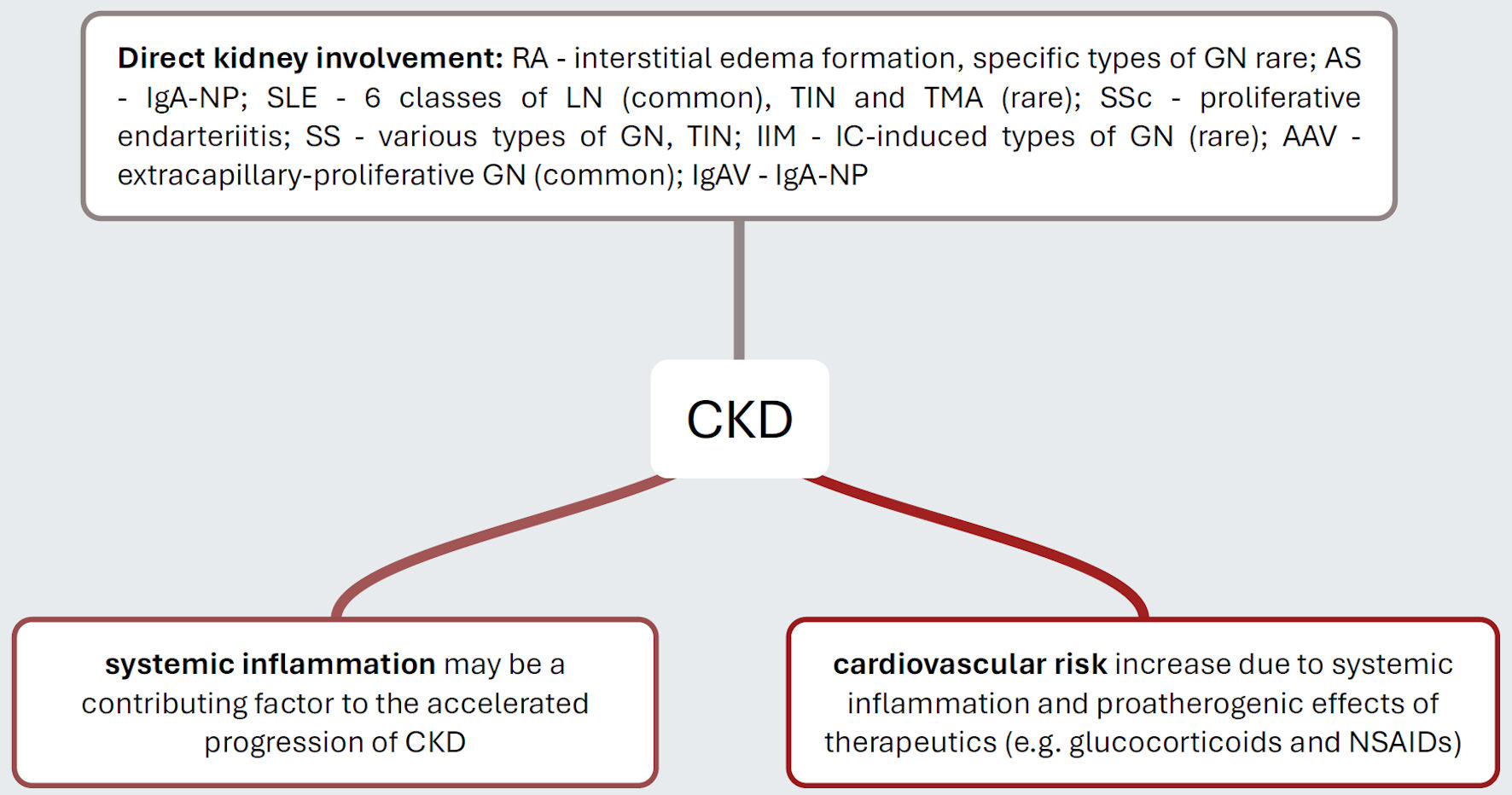 Click for large image | Figure 1. Pathogenetic mechanisms that explain the increased risk of CKD in distinct inflammatory rheumatic diseases. Rheumatic autoimmunopathies manifest themselves with varying frequency directly on the kidneys, with SLE and AAV being disproportionately common. It is now considered certain that individuals with rheumatic autoimmunopathies suffer from a significantly increased cardiovascular risk, which ultimately increases the risk of hypertensive atherosclerotic nephropathy. Presumably, the inflammatory activity of the diseases themselves is also a progression-promoting factor. CKD: chronic kidney disease; RA: rheumatoid arthritis; GN: glomerulonephritis; IgA-NP: IgA nephropathy; SLE: systemic lupus erythematosus; LN: lupus nephritis; TIN: tubulo-interstitial nephritis; TMA: thrombotic microangiopathy; SSc: systemic sclerosis; SS: Sjogren’s syndrome; IIM: idiopathic inflammatory myopathy; IC: immunocomplex; AAV: ANCA-associated vasculitis; IgAV: IgA vasculitis; NSAIDs: nonsteroidal anti-inflammatory drugs. |
| Conclusions | ▴Top |
The prevalence of CKD is elevated in specific populations with autoimmune-mediated inflammatory rheumatic diseases when compared to the Central European average. This elevated risk is attributable, at least in part, to the presence of direct extra-articular manifestations of kidney disease. In certain cases, the kidneys are rarely directly affected (e.g., RA); in others, they are regularly affected (e.g., AAV, SLE). However, generalized inflammation-associated processes and the increased cardiovascular risk also presumably contribute to the increase in CKD prevalence. Recent recommendations for monitoring patients with manifest CKD or those at increased risk of CKD have been issued. In accordance with the latest KDIGO guidelines on CKD [2], the measurement of serum creatinine concentration, along with the estimation of eGFR using methods such as CKD-EPI, is still recommended. If creatinine measurement is not feasible for specific reasons, such as in individuals with severe hyperbilirubinemia, eGFR should be estimated based on cystatin C. The second key parameter is the urine albumin-to-creatinine ratio (UACR) expressed in grams of albumin per gram of creatinine (g/g). The UACR is quantified from spot urine and approximately reflects the daily proteinuria. Since individual creatinine excretion varies, the UACR also varies among patients. It is important to note that the UACR is often not recorded, particularly in cases where the risk of CKD is significantly elevated [96]. There is certainly room for improvement in this area.
Acknowledgments
None to declare.
Financial Disclosure
No funding was provided for the study.
Conflict of Interest
The authors declare that they do not have any conflict of interest.
Author Contributions
D. Patschan, B. Marahrens, and I. Matyukhin searched for references. D. Patschan assisted in writing. H. Hansen-Nootbaar and W. Safi selected references from the pre-selection. O. Ritter prepared figure and legends. S. Patschan designed and wrote the article.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Eckardt KU. Chronic kidney disease (not) on the agenda. Dtsch Aerzteblatt Online [Internet]. 2016 [cited Sept 27, 2021]; Available from: https://www.aerzteblatt.de/10.3238/arztebl.2016.0083.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105(4S):S117-S314.
doi pubmed - Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305.
doi pubmed - Sharma A, Dhooria A, Aggarwal A, Rathi M, Chandran V. Connective tissue disorder-associated vasculitis. Curr Rheumatol Rep. 2016;18(6):31.
doi pubmed - Ponticelli C, Doria A, Moroni G. Renal disorders in rheumatologic diseases: the spectrum is changing (part 2. Arthridides). J Nephrol. 2021;34(4):1081-1090.
doi pubmed - Wartolowska K, Hough MG, Jenkinson M, Andersson J, Wordsworth BP, Tracey I. Structural changes of the brain in rheumatoid arthritis. Arthritis Rheum. 2012;64(2):371-379.
doi pubmed - Strobel ES, Fritschka E. Renal diseases in ankylosing spondylitis: review of the literature illustrated by case reports. Clin Rheumatol. 1998;17(6):524-530.
doi pubmed - Hoi A, Igel T, Mok CC, Arnaud L. Systemic lupus erythematosus. Lancet. 2024;403(10441):2326-2338.
doi pubmed - Kitching AR, Anders HJ, Basu N, Brouwer E, Gordon J, Jayne DR, Kullman J, et al. ANCA-associated vasculitis. Nat Rev Dis Primers. 2020;6(1):71.
doi pubmed - Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179-184.
doi pubmed - Anders HJ, Vielhauer V. Renal co-morbidity in patients with rheumatic diseases. Arthritis Res Ther. 2011;13(3):222.
doi pubmed - Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713-735.
doi pubmed - Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5(6):1003-1009.
doi pubmed - Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389(10075):1238-1252.
doi pubmed - Murray CJL. The global burden of disease study at 30 years. Nat Med. 2022;28(10):2019-2026.
doi pubmed - Collaboration GBDCKD. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709-733.
doi pubmed - Shimizu M, Paudel ML, Shadick N, Weinblatt M, Solomon DH. Age of onset of rheumatoid arthritis and radiographic changes. Semin Arthritis Rheum. 2025;71:152635.
doi pubmed - Finckh A, Gilbert B, Hodkinson B, Bae SC, Thomas R, Deane KD, Alpizar-Rodriguez D, et al. Global epidemiology of rheumatoid arthritis. Nat Rev Rheumatol. 2022;18(10):591-602.
doi pubmed - Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390(10089):73-84.
doi pubmed - Boel A, Lopez-Medina C, van der Heijde D, van Gaalen FA. Age at onset in axial spondyloarthritis around the world: data from the Assessment in SpondyloArthritis international Society Peripheral Involvement in Spondyloarthritis study. Rheumatology (Oxford). 2022;61(4):1468-1475.
doi pubmed - Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Time trends in epidemiology and characteristics of psoriatic arthritis over 3 decades: a population-based study. J Rheumatol. 2009;36(2):361-367.
doi pubmed - Bentaleb I, Abdelghani KB, Rostom S, Amine B, Laatar A, Bahiri R. Reactive Arthritis: Update. Curr Clin Microbiol Rep. 2020;7(4):124-132.
doi pubmed - Sassi RH, Hendler JV, Piccoli GF, Gasparin AA, da Silva Chakr RM, Brenol JC, Monticielo OA. Age of onset influences on clinical and laboratory profile of patients with systemic lupus erythematosus. Clin Rheumatol. 2017;36(1):89-95.
doi pubmed - Alba MA, Velasco C, Simeon CP, Fonollosa V, Trapiella L, Egurbide MV, Saez L, et al. Early- versus late-onset systemic sclerosis: differences in clinical presentation and outcome in 1037 patients. Medicine (Baltimore). 2014;93(2):73-81.
doi pubmed - Sebastian A, Madej M, Sebastian M, Morgiel E, Wawryka P, Wiland P. Differences in clinical phenotypes of primary Sjogren's syndrome depending on early or late onset. Adv Clin Exp Med. 2021;30(11):1141-1146.
doi pubmed - Mixed Connective-Tissue Disease (MCTD): practice essentials, pathophysiology, etiology. 2024 [cited July 19, 2025]; Available from: https://emedicine.medscape.com/article/335815-overview#a6.
- Monti S, Craven A, Klersy C, Montecucco C, Caporali R, Watts R, Merkel PA, et al. Association between age at disease onset of anti-neutrophil cytoplasmic antibody-associated vasculitis and clinical presentation and short-term outcomes. Rheumatology (Oxford). 2021;60(2):617-628.
doi pubmed - Kapoor T, Bathon J. Renal manifestations of rheumatoid arthritis. Rheum Dis Clin North Am. 2018;44(4):571-584.
doi pubmed - Gubner R, August S, Ginsberg V. Therapeutic suppression of tissue reactivity. II. Effect of aminopterin in rheumatoid arthritis and psoriasis. Am J Med Sci. 1951;221(2):176-182.
pubmed - Curtis JR, Singh JA. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther. 2011;33(6):679-707.
doi pubmed - Davies DJ, Dowling J, Xipell JM. Gold nephropathy. Pathology. 1977;9(4):281-288.
doi pubmed - Chiu HY, Huang HL, Li CH, Chen HA, Yeh CL, Chiu SH, Lin WC, et al. Increased Risk of Chronic Kidney Disease in Rheumatoid Arthritis Associated with Cardiovascular Complications - A National Population-Based Cohort Study. PLoS One. 2015;10(9):e0136508.
doi pubmed - Tokoroyama T, Ando M, Setoguchi K, Tsuchiya K, Nitta K. Prevalence, incidence and prognosis of chronic kidney disease classified according to current guidelines: a large retrospective cohort study of rheumatoid arthritis patients. Nephrol Dial Transplant. 2017;32(12):2035-2042.
doi pubmed - Horak P, Smrzova A, Krejci K, Tichy T, Zadrazil J, Skacelova M. Renal manifestations of rheumatic diseases. A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013;157(2):98-104.
doi pubmed - Wegelius O, Klockars M. Reversible acute renal failure complicating rheumatoid arthritis. Scand J Rheumatol. 1987;16(1):161-168.
doi pubmed - Savadogo B, Fahed H, Sellam J, Georgin-Lavialle S, Fautrel B, Mitrovic S. AA amyloidosis in inflammatory joint diseases: A systematic review. Semin Arthritis Rheum. 2025;74:152762.
doi pubmed - Simms RW, Prout MN, Cohen AS. The epidemiology of AL and AA amyloidosis. Baillieres Clin Rheumatol. 1994;8(3):627-634.
doi pubmed - Karam S, Haidous M, Royal V, Leung N. Renal AA amyloidosis: presentation, diagnosis, and current therapeutic options: a review. Kidney Int. 2023;103(3):473-484.
doi pubmed - Bittar M, Maksymowych WP. A review on imaging in axial spondyloarthritis: spartan 2024 annual meeting proceedings. Curr Rheumatol Rep. 2025;27(1):12.
doi pubmed - Braun J. Axial spondyloarthritis: thoughts about nomenclature and treatment targets. Clin Exp Rheumatol. 2012;30(4 Suppl 73):S132-135.
pubmed - Carron P, De Craemer AS, Van den Bosch F. Peripheral spondyloarthritis: a neglected entity-state of the art. RMD Open. 2020;6(1):e001136.
doi pubmed - Akkoc N, Khan MA. Is axial spondyloarthritis more common than rheumatoid arthritis? Curr Rheumatol Rep. 2020;22(9):54.
doi pubmed - Strand V, Rao SA, Shillington AC, Cifaldi MA, McGuire M, Ruderman EM. Prevalence of axial spondyloarthritis in United States rheumatology practices: Assessment of SpondyloArthritis International Society criteria versus rheumatology expert clinical diagnosis. Arthritis Care Res (Hoboken). 2013;65(8):1299-1306.
doi pubmed - Shbeeb M, Uramoto KM, Gibson LE, O'Fallon WM, Gabriel SE. The epidemiology of psoriatic arthritis in Olmsted County, Minnesota, USA, 1982-1991. J Rheumatol. 2000;27(5):1247-1250.
pubmed - Champtiaux N, Liote F, El Karoui K, Vigneau C, Miceli C, Cornec-Le Gall E, Remy P, et al. Spondyloarthritis-Associated IgA Nephropathy. Kidney Int Rep. 2020;5(6):813-820.
doi pubmed - Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, Liu ZH, et al. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91(5):1014-1021.
doi pubmed - Hwang S, Kim YJ, Ahn SM, Koo BS. Incidence rate of chronic kidney disease and its association with long-term nonsteroidal anti-inflammatory drug use in ankylosing spondylitis: A nationwide population-based study. Int J Rheum Dis. 2024;27(8):e15310.
doi pubmed - Wanders A, Heijde D, Landewe R, Behier JM, Calin A, Olivieri I, Zeidler H, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005;52(6):1756-1765.
doi pubmed - Ramiro S, Nikiphorou E, Sepriano A, Ortolan A, Webers C, Baraliakos X, Landewe RBM, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023;82(1):19-34.
doi pubmed - Kharouf F, Gao S, Al-Matar S, Cook RJ, Chandran V, Gladman DD. Chronic kidney disease in patients with psoriatic arthritis: a cohort study. RMD Open. 2024;10(4):.
doi pubmed - Quarenghi MI, Del Vecchio L, Casartelli D, Manunta P, Rossi R. MPO antibody-positive vasculitis in a patient with psoriatic arthritis and gold-induced membranous glomerulonephritis. Nephrol Dial Transplant. 1998;13(8):2104-2106.
doi pubmed - Arnaud L, Tektonidou MG. Long-term outcomes in systemic lupus erythematosus: trends over time and major contributors. Rheumatology (Oxford). 2020;59(Suppl5):v29-v38.
doi pubmed - Anders HJ, Saxena R, Zhao MH, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nat Rev Dis Primers. 2020;6(1):7.
doi pubmed - Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65(2):521-530.
doi pubmed - Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15(2):241-250.
doi pubmed - Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, D'Agati VD, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93(4):789-796.
doi pubmed - Parikh SV, Almaani S, Brodsky S, Rovin BH. Update on Lupus Nephritis: Core Curriculum 2020. Am J Kidney Dis. 2020;76(2):265-281.
doi pubmed - Yuan M, Tan Y, Zhao MH. The Role of Anti-mCRP Autoantibodies in Lupus Nephritis. Kidney Dis (Basel). 2023;9(5):317-325.
doi pubmed - Almaani S, Meara A, Rovin BH. Update on lupus nephritis. Clin J Am Soc Nephrol. 2017;12(5):825-835.
doi pubmed - Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685-1699.
doi pubmed - Cole A, Ong VH, Denton CP. Renal disease and systemic sclerosis: an update on scleroderma renal crisis. Clin Rev Allergy Immunol. 2023;64(3):378-391.
doi pubmed - Bose N, Chiesa-Vottero A, Chatterjee S. Scleroderma renal crisis. Semin Arthritis Rheum. 2015;44(6):687-694.
doi pubmed - Iliopoulos G, Daoussis D. Renal dysfunction in systemic sclerosis beyond scleroderma renal crisis. Rheumatol Int. 2021;41(7):1203-1208.
doi pubmed - Gigante A, Barbano B, Granata G, Quarta S, Amoroso A, Salsano F, Cianci R, et al. Evaluation of estimated glomerular filtration rate and clinical variables in systemic sclerosis patients. Clin Nephrol. 2016;85(6):326-331.
doi pubmed - Ramos-Casals M, Brito-Zeron P, Seror R, Bootsma H, Bowman SJ, Dorner T, Gottenberg JE, et al. Characterization of systemic disease in primary Sjogren's syndrome: EULAR-SS Task Force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology (Oxford). 2015;54(12):2230-2238.
doi pubmed - Seror R, Theander E, Brun JG, Ramos-Casals M, Valim V, Dorner T, Bootsma H, et al. Validation of EULAR primary Sjogren's syndrome disease activity (ESSDAI) and patient indexes (ESSPRI). Ann Rheum Dis. 2015;74(5):859-866.
doi pubmed - Goules A, Geetha D, Arend LJ, Baer AN. Renal involvement in primary Sjogren's syndrome: natural history and treatment outcome. Clin Exp Rheumatol. 2019;37(3 Suppl 118):123-132.
pubmed - Lundberg IE, Fujimoto M, Vencovsky J, Aggarwal R, Holmqvist M, Christopher-Stine L, Mammen AL, et al. Idiopathic inflammatory myopathies. Nat Rev Dis Primers. 2021;7(1):86.
doi pubmed - Couvrat-Desvergnes G, Masseau A, Benveniste O, Bruel A, Hervier B, Mussini JM, Buob D, et al. The spectrum of renal involvement in patients with inflammatory myopathies. Medicine (Baltimore). 2014;93(1):33-41.
doi pubmed - Hanaoka H, Ishigaki S, Takei H, Hiramoto K, Saito S, Kondo Y, Kikuchi J, et al. Early combination of pulmonary vasodilators prevents chronic kidney disease progression in connective tissue disease-associated pulmonary hypertension. Int J Rheum Dis. 2021;24(11):1419-1426.
doi pubmed - Watts RA, Hatemi G, Burns JC, Mohammad AJ. Global epidemiology of vasculitis. Nat Rev Rheumatol. 2022;18(1):22-34.
doi pubmed - Chakraborty RK, Rout P. Eosinophilic granulomatosis with polyangiitis (churg-strauss syndrome). In: StatPearls. Treasure Island (FL) ineligible companies. 2025.
pubmed - Sinico RA, Di Toma L, Radice A. Renal involvement in anti-neutrophil cytoplasmic autoantibody associated vasculitis. Autoimmun Rev. 2013;12(4):477-482.
doi pubmed - Sachez-Alamo B, Moi L, Bajema I, Berden A, Flossmann O, Hruskova Z, Jayne D, et al. Long-term outcome of kidney function in patients with ANCA-associated vasculitis. Nephrol Dial Transplant. 2024;39(9):1483-1493.
doi pubmed - Kramer S, Vogt K, Schreibing TM, Busch M, Schmitt T, Bergner R, Mosberger S, et al. Remission induction therapies and long-term outcomes in granulomatosis with polyangiitis and microscopic polyangiitis: real-world data from a European cohort. Rheumatol Int. 2024;45(1):7.
doi pubmed - Pillebout E, Sunderkotter C. IgA vasculitis. Semin Immunopathol. 2021;43(5):729-738.
doi pubmed - Levanon S, Gotloib V, Kraus Y, Novofastovski I, Brikman S, Fawaz A, Egbaria M, et al. IgA vasculitis in adults, pediatrics and non-vasculitic IgA nephropathy, retrospective analysis from 2 centers. Medicine (Baltimore). 2023;102(50):e36521.
doi pubmed - Kochi M, Kohagura K, Shiohira Y, Iseki K, Ohya Y. Inflammation as a Risk of Developing Chronic Kidney Disease in Rheumatoid Arthritis. PLoS One. 2016;11(8):e0160225.
doi pubmed - Haroon M, Adeeb F, Devlin J, D OG, Walker F. A comparative study of renal dysfunction in patients with inflammatory arthropathies: strong association with cardiovascular diseases and not with anti-rheumatic therapies, inflammatory markers or duration of arthritis. Int J Rheum Dis. 2011;14(3):255-260.
doi pubmed - Mangoni AA, Zinellu A, Sotgia S, Carru C, Piga M, Erre GL. Protective effects of methotrexate against proatherosclerotic cytokines: a review of the evidence. Mediators Inflamm. 2017;2017:9632846.
doi pubmed - Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJ, Kvien TK, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76(1):17-28.
doi pubmed - Romagnani P, Remuzzi G, Glassock R, Levin A, Jager KJ, Tonelli M, Massy Z, et al. Chronic kidney disease. Nat Rev Dis Primers. 2017;3:17088.
doi pubmed - Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11(5):264-276.
doi pubmed - Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J Am Soc Nephrol. 2015;26(8):1765-1776.
doi pubmed - Forni LG, Darmon M, Ostermann M, Oudemans-van Straaten HM, Pettila V, Prowle JR, Schetz M, et al. Renal recovery after acute kidney injury. Intensive Care Med. 2017;43(6):855-866.
doi pubmed - Patschan D, Schmalz G, Safi W, Stasche F, Matyukhin I, Ritter O, Patschan S. Acute kidney injury in autoimmune-mediated rheumatic diseases. J Clin Med Res. 2025;17(2):67-75.
doi pubmed - Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193-207.
doi pubmed - Nanra RS. Analgesic nephropathy. Med J Aust. 1976;1(20):745-748.
pubmed - Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3):531-539.
doi pubmed - Muriithi AK, Leung N, Valeri AM, Cornell LD, Sethi S, Fidler ME, Nasr SH. Biopsy-proven acute interstitial nephritis, 1993-2011: a case series. Am J Kidney Dis. 2014;64(4):558-566.
doi pubmed - Bir K, Herzenberg AM, Carette S. Azathioprine induced acute interstitial nephritis as the cause of rapidly progressive renal failure in a patient with Wegener's granulomatosis. J Rheumatol. 2006;33(1):185-187.
pubmed - Augusto JF, Sayegh J, Simon A, Croue A, Chennebault JM, Cousin M, Subra JF. A case of sulphasalazine-induced DRESS syndrome with delayed acute interstitial nephritis. Nephrol Dial Transplant. 2009;24(9):2940-2942.
doi pubmed - Issa N, Kukla A, Ibrahim HN. Calcineurin inhibitor nephrotoxicity: a review and perspective of the evidence. Am J Nephrol. 2013;37(6):602-612.
doi pubmed - Perazella MA, Luciano RL. Review of select causes of drug-induced AKI. Expert Rev Clin Pharmacol. 2015;8(4):367-371.
doi pubmed - Kim HJ, Kim MJ, Lee CK, Hong YH. Effects of Methotrexate on Carotid Intima-media Thickness in Patients with Rheumatoid Arthritis. J Korean Med Sci. 2015;30(11):1589-1596.
doi pubmed - Wanner C, Schaeffner E, Frese T, Weber C, Stahl P, Scherg F, Burckhardt F, et al. [InspeCKD - Analysis of the use of diagnostics in patients at high risk for chronic kidney disease in German general practitioner (GP) practices]. MMW Fortschr Med. 2024;166(Suppl 4):9-17.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.