| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 11, November 2025, pages 608-617
Clinical Features and Prediction Model of Secondary Infection Risk in Adult Patients With Chronic Respiratory Diseases: A Case-Control Study
He Qing Huanga, b, c, Hong Lua, b, Yi Ling Chenb, Mei Li Shend, Zu Tao Chenb, c, e, f, Jie Xua, f
aCenter of Clinical Laboratory, the First Affiliated Hospital of Soochow University, Suzhou, China
bDepartment of Infectious Diseases, The First Affiliated Hospital of Soochow University, Suzhou, China
cDepartment of Infectious Diseases, The Fourth Affiliated Hospital of Soochow University, Suzhou, Jiangsu Province, China
dMedical Department, Nanjing Dinfectome Technology Inc., Nanjing, China
eMOE Key Laboratory of Geriatric Diseases and Immunology, Suzhou Key Laboratory of Pathogen Bioscience and Anti-infective Medicine, Soochow University, Suzhou, China
fCorresponding Authors: Jie Xu, Center of Clinical Laboratory, the First Affiliated Hospital of Soochow University, Suzhou 215006, China; Zu Tao Chen, Department of Infectious Diseases, The First Affiliated Hospital of Soochow University, Suzhou 215006, China
Manuscript submitted June 5, 2025, accepted November 11, 2025, published online November 26, 2025
Short title: Infection Risk of Chronic Respiratory Diseases
doi: https://doi.org/10.14740/jocmr6291
| Abstract | ▴Top |
Background: There are limited investigations on the general pathogen features, clinical characteristics, and predicted clinical markers of secondary lower respiratory tract infection of chronic respiratory disorders.
Methods: A total of 154 adult inpatients with chronic respiratory diseases between 2019 and 2022 were enrolled. Clinical data were retrospectively collected and analyzed. Multivariate logistic regression analysis was used to analyze the susceptibility factors of infection secondary to chronic respiratory diseases.
Results: Among the patients with chronic respiratory diseases, the most prevalent condition was chronic obstructive pulmonary disease (44.2%, 68/154). Cough, expectoration, chest tightness, and wheezing were the predominant symptoms irrespective of infection. Pseudomonas aeruginosa accounted for 37% (20/54) in pathogen infection. Aspergillus fumigatus was the primary cause of filamentous fungal infection. The combination of low body mass index, increased tricuspid regurgitation pressure, and decreased lymphocyte count could accurately predict infection secondary to chronic respiratory diseases (area under curve (AUC): 0.788, 95% confidence interval (CI): 0.689 - 0.887, P = 0.000).
Conclusions: This study focused and explored the common features between secondary infections of various chronic respiratory diseases. The prediction model is expected to enable timely detection and treatment of secondary infections in clinical practice.
Keywords: Chronic respiratory disease; Secondary infection; Lower respiratory tract infection; Clinical features; Prediction model
| Introduction | ▴Top |
Chronic respiratory diseases are still the third leading cause of disability and death worldwide, after cardiovascular diseases and tumors, including chronic obstructive pulmonary disease (COPD), bronchiectasis, bronchial asthma, cystic pulmonary fibrosis, interstitial pneumonia, pulmonary fibrosis, pulmonary cavity, chronic tuberculosis, and other diseases [1]. Pulmonary inflammation predisposes to secondary respiratory tract infections [2]. The timing of diagnosing infection, the characteristics of pathogen infection, and the choice of empirical antibiotics have brought challenges to clinical treatment.
Clinical characteristics, rapid clinical diagnosis, and antibiotic treatment options have been better understood for community-acquired pneumonia [3-5]. Previous studies on chronic respiratory diseases with secondary infections have often focused on specific conditions, such as COPD [6, 7]. However, given the current global aging population and the pervasive use of antibiotics, there is a lack of research examining the prevalence, epidemiology, clinical manifestations, and predictive markers of infections associated with various chronic respiratory diseases. Meanwhile, efforts have been made to illustrate the potential efficacy of early interventions in preventing the severe or rapid deterioration of the disease, particularly in avoiding the increased medical and economic burden that may result from delays in the timing of treatment. Further research into the management of secondary infections in patients with chronic respiratory diseases would be valuable and warrants further investigation.
In the study, we retrospectively included adult patients diagnosed with chronic respiratory diseases and collected general information, underlying diseases, clinical symptoms, laboratory infection indicators, microbiological tests, echocardiography results, imaging characteristics, and information on infections during hospitalization. The aim was to describe the clinical features of secondary infections in patients with chronic respiratory diseases and to identify predictive risk factors for infections.
| Materials and Methods | ▴Top |
Patients’ enrollment
Patients diagnosed with chronic respiratory diseases were retrospectively collected from the First Affiliated Hospital of Soochow University between 2019 and 2022. The chronic respiratory diseases included asthma, COPD, interstitial lung disease (ILD), pulmonary nodular disease (PND), pneumoconiosis, and other chronic respiratory diseases [8]. Diagnostic criteria were in accordance with the Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) guidelines [9]. The inclusion criteria were: 1) patients with a confirmed diagnosis of chronic respiratory disease; 2) age ≥ 18 years. Exclusion criteria were: 1) patients with repeated hospitalization (> 4 times in 90 days); 2) patients with right ventricular outflow tract obstruction and/or mitral stenosis; 3) patients with comorbid malignant hematological disorders; 4) patients with consumptive diseases such as combined malignant tumors, human acquired immunodeficiency, etc. The number of cases in the center during the study period determined the sample size. The diagnosis of infection and pathogen findings of the patients with chronic respiratory diseases were reviewed and they were divided into observation and control groups. The observation group had a combined lower respiratory tract infection with evidence of pathogenesis. The control group did not have a combined lower respiratory tract infection and the culture results of the respiratory specimens showed normal flora. The diagnosis of lower respiratory tract infection referred to guideline of IDSA/ATS [10].
Ethical compliance with human/animal study
This study involving human participants was performed in accordance with the Declaration of Helsinki and was approved by Ethics Committee of the First Affiliated Hospital of Soochow University (No. 2024056). Informed consent was obtained from all participants and/or their legal guardians. All methods were performed in accordance with the relevant guidelines and regulations.
Clinical data collection
The following clinical information were collected: age, gender, underlying diseases such as hypertension, diabetes, body mass index (BMI), age of onset, laboratory indicators including white blood cell count (WBC, (3.5 - 9.5) × 109/L), lymphocyte count (LY, (1.10 - 3.20) × 109/L), neutrophil count (NE, (1.80 - 6.30) × 109/L), percentage of neutrophil count (NE%, 40.0-75.0%), C-reactive protein (CRP, 0 - 4 mg/L), procalcitonin (PCT, 0 - 0.5 ng/mL), albumin (ALB, 40-55g/L), and N-terminal pro-brain natriuretic peptide (NT-proBNP, 0 - 125 pg/mL). Additional information included echocardiography results such as left ventricular ejection fraction (LVEF, 52-74%), tricuspid regurgitation pressure gradient (TRPG), and findings from the initial chest computed tomography (CT) examination.
Detection of pathogens
Pathogen detection and antimicrobial susceptibility assessment were both conducted in the initial phase of hospitalization, using the first qualified sputum or bronchoalveolar lavage fluid samples collected from patients upon admission. The Columbia blood agar and chocolate-colored blood agar plates were inoculated respectively at 35 °C with 5% carbon dioxide to isolate common Gram-positive and Gram-negative bacteria. Aspergillus and Candida were isolated and cultivated on Sabouraud’s agar plates at 28 and 35 °C, respectively. The isolated strains were identified by biological Merieux Vitek MS mass spectrometer. Viridans group streptococci, non-pathogenic Neisseria and Rothia mucilaginosa were reported in the form of “normal flora”. Acid-fast staining was used to find Mycobacterium tuberculosis. In instances where multiple sputum or bronchoalveolar lavage fluid samples yielded positive cultures and the same strain was identified, only the initial data were subjected to analysis.
Antimicrobial susceptibility testing
The Vitek 2 compact 60 automatic microbial identification and drug sensitivity analyzer (Biomerieux, France) was used to test the drug sensitivity of the strains in vitro, and the disk diffusion method was used to supplement and review some drug sensitivity results. The test results were judged and explained according to the CLSI-M100 30th document of the American Clinical Laboratory Standardization Committee. The quality control strains were Escherichia coli ATCC25922 and ATCC8739, Pseudomonas aeruginosa ATCC27853, and Staphylococcus aureus ATCC29213.
Statistical analysis
SPSS29.0 statistical software was used for statistical analysis. Considering retrospective collection of clinical data, patients with more than four missing data were excluded from statistical analyses, and the remaining default values were expressed as within-group means. If the continuous variables were normally distributed, they were expressed as the mean ± standard deviation (SD). The independent sample t-test was used for comparison between groups. The skewed distribution was expressed as median and quartile. The Mann-Whitney U test was used for comparison between groups. The categorical variables were expressed as frequency and percentage. The Chi-square test or Fisher exact probability method was used for comparison between groups. Multivariate logistic regression analysis was used to analyze the susceptibility factors of infection secondary to chronic respiratory diseases. The receiver operating characteristic (ROC) curve was drawn with SPSS software to evaluate the predictive value. P value < 0.05 was considered statistically significant.
| Results | ▴Top |
Patients’ enrollment
An initial retrospective collection of 298 adult patients with chronic respiratory diseases diagnosed at the First Affiliated Hospital of Soochow University between 2019 and 2022 was performed. One hundred nine patients were excluded according to the exclusion criteria, including four pediatric patients, 33 multiple hospitalizations, 27 right ventricular outflow tract obstruction and/or mitral stenosis, two patients with hematological disorders, and 43 patients combined with consumptive diseases. Then 189 patients fulfilled the enrolment criteria and clinical diagnosis of infection, etiological, and clinical data were collected. After the final exclusion of 35 patients with excessive missing clinical data, 154 patients were included in the final data analysis. Based on the clinical infection diagnosis and pathogen findings of sputum or bronchoalveolar lavage fluid, 154 patients with chronic respiratory diseases were split into two groups: the observation group with infection (n = 54) and the control group without infection (n = 100) (Fig. 1).
 Click for large image | Figure 1. Enrolment flowchart. |
Among 154 patients with chronic respiratory diseases, there were 68 cases of COPD, five cases of bronchiectasis, seven cases of bronchial asthma, 55 cases of interstitial pneumonia, five cases of pulmonary cavity, two cases of pulmonary tuberculosis and 15 cases of pulmonary fibrosis, and four patients with two chronic respiratory diseases (Table 1).
 Click to view | Table 1. The Diseases Type and Culture Result of 154 Patients With Chronic Respiratory Diseases |
General features between the observation group and the control group
The control group consisted of 100 uninfected cases (64.9%) with normal flora (viridans group Streptococcus viridans, non-pathogenic Neisseria and Rothia mucilaginosa), and the observation group covered 54 cases with pathogenetic evidence of infection (35.1%) (Table 2). The ratio of male to female was 2.21:1, with 106 males and 48 females. The range of ages was 30 to 100. The control group’s median age was 67 years old, while the observation group’s median age was 72 years old, and the difference was statistically significant (P < 0.001). There was no significant difference in the age and gender between the two groups at the time of diagnosis of chronic respiratory diseases (P > 0.05). The onset age range ≥ 55 years old was the highest in both groups. The BMI in the observation group was significantly lower than the control group (19.65 vs. 23.13, P < 0.001). Smoking history, diabetes, and hypertension rates in the observation group were slightly higher than those the control group, but with no significant difference (P > 0.05). Both groups had similar rates of diabetes and hypertension, and there was no significant difference between them (P > 0.05). The number of acute exacerbation ≥ 2/year did not significantly differ between the two groups (P > 0.05).
 Click to view | Table 2. The Common Clinical Characteristics of the Observation Group and the Control Group |
In the observation group, there were 32 cases of non-fermenting bacteria, eight cases of Aspergillus fumigatus, six cases of Enterobacteriaceae, five cases of acid-fast bacilli (suspected Mycobacterium tuberculosis), three cases of Candida, one case of Staphylococcus aureus, one case of Corynebacterium striatum, and one case of Hemophilus. Pseudomonas aeruginosa constituted the majority of non-fermenting bacteria in 20 instances, and only found in COPD. Klebsiella pneumoniae, the predominant species in the Enterobacteriaceae family, was present in three cases and was more common in pulmonary fibrosis. Among Candida, two cases were Candida albicans and one case was Candida tropicalis, which were mostly distributed in interstitial pneumonia. Three cases of mixed infections were found in COPD, two of which were Burkholderia cepacia and Acinetobacter baumannii, and the other case was Corynebacterium striatum and Burkholderia contaminans.
Clinical presentation and laboratory indicators between the two groups
Cough and expectoration were the most prevalent clinical signs in both groups with similar incidences of > 75%. Chest tightness and wheezing were also frequent clinical presentations. The proportions of fever and respiratory distress in both groups were lower than 30%. There was no significant difference of these presentations between the two groups (P > 0.05) (Table 2).
The laboratory indicators including NE% (P < 0.001), CRP (P = 0.003), PCT (P = 0.005), ALB (P < 0.001), and TRPG (P = 0.001) in the observation group were significantly higher than those in the control group. While the LY of the observation group was significantly lower than that of the control group (P < 0.001). There was no significant difference in other indicators between the two groups, such as WBC, NE, NT-proBNP, and LVEF (P > 0.05) (Table 2).
Drug resistance characteristics of Pseudomonas aeruginosa infection secondary to chronic respiratory diseases
Pseudomonas aeruginosa constituted the majority of infection in the observation group and we further collected its susceptibility test results. Pseudomonas aeruginosa exhibited resistance rates of 35.0%, 35.0%, 30.0%, and 33.3% to imipenem, meropenem, quinolones ciprofloxacin, and levofloxacin, respectively. And it was susceptible to amikacin, tobramycin, ceftazidime, and cefepime, with the susceptibility rates of 90.0%, 94.4%, 80.0%, and 80.0%, respectively (Table 3).
 Click to view | Table 3. Susceptibility Results of Pseudomonas aeruginosa to Common Antibiotics |
Multivariate analysis of infection secondary to chronic respiratory diseases
The meaningful indicators of univariate analysis were incorporated into the multivariate binary logistic regression analysis model as independent variable X, including BMI, TRPG, NE%, CRP, age, LY, and ALB, and secondary infection of chronic respiratory diseases was used as the dependent variable Y. There was a negative correlation between infection and LY (odds ratio (OR) = 0.122, P = 0.024), BMI (OR = 0.789, P = 0.023), and NE% (OR = 0.908, P = 0.015), and positively correlated with TRPG (OR = 1.116, P = 0.025) (Table 4).
 Click to view | Table 4. Multivariate Logistic Regression Analysis of Chronic Respiratory Diseases With Infections |
Prediction model
BMI and peripheral blood LY had high predictive value for infection in hospitalized patients with chronic respiratory diseases. The predictive value of TRPG was general. The combined prediction model (area under curve (AUC) = 0.788, 95% confidence interval (CI): 0.689 - 0.887, P < 0.001) performed better than any single indicator (Table 5, Fig. 2).
 Click to view | Table 5. The Predictive Value of BMI, LY, and TRPG for Infection in Patients With Chronic Respiratory Diseases |
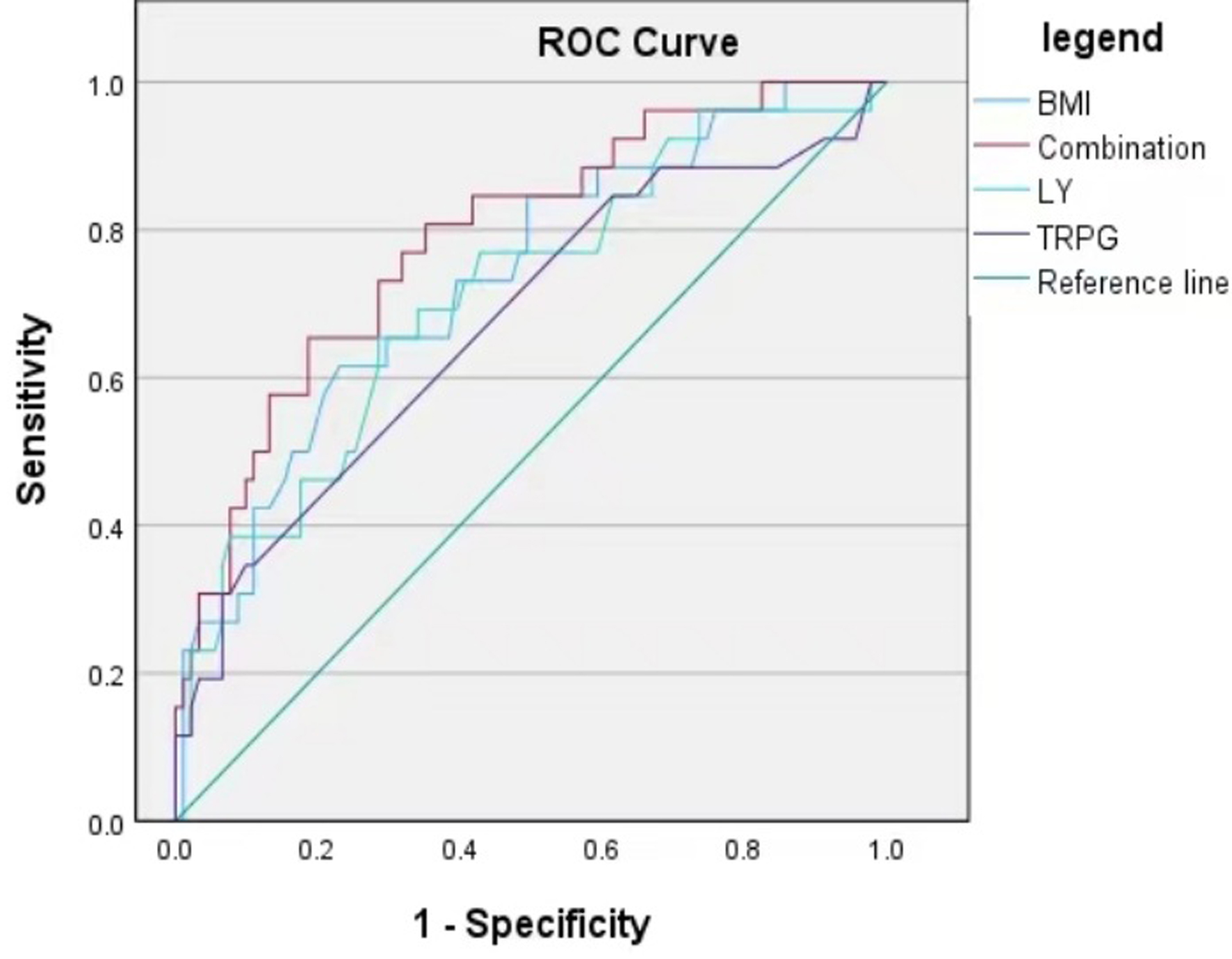 Click for large image | Figure 2. ROC curve of a predictive model for infections in patients with chronic respiratory diseases. BMI: body mass index; LY: lymphocyte count; ROC: receiver operating characteristic; TRPG: tricuspid regurgitation pressure gradient. |
| Discussion | ▴Top |
Chronic respiratory diseases have a high global incidence and death rate, which places a significant financial and clinical treatment burden. This article aims to reflect the general epidemiological features, clinical characteristics, and establishment of predictive infection models of secondary pathogenic bacteria infection in chronic respiratory diseases. The combined model of low body weight, elevated tricuspid regurgitant pressure, and reduced LY had a good predictive value for secondary infections in chronic respiratory disease.
Patients with chronic respiratory diseases had repeated airway inflammation and airway structure damage, which weakened their clean self-purification ability and low local resistance. Pathogens are challenging to eradicate yet simple to colonize [11]. The research showed that long-term damage to the anatomy of the airways has been found to increase the risk of recurrent hospitalization for antibiotic therapy [12]. The majority of Gram-negative bacteria are resistant to many drugs, making them the most prevalent cause of bacterial illnesses. Therefore, it is crucial for the prevention and treatment of patients with this group of diseases, early detection of infection and empirical treatment to prevent disease exacerbation and reduce the medical burden by analyzing the distribution of pathogenic bacteria, drug resistance characteristics, and susceptibility factors of pathogen in patients with chronic respiratory diseases. In this study, the secondary infection proportion of patients with secondary infection of chronic respiratory illnesses was 35.1%, mainly bacterial infection. Pseudomonas aeruginosa was the most common bacteria, followed by Klebsiella pneumoniae. Aspergillus fumigatus was the leading cause of fungal infection. According to studies, Pseudomonas aeruginosa is hard to get rid of in COPD [13, 14]. It can also be easily re-infected, and its strains’ rates of mutation and antibiotic resistance are trending upward. The pathogenicity of Aspergillus depends on the host’s capacity to fight against infection. In addition to the host immunological status, the initial condition of the lung structure is a significant influence, and the risk of it will rise with the deterioration of lung parenchyma and airway structure [15]. Fungal infection is more common in autoimmune interstitial pneumonia, and can also be related to long-term oral hormones in patients. The high resistance that Pseudomonas aeruginosa exhibits to carbapenems (such as imipenem and meropenem) and quinolones (such as ciprofloxacin and levofloxacin), may be attributed to the widespread use of these drugs in clinical settings. The high susceptibility of ceftazidime and cefepime may be attributed to the medication’s properties as well as Pseudomonas aeruginosa’s drug resistance mechanism. Currently, aminoglycosides have relatively few clinical applications and remain highly sensitive. They can be used as an option or in combination with other drugs in anti-infective treatment.
Patients with chronic respiratory diseases are more likely to be middle-aged and older men, with age of over 55. Patients with infections were typically malnourished (lower BMI) and exhibit non-typical clinical manifestations, such as cough and expectoration, tightness in the chest, and wheezing. Fever is less common than simple pulmonary infections, which may be related to the complementary use of antibiotics, long-term use of oral hormones, and decreased immunity in patients with these conditions [16-18]. This also makes it difficult for such patients to detect infection in time in clinical practice, delaying the timing of treatment. The immune function of diabetic patients is low, and hyperglycemia is beneficial to bacterial reproduction [19]. However, this study did not find significant association between the diabetes and the presence of harmful microorganisms. Hypertension, smoking history, and acute exacerbations ≥ 2 times/year were not associated with the secondary infection of chronic respiratory diseases in this study. But some studies have shown that long-term smoking could lead to the dysfunction of alveolar macrophages, and the destruction of airway structure is prone to bacterial infection [20]. This may be due to the fact that this was a retrospective case-control study and the patient was in a state of smoking cessation when the history was taken, with recall bias.
Among the laboratory indicators of the two groups, there was no significant difference in the WBC. PCT was statistically higher in the observation group, but it was distributed in the normal range (< 0.5 ng/mL). This phenomenon may be associated with the premature use of broad-spectrum antibiotics in patients with chronic respiratory diseases: early intervention with antibiotics prevents the sustained release of inflammatory factors, and the PCT concentration requires 12 - 24 h to reach its peak, thus posing certain challenges to the detection of early infection signs [21-23]. Detecting infection signs is more challenging in this situation. CRP response is more sensitive than PCT in individuals with chronic respiratory illnesses coupled with infection. In the early stages, CRP was significantly higher than control group and more suggestive. CRP is a sensitive indicator of the systemic inflammatory response. A previous study suggested that elevated CRP levels are not affected by steroids and PCT is more related to the severity of the disease [24].
In clinical practice, TRPG is frequently utilized as a non-invasive technique to assess pulmonary hypertension, chronic respiratory diseases often have irreversible changes in the lung structure, and are prone to repeated airway infections. The normal airway structure is gradually destroyed. Hypoxia causes pulmonary vasoconstriction and vascular remodeling. Long-term recurrent peribronchial inflammation accumulates adjacent pulmonary arterioles, resulting in pulmonary vascular stenosis, occlusion, vascular fibrosis, and pulmonary capillary bed reduction. Pulmonary hypertension aggravates right ventricular load [25, 26]. Numerous inflammatory mediators are simultaneously produced during the acute infection phase, leading to myocardial damage, exacerbating pulmonary hypertension, and intensifying moderate-to-severe tricuspid regurgitation, all of which raise TPRG [27, 28]. Furthermore, research has demonstrated a negative correlation between pulmonary function and TRPG [29]. Patients with chronic respiratory diseases, particularly those with COPD, exhibited underweight, atrophy of the skeletal muscle, and gradually increased LY and disease duration and progression [30-32]. Malnutrition, poor respiratory muscle function, and low immunity lead to increased risk of pathogen infection [33-35]. The results of this study demonstrated that the secondary infection of chronic respiratory diseases could significantly affect the levels of TPGR and LY, and BMI could be used as a long-term index to evaluate the status of patients. The combination of these could have a good predictive value for the occurrence of pathogen infection in this group of patients.
There are not many studies on the general characteristics of secondary infection and early prediction of infection in patients with chronic respiratory diseases. This study reflected the clinical features, and facilitated the rapid identification of infection, enabling prompt treatment. But it also had certain limitations. Due to its retrospective case-control design, this study was susceptible to recollection and selection bias. Additionally, the samples were retrospectively collected from a single center, and data collection may result in the absence of clinical information for some patients, thereby reducing the sample size in the observation group. The observation group comprises only 54 patients, a size smaller than the minimum sample size recommended in similar studies. Therefore, the study conclusions may only be applicable to patients with similar characteristics in this center and are difficult to generalize to patients with chronic respiratory diseases in other regions or hospitals with varying levels of diagnosis and treatment. For future research, a multi-center prospective cohort design is recommended. Establishing standardized data collection protocols in advance will help minimize potential biases, such as clarifying the timing of measurements for indicators like vital capacity and oxygen saturation, and incorporating additional influencing factors like hemoglobin levels and medication exposure history.
Acknowledgments
We would like to express our sincere appreciation to the patients and their families for their participation in this study. We are also grateful to thank all the staff involved in this study for their support in patient confidentiality and data collection.
Financial Disclosure
This study was supported by the Pfizer Global Medical Grants (76080151).
Conflict of Interest
Author Mei Li Shen was employed by the Nanjing Dinfectome Technology Inc., Nanjing, China. The remaining authors declare no potential conflict of interest.
Informed Consent
Confirms that informed consent was obtained from all participants and/or their legal guardians.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by He Qing Huang and Hong Lu. The manuscript was written by He Qing Huang and revised by Mei Li Shen. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
ALB: albumin; AUC: area under curve; BMI: body mass index; 95% CI: 95% confidence interval; COPD: chronic obstructive pulmonary disease; CRP: C-reactive protein; IDSA/ATS: Infectious Diseases Society of America/American Thoracic Society; LVEF: left ventricular ejection fraction (52-74%); LY: lymphocyte count; NE: neutrophil count; NT-proBNP: N-terminal pro-brain natriuretic peptide; OR: odds ratio; PCT: procalcitonin; TRPG: tricuspid regurgitation pressure gradient; WBC: white blood cell count
| References | ▴Top |
- Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789-1858.
doi pubmed - Trottein F, Alcorn JF. Editorial: secondary respiratory infections in the context of acute and chronic pulmonary diseases. Front Immunol. 2019;10:2764.
doi pubmed - Liu YN, Zhang YF, Xu Q, Qiu Y, Lu QB, Wang T, Zhang XA, et al. Infection and co-infection patterns of community-acquired pneumonia in patients of different ages in China from 2009 to 2020: a national surveillance study. Lancet Microbe. 2023;4(5):e330-e339.
doi pubmed - Torres A, Chalmers JD, Dela Cruz CS, Dominedo C, Kollef M, Martin-Loeches I, Niederman M, et al. Challenges in severe community-acquired pneumonia: a point-of-view review. Intensive Care Med. 2019;45(2):159-171.
doi pubmed - Prina E, Ranzani OT, Polverino E, Cilloniz C, Ferrer M, Fernandez L, Puig de la Bellacasa J, et al. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann Am Thorac Soc. 2015;12(2):153-160.
doi pubmed - Shukla SD, Walters EH, Simpson JL, Keely S, Wark PAB, O'Toole RF, Hansbro PM. Hypoxia-inducible factor and bacterial infections in chronic obstructive pulmonary disease. Respirology. 2020;25(1):53-63.
doi pubmed - Love ME, Proud D. Respiratory viral and bacterial exacerbations of COPD - the role of the airway epithelium. Cells. 2022;11(9).
doi pubmed - GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585-596.
doi pubmed - Rochester CL, Alison JA, Carlin B, Jenkins AR, Cox NS, Bauldoff G, Bhatt SP, et al. Pulmonary rehabilitation for adults with chronic respiratory disease: an official American thoracic society clinical practice guideline. Am J Respir Crit Care Med. 2023;208(4):e7-e26.
doi pubmed - Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, Cooley LA, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67.
doi pubmed - Brandsma CA, Van den Berge M, Hackett TL, Brusselle G, Timens W. Recent advances in chronic obstructive pulmonary disease pathogenesis: from disease mechanisms to precision medicine. J Pathol. 2020;250(5):624-635.
doi pubmed - Lin J, He SS, Xu YZ, Li HY, Wu XM, Feng JX. Bacterial etiology in early re-admission patients with acute exacerbation of chronic obstructive pulmonary disease. Afr Health Sci. 2019;19(2):2073-2081.
doi pubmed - Martinez-Solano L, Macia MD, Fajardo A, Oliver A, Martinez JL. Chronic Pseudomonas aeruginosa infection in chronic obstructive pulmonary disease. Clin Infect Dis. 2008;47(12):1526-1533.
doi pubmed - Rakhimova E, Wiehlmann L, Brauer AL, Sethi S, Murphy TF, Tummler B. Pseudomonas aeruginosa population biology in chronic obstructive pulmonary disease. J Infect Dis. 2009;200(12):1928-1935.
doi pubmed - Al-Alawi A, Ryan CF, Flint JD, Muller NL. Aspergillus-related lung disease. Can Respir J. 2005;12(7):377-387.
doi pubmed - Yahav D, Schlesinger A, Daitch V, Akayzen Y, Farbman L, Abu-Ghanem Y, Paul M, et al. Presentation of infection in older patients—a prospective study. Ann Med. 2015;47(4):354-358.
doi pubmed - Liu DS, Han XD, Liu XD. Current status of community-acquired pneumonia in patients with chronic obstructive pulmonary disease. Chin Med J (Engl). 2018;131(9):1086-1091.
doi pubmed - Boe DM, Boule LA, Kovacs EJ. Innate immune responses in the ageing lung. Clin Exp Immunol. 2017;187(1):16-25.
doi pubmed - Glaser S, Kruger S, Merkel M, Bramlage P, Herth FJ. Chronic obstructive pulmonary disease and diabetes mellitus: a systematic review of the literature. Respiration. 2015;89(3):253-264.
doi pubmed - Lugg ST, Scott A, Parekh D, Naidu B, Thickett DR. Cigarette smoke exposure and alveolar macrophages: mechanisms for lung disease. Thorax. 2022;77(1):94-101.
doi pubmed - Duman D, Aksoy E, Agca MC, Kocak ND, Ozmen I, Akturk UA, Gungor S, et al. The utility of inflammatory markers to predict readmissions and mortality in COPD cases with or without eosinophilia. Int J Chron Obstruct Pulmon Dis. 2015;10:2469-2478.
doi pubmed - Sager R, Kutz A, Mueller B, Schuetz P. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15(1):15.
doi pubmed - Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754.
doi pubmed - Zhou W, Tan J. The expression and the clinical significance of eosinophils, PCT and CRP in patients with acute exacerbation of chronic obstructive pulmonary disease complicated with pulmonary infection. Am J Transl Res. 2021;13(4):3451-3458.
pubmed - Cavailles A, Brinchault-Rabin G, Dixmier A, Goupil F, Gut-Gobert C, Marchand-Adam S, Meurice JC, et al. Comorbidities of COPD. Eur Respir Rev. 2013;22(130):454-475.
doi pubmed - Olsson KM, Corte TJ, Kamp JC, Montani D, Nathan SD, Neubert L, Price LC, et al. Pulmonary hypertension associated with lung disease: new insights into pathomechanisms, diagnosis, and management. Lancet Respir Med. 2023;11(9):820-835.
doi pubmed - Hioka T, Kaga S, Mikami T, Okada K, Murayama M, Masauzi N, Nakabachi M, et al. Overestimation by echocardiography of the peak systolic pressure gradient between the right ventricle and right atrium due to tricuspid regurgitation and the usefulness of the early diastolic transpulmonary valve pressure gradient for estimating pulmonary artery pressure. Heart Vessels. 2017;32(7):833-842.
doi pubmed - Omote K, Nagai T, Kamiya K, Aikawa T, Tsujinaga S, Kato Y, Komoriyama H, et al. Long-term prognostic significance of admission tricuspid regurgitation pressure gradient in hospitalized patients with heart failure with preserved ejection fraction: a report from the Japanese real-world multicenter registry. J Card Fail. 2019;25(12):978-985.
doi pubmed - Lan CC, Yeh KH, Tzeng IS, Hsieh PC, Yang MC, Wu CW, Su WL, et al. Evaluation of the relationship of tricuspid regurgitation peak gradient/tricuspid annulus plane systolic excursion to exercise capacity, cardiac index, and ventilatory function during exercise in patients with COPD. Heart Lung. 2023;62:22-27.
doi pubmed - Baccioglu A, Gulbay BE, Acican T. Body composition in patients with stable chronic obstructive pulmonary disease: comparison with malnutrition in healthy smokers. Eurasian J Med. 2014;46(3):169-175.
doi pubmed - Bailey KV, Ferro-Luzzi A. Use of body mass index of adults in assessing individual and community nutritional status. Bull World Health Organ. 1995;73(5):673-680.
pubmed - Semenzato U, Biondini D, Bazzan E, Tine M, Balestro E, Buldini B, Carizzo SJ, et al. Low-blood lymphocyte number and lymphocyte decline as key factors in COPD outcomes: a longitudinal cohort study. Respiration. 2021;100(7):618-630.
doi pubmed - Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4(5):e115.
doi pubmed - Bone AE, Hepgul N, Kon S, Maddocks M. Sarcopenia and frailty in chronic respiratory disease. Chron Respir Dis. 2017;14(1):85-99.
doi pubmed - Angulo J, El Assar M, Rodriguez-Manas L. Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol Aspects Med. 2016;50:1-32.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.