| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 8, August 2025, pages 437-444
Safety of Molnupiravir in Hospitalized Patients With Coronavirus Disease 2019: A Retrospective, Single-Center, Cohort Study
Yota Yamadaa, b, f, Maresuke Oyac, Motoyasu Miyazakia, b, d, Hitomi Hirataa, b, Arata Ogawaa, b, Chika Hagiwaraa, Akio Nakashimaa, d, Hisako Kushimab, e, Hiroshi Ishiib, e, Osamu Imakyurea, d
aDepartment of Pharmacy, Fukuoka University Chikushi Hospital, Fukuoka 818-8502, Japan
bDepartment of Infection Control and Prevention, Fukuoka University Chikushi Hospital, Fukuoka 818-8502, Japan
cDepartment of Pharmacy, Saiseikai Fukuoka General Hospital, Fukuoka 810-0001, Japan
dDepartment of Hospital Pharmacy, Faculty of Pharmaceutical Sciences, Fukuoka University, Fukuoka 814-0180, Japan
eDepartment of Respiratory Medicine, Fukuoka University Chikushi Hospital, Fukuoka 818-8502, Japan
fCorresponding Author: Yota Yamada, Department of Pharmacy, Fukuoka University Chikushi Hospital, Fukuoka 818-8502, Japan
Manuscript submitted June 11, 2025, accepted August 7, 2025, published online August 31, 2025
Short title: MOV Safety in Inpatients With COVID-19
doi: https://doi.org/10.14740/jocmr6297
| Abstract | ▴Top |
Background: Molnupiravir (MOV) is recommended for the treatment of patients with coronavirus disease 2019 (COVID-19) who are ineligible for remdesivir treatment. However, data regarding laboratory-based adverse events (AEs) associated with MOV use in hospitalized patients are limited. In this study, we evaluated MOV-associated laboratory abnormalities, including increased creatine phosphokinase (CPK) and decreased hemoglobin (Hb) levels (e.g., anemia), and evaluated related risk factors in hospitalized COVID-19 patients.
Methods: We reviewed retrospective data for 78 adult inpatients with COVID-19 who received MOV upon admission at Fukuoka University Chikushi Hospital. Individuals with MOV treatment history, early discontinuation, or premature discharge were excluded. Data were collected on demographics, Charlson Comorbidity Index, bacterial coinfection, concomitant medications, COVID-19 severity, oxygen therapy, and length of hospital stay. The AEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 Japanese Clinical Oncology Group. Multivariate analyses were conducted to identify risk factors for elevated CPK levels and anemia.
Results: The median age of the study population was 82 years (interquartile range: 74 - 89 years; men: 56.4%), and 17.9% had bacterial coinfection. Twenty-seven patients (34.6%) experienced ≥ 1 AE, and MOV was discontinued in two patients because of a mild rash and CPK elevation. Elevated CPK levels and anemia each occurred in 11 patients (14.1%). Severe AEs (grade ≥ 3) were observed in one patient with grade 4 CPK elevation and in another with grade 3 anemia. Multivariate analysis showed that bacterial coinfection tended to increase CPK levels (adjusted odds ratio (aOR): 3.30, 95% confidence interval (CI): 0.75 - 13.32, P = 0.10) and was significantly associated with anemia (aOR: 5.40, 95% CI: 1.27 - 23.69, P = 0.022).
Conclusions: MOV exhibits a generally favorable safety profile in hospitalized COVID-19 patients, with low treatment discontinuation rates and mild laboratory abnormalities. Elevated CPK levels and anemia may reflect the complications of bacterial coinfection rather than direct MOV toxicity; however, these results should be interpreted with caution because of the small sample size and single-center, non-controlled study design. Further multicenter prospective studies are warranted to determine the relationship between CPK elevation, anemia, and MOV treatment in COVID-19 patients.
Keywords: Coronavirus disease 2019; Molnupiravir; Adverse events; Creatine phosphokinase; Anemia
| Introduction | ▴Top |
Since its emergence in late 2019, coronavirus disease 2019 (COVID-19) has infected > 770 million individuals and claimed > 7 million lives worldwide as of April 2025 [1]. This pandemic continues to impose a substantial burden on healthcare systems, not only because of acute infections, but also because of long-term sequelae and the emergence of new variants [1, 2]. Although the Omicron variant and its sublineages are associated with less severe pulmonary disease compared with earlier variants, advanced age and the presence of comorbidities increase the risk of clinical deterioration, hospitalization, and prolonged complications [3-5]. Consequently, regulatory agencies in Japan and abroad recommend the prompt administration of direct-acting oral antivirals for outpatients at increased risk and for hospitalized patients who are ineligible for remdesivir treatment [6, 7]. Of these, molnupiravir (MOV) is considered a viable option for patients requiring multiple concomitant medications due to comorbidities and for older adults, particularly because of its minimal potential for drug-drug interactions. MOV is a prodrug of N4-hydroxycytidine, which is a ribonucleoside analog. Following administration, its active metabolite, NHC triphosphate, is incorporated into viral RNA by the viral RNA-dependent RNA polymerase. This results in the accumulation of replication errors and subsequent inhibition of viral proliferation [8].
MOV is an antiviral agent that inhibits severe acute respiratory syndrome coronavirus 2 replication [8]. In the phase III MOVe-OUT trial, nonhospitalized adults treated with MOV experienced a 30% reduction in the composite endpoint of hospitalization or death compared with those treated with placebo [9]. In this trial, adverse events (AEs) occurred in approximately 30% of patients receiving MOV, which was similar to that observed in the placebo group. The most frequently reported events in the MOV arm included diarrhea, nausea, and dizziness. Severe (grade 3 or higher) AEs were uncommon and comparable between groups. In the UK PANORAMIC trial, the median time to self-reported resolution of all symptoms was approximately 4 days shorter in the MOV plus usual care group compared with that in the usual care group [10]. The safety profile observed in the PANORAMIC trial was similar to that of other clinical and observational studies. Gastrointestinal symptoms, including diarrhea, nausea, and vomiting, were the most frequently reported AEs, and most were mild to moderate in severity. No serious AEs related to the study drug were observed, indicating that tolerability was generally favorable in this trial.
Nevertheless, safety concerns remain, whereas clinical trials and real-world studies have provided limited data on laboratory-based AEs. A post-marketing safety surveillance analysis using the Food and Drug Administration Adverse Event Reporting System recorded 612 MOV-related adverse drug reactions as of March 31, 2022, which included diarrhea, rash, nausea, and COVID-19 pneumonia; however, reports of laboratory abnormalities remain scarce [11]. Although the overall profile of AEs was captured in a large registry study (n = 3,179), a detailed evaluation of the individual changes in the laboratory data and Common Terminology Criteria for Adverse Events (CTCAE) Grade assignments was limited [12].
In this single-center, retrospective study of hospitalized patients treated with MOV, we used the CTCAE v5.0 Japanese Clinical Oncology Group (JCOG) edition criteria to analyze laboratory-based AEs that are difficult to detect in outpatient settings.
| Materials and Methods | ▴Top |
Study design and participants
This study was a retrospective cohort analysis of patients with COVID-19 who received MOV as initial treatment during hospitalization between May 2021 and September 2023 at Fukuoka University Chikushi Hospital. The exclusion criteria included patients who had received MOV before admission, those who discontinued treatment because of swallowing difficulties, and those who were discharged before completing the regimen. At our institution, elderly individuals with multiple comorbidities and reduced renal function were preferentially hospitalized and treated with MOV. Thus, the majority of the study population consisted of patients in their late seventies and eighties. The sample size was not determined prospectively, but was based on the number of eligible cases. Accordingly, no a priori sample size calculation or power analysis was performed. The study was approved by the Ethics Committee of the Fukuoka University School of Medicine (No. C23-11-001). It complied with the ethical standards of the responsible institution on human subjects and the Declaration of Helsinki.
Data collection
Patient demographic and clinical data, including age, sex, history of AEs or allergies, Charlson Comorbidity Index (CCI) [13], presence of bacterial coinfection, concomitant medications, COVID-19 severity, length of hospital stay, and requirement for oxygen therapy, were collected from electronic medical records. Bacterial coinfection was defined as cases in which the attending physician determined the presence of bacterial infection. This was based on a comprehensive assessment of clinical symptoms and findings, inflammatory markers, such as procalcitonin, radiological findings, and treatment with new antibiotics. The following clinical laboratory parameters were evaluated: creatine phosphokinase (CPK) concentration, liver function tests, including aspartate aminotransferase (AST) and alanine aminotransferase (ALT), renal function, assessed by serum creatinine concentration, and hematological parameters, including white blood cell (WBC) counts, hemoglobin (Hb), and platelet counts.
Safety assessment
Because this study lacked a control group, it was challenging to separate AEs attributable to MOV from those resulting from COVID-19. Safety assessments were performed by comparing the clinical laboratory findings before MOV administration with those of day 7 post-treatment initiation. For patients who were discharged before day 7, laboratory values at discharge were used as the final data point. AEs, including laboratory abnormalities, were evaluated and graded according to the CTCAE v5.0 JCOG edition. Events corresponding to grade 1 or higher were considered abnormal. In addition, because increased CPK levels and anemia were the most frequent and severe events, we performed univariate analyses using a Chi-square test for categorical variables and the Mann-Whitney U test for numerical variables to identify risk factors for both outcomes. The criteria for grading a CPK increase and anemia were based on CTCAE v5.0. The upper limit of normal (ULN) for CPK was 248 IU/L for men and 153 IU/L for women. Hereafter, ULN refers to these sex-specific values. The lower limit of normal (LLN) for Hb was defined as 13.7 g/dL for men and 11.6 g/dL for women. A CPK increase was graded as follows: grade 1, > ULN to 2.5 × ULN; grade 2, > 2.5 × ULN to 5.0 × ULN; grade 3, > 5.0 × ULN to 10.0 × ULN; and grade 4, > 10.0 × ULN.
The reference range for Hb was 13.7 - 16.8 g/dL for men and 11.6 - 14.8 g/dL for women. Anemia was graded as follows: grade 1, Hb < LLN to 10.0 g/dL; grade 2, < 10.0 to 8.0 g/dL; grade 3, < 8.0 g/dL; and grade 4, life-threatening anemia or urgent intervention (e.g., transfusion) required.
Statistical analysis
Categorical variables were analyzed using a Chi-square test or Fisher’s exact test, whereas numerical variables were evaluated using the Mann-Whitney U test. Variables included in the multivariate models had a P-value of less than 0.15 in the univariate analysis. For the analysis of CPK elevation, clinically important factors, such as the use of lipid-lowering agents, were included. P-values < 0.05 were considered statistically significant. All statistical analyses were conducted using JMP Pro statistical software (SAS Institute, Inc., Cary, NC, USA).
| Results | ▴Top |
Patient characteristics
Ninety-five patients were screened, and 78 were enrolled (Fig. 1). The median age was 82 years (interquartile range (IQR): 74 - 89), 56.4% were men, and 20.5% had a history of AEs or allergies before admission. The median CCI score was 2 (IQR: 1.0 - 4.0; range: 0 - 11). Bacterial coinfection occurred in 17.9% of the cases. COVID-19 severity, which was classified based on Japanese Ministry of Health criteria [14], was mild in 57 patients (73.1%), moderate I in 14 patients (17.9%), and moderate II in seven patients (9.0%). During hospitalization, 20.5% of the patients required oxygen therapy, and the median length of hospital stay was 12 days (IQR: 10 - 21.3).
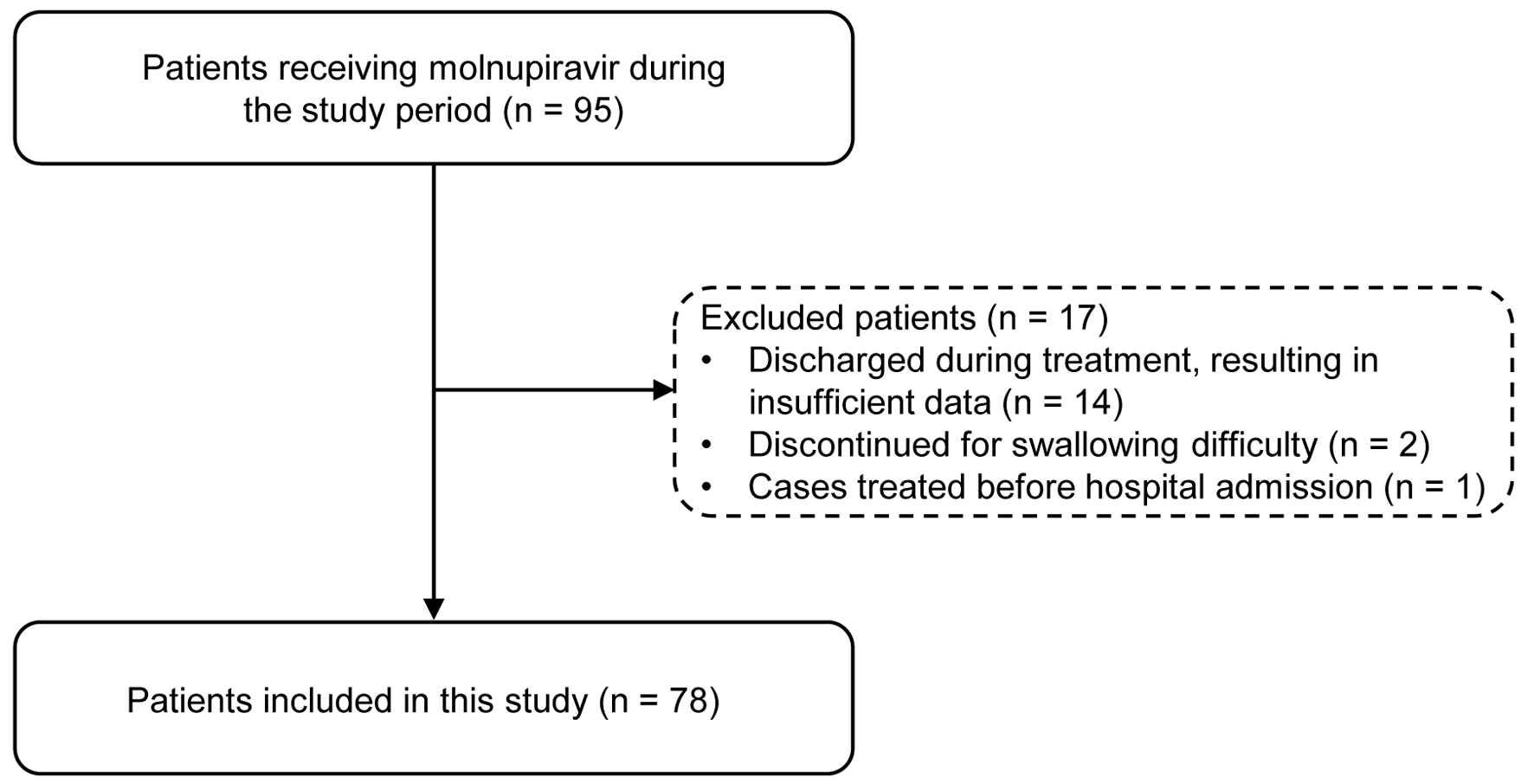 Click for large image | Figure 1. Flowchart of patient selection. |
Incidence and severity of AEs
Of the enrolled patients, 27 (34.6%) experienced ≥ 1 AE. The frequency and severity of these events are listed in Table 1. Treatment was discontinued because of AEs in two patients (2.6%), one for a grade 1 rash and the other for a grade 1 CPK elevation. Of the 27 patients with AEs, the most common laboratory changes were increased CPK levels and anemia, which were observed in 11 patients each (14.1% of the analyzed cohort). Severe AEs of grade ≥ 3 occurred in two patients (2.6%). One experienced a grade 4 CPK elevation, with CPK rising from 176 to 3,964 U/L, and another experienced grade 3 anemia, with Hb decreasing from 8.7 to 7.9 g/dL.
 Click to view | Table 1. Incidence and Severity of Adverse Events |
Distribution of AE combinations
The distribution of AE combinations is presented in Figure 2. The most frequent event was an isolated increase in CPK levels, which was observed in five patients (18.5%). Isolated anemia was also observed in five patients (18.5%). An isolated decrease in WBC occurred in four patients (14.8%), and AST and/or ALT elevation, as well as elevated CPK, anemia, and increased AST and/or ALT were observed in two patients each (7.4%). Composite events categorized as “others” occurred in nine patients (33.3%) and included various combinations, such as CPK increase with anemia or hepatic enzyme elevation, as well as single cases of creatinine increase and rash. These results indicate a heterogeneous AE profile following MOV administration.
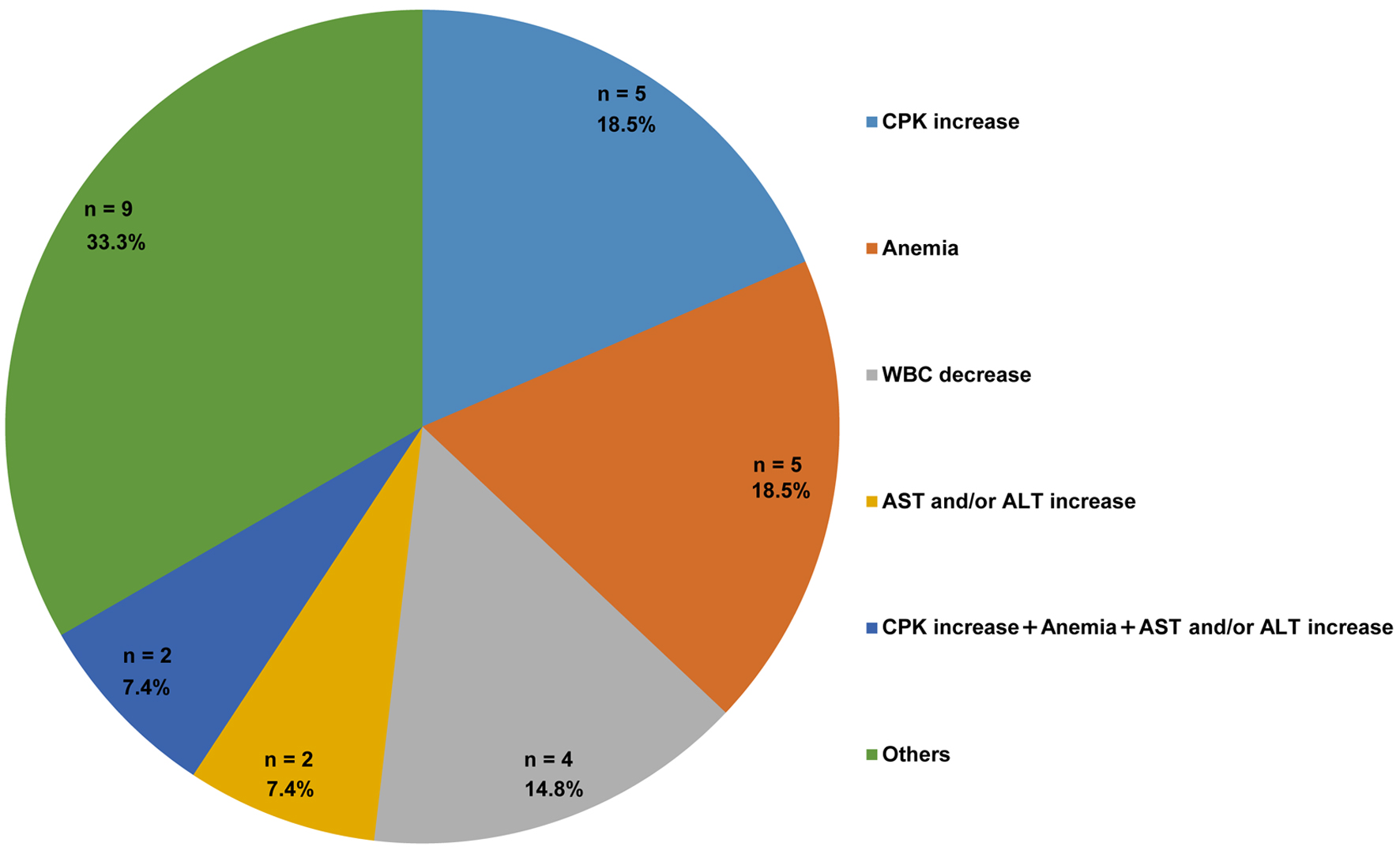 Click for large image | Figure 2. Distribution of adverse events among patients receiving molnupiravir (n = 27). The number and percentage of patients are indicated in each category. Patients with missing AE data were excluded from this analysis. “Others” include CPK increase with anemia (n = 1), CPK increase with AST and/or ALT increase (n = 1), CPK increase with AST and/or ALT increase and WBC decrease (n = 1), CPK increase with anemia and platelet count decrease (n = 1), anemia with WBC decrease (n = 1), anemia with AST and/or ALT increase (n = 1), AST and/or ALT increase with rash (n = 1), creatinine increase (n = 1), and one rash (n = 1). ALT: alanine aminotransferase; AST: aspartate aminotransferase; CPK: creatine phosphokinase; WBC: white blood cell count. |
Factors associated with AEs
Table 2 lists the factors associated with increased CPK levels. In the univariate analyses, none of the predefined risk factors, such as age, sex, history of AEs or allergies, heart disease, CCI, concomitant medications, COVID-19 severity, or oxygen therapy, were significantly associated with CPK elevation; however, there was a trend toward higher rates of CPK elevation in patients with bacterial coinfection (Table 2). Multivariate analysis revealed that anemia was significantly associated with bacterial coinfection (Table 3).
 Click to view | Table 2. Factors Associated With CPK Elevation in Patients Receiving Molnupiravir |
 Click to view | Table 3. Factors Associated With Hb Decrease in Patients Receiving Molnupiravir |
| Discussion | ▴Top |
In this single-center retrospective cohort study of hospitalized COVID-19 patients, MOV treatment showed a favorable safety and tolerability profile. The treatment discontinuation rate was 2.6%, which was consistent with the 1.0% and 1.64% discontinuation rates reported in the phase III MOVe-OUT trial and the Japanese post-marketing surveillance, respectively [9, 12]. No severe hepatic or renal AEs were observed, which indicates that concerns regarding the safety of MOV in real-world inpatients are mild.
The most frequently observed laboratory abnormalities included increased CPK levels and decreased Hb levels, each occurring in 14.1% of the patients. Notably, only two patients experienced grade ≥ 3 laboratory abnormalities (one with grade 4 CPK elevation and one with grade 3 anemia). This suggests that most observed events were mild and of limited clinical impact. Increased serum CPK levels resulting from skeletal muscle injury are frequently reported in COVID-19. One study reported CPK levels above the upper limit of the normal range in approximately 9-33% of COVID-19 patients [15]. Although mild increases in CPK levels have been described, even in asymptomatic statin users with COVID-19 [16], we did not observe an association in the present study. Univariate analysis revealed no significant risk factors for increased CPK levels. Nevertheless, a trend toward higher CPK levels was observed in patients coinfected with bacteria (P = 0.10), which suggests that bacterial coinfection may increase the risk of CPK elevation. The development of sepsis or systemic inflammatory response syndrome may induce tissue hypoxia and metabolic disturbances, which can result in muscle injury and CPK elevation [17]. Moreover, secondary factors associated with infection, such as high fever, dehydration, and seizures, may result in rhabdomyolysis and further contribute to increased CPK levels [18]. Because these mechanisms may have induced CPK elevation, it is difficult to conclude that it is a direct AE of MOV. Although the general natural course of CPK elevation following COVID-19 infection has not been established, Friedman et al reported that 48% of patients showed elevated CK levels at the initial measurement, which was typically performed within 1 week of symptom onset. Consistent with our findings, this suggests that CK elevation frequently occurs during the early phase of COVID-19 [15]. Moreover, anemia is frequently observed in COVID-19 patients. Early pandemic studies revealed that approximately 30-60% of patients experienced a decrease in Hb levels during acute infection [18]. For example, one hospital series reported anemia in 61% of hospitalized patients with COVID-19 at admission compared with 45% in non-COVID controls [19, 20]. A previous report showed that Hb levels gradually decreased after symptom onset in survivors and non-survivors with COVID-19, but only non-survivors developed mild anemia (Hb < 10 g/dL) by the end of follow-up [21]. Similarly, in the present study, a decrease in Hb levels was observed during the early phase following symptom onset. Moreover, a significant decrease in Hb levels was reported in patients with COVID-19 and bacterial coinfections [22]. In the present study, only bacterial coinfection was significantly associated with anemia. Therefore, it would be desirable for future studies to stratify patients based on their risk of bacterial coinfection to identify those at higher risk for hematological abnormalities. This indicates that the observed reduction in Hb levels cannot be definitively attributed to MOV. The prolonged persistence of anemia was reported in COVID-19 patients, particularly among those with severe disease that required hospitalization [23, 24]. Accordingly, long-term hematological monitoring, particularly of CPK and Hb levels, may be warranted in patients receiving MOV, particularly in those with bacterial coinfection.
This study had several limitations. First, as a noninterventional, single-arm retrospective cohort study, we were not able to perform statistical comparisons of MOV safety with other treatments or a placebo. Therefore, interpretation of causality should be made with caution because of the absence of a control group. Second, the inability to definitively distinguish drug-induced effects from COVID-19-related muscle injury limited causal inference regarding increased CPK levels. Third, the single-center setting and relatively small sample size restricted the generalizability of these findings and decreased the statistical power for detecting modest risk factors. Fourth, because follow-up was confined to the hospitalization period, we could not assess delayed AEs that could have manifested following patient discharge. Fifth, the retrospective design prevented the systematic evaluation of certain AEs, such as gastrointestinal symptoms like diarrhea. Sixth, as this study involved multiple statistical tests in an exploratory setting, no adjustment for multiple comparisons was made. Thus, the possibility of type I errors should be considered when interpreting the results.
Nonetheless, the key strengths of this study include the systematic grading of changes in the laboratory results (CTCAE v5.0, JCOG edition) and the comprehensive reporting of AEs in a high-risk cohort of hospitalized patients.
Conclusion
In conclusion, this study highlights the favorable safety profile and manageable laboratory abnormalities associated with MOV in hospitalized COVID-19 patients. These findings suggest that MOV appears to be safe and well-tolerated in clinical practice; however, the small sample size and single-center, retrospective design limited the statistical power and generalizability of our results. The observed increased CPK levels and anemia likely reflect factors other than direct MOV toxicity, particularly those associated with bacterial coinfection. Further multicenter, prospective studies are warranted to clarify the causal relationships between CPK elevation, anemia, and MOV treatment in COVID-19 patients.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they have no competing interests.
Informed Consent
Not applicable.
Author Contributions
YY, MM, and OI contributed to the concept and design of the study. YY, MO, HH, AO, and CH conducted the study. YY, MM, and AN were involved with data analysis and interpretation of the results. YY, MM, and HI drafted the manuscript. MM, HK, HI, and OI supervised the entire project and reviewed the manuscript. All the authors approved the final version of the manuscript.
Data Availability
Any queries regarding the availability of data supporting this study should be directed to the corresponding author.
Abbreviations
AEs: adverse events; aOR: adjusted odds ratio; CCI: Charlson Comorbidity Index; COVID-19: coronavirus disease 2019; CPK: creatine phosphokinase; CTCAE: Common Terminology Criteria for Adverse Events; Hb: hemoglobin; IQR: interquartile range; JCOG: Japanese Clinical Oncology Group; OR: odds ratio; MOV: molnupiravir; 95% CI: 95% confidence interval
| References | ▴Top |
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard [Internet]. World Health Organization; 2025. Available from: https://covid19.who.int/. Accessed Jun 10, 2025.
- Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133-146.
doi pubmed - Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M, Amoako DG, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399(10323):437-446.
doi pubmed - Madhi SA, Kwatra G, Myers JE, Jassat W, Dhar N, Mukendi CK, Nana AJ, et al. Population immunity and COVID-19 severity with omicron variant in South Africa. N Engl J Med. 2022;386(14):1314-1326.
doi pubmed - Ortiz-de-Lejarazu R, Quiroga Gili B, Lopez Garcia A. Burden of COVID-19 variant omicron in immunocompromised patients in Spain: systematic review. Med Clin (Barc). 2024;163(7):347-359.
doi pubmed - Japanese Association for Infectious Diseases. Guidance on Pharmacotherapy for COVID-19, Version 15.1 [in Japanese]. Japanese Association for Infectious Diseases; 2023. Available from: https://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19_drug_230217.pdf. Accessed Jun 10, 2023.
- Bhimraj A, Morgan RL, Shumaker AH, Baden LR, Cheng VC, Edwards KM, Gallagher JC, et al. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19 (September 2022). Clin Infect Dis. 2024;78(7):e250-e349.
doi pubmed - Kabinger F, Stiller C, Schmitzova J, Dienemann C, Kokic G, Hillen HS, Hobartner C, et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol. 2021;28(9):740-746.
doi pubmed - Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, Martin-Quiros A, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509-520.
doi pubmed - Butler CC, Hobbs FDR, Gbinigie OA, Rahman NM, Hayward G, Richards DB, Dorward J, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet. 2023;401(10373):281-293.
doi pubmed - Santi Laurini G, Montanaro N, Motola D. Safety profile of molnupiravir in the treatment of COVID-19: a descriptive study based on FAERS data. J Clin Med. 2022;12(1):34.
doi pubmed - Shinozaki S, Watanabe A, Kimata M, Miyazaki M, Maekawa S. Safety and effectiveness of molnupiravir in Japanese patients with COVID-19: final report of post-marketing surveillance in Japan. Infect Dis Ther. 2024;13(1):189-205.
doi pubmed - Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
doi pubmed - Ministry of Health, Labour and Welfare. Clinical management of patients with COVID-19 [in Japanese]. Ministry of Health, Labour and Welfare; 2023. Available from: https://www.mhlw.go.jp/content/000936655.pdf. Accessed Jun 10, 2023.
- Friedman SA, Charmchi Z, Silver M, Jacoby N, Perk J, Anziska Y. Skeletal muscle manifestations and creatine kinase in COVID-19. Neurohospitalist. 2022;12(4):597-606.
doi pubmed - Schetz D, Sztormowska-Achranowicz K, Foerster J, Kocic I. Muscle pain and muscle weakness in COVID19 patients: cross-talk with statins - preliminary results. Biomed Pharmacother. 2022;148:112757.
doi pubmed - Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591-1600.
doi pubmed - Melli G, Chaudhry V, Cornblath DR. Rhabdomyolysis: an evaluation of 475 hospitalized patients. Medicine (Baltimore). 2005;84(6):377-385.
doi pubmed - Rahman A, Niloofa R, Jayarajah U, De Mel S, Abeysuriya V, Seneviratne SL. Hematological abnormalities in COVID-19: a narrative review. Am J Trop Med Hyg. 2021;104(4):1188-1201.
doi pubmed - Bergamaschi G, Borrelli de Andreis F, Aronico N, Lenti MV, Barteselli C, Merli S, Pellegrino I, et al. Anemia in patients with COVID-19: pathogenesis and clinical significance. Clin Exp Med. 2021;21(2):239-246.
doi pubmed - Lanini S, Montaldo C, Nicastri E, Vairo F, Agrati C, Petrosillo N, Scognamiglio P, et al. COVID-19 disease-Temporal analyses of complete blood count parameters over course of illness, and relationship to patient demographics and management outcomes in survivors and non-survivors: A longitudinal descriptive cohort study. PLoS One. 2020;15(12):e0244129.
doi pubmed - Alqahtani A, Alamer E, Mir M, Alasmari A, Alshahrani MM, Asiri M, Ahmad I, et al. Bacterial coinfections increase mortality of severely ill COVID-19 patients in Saudi Arabia. Int J Environ Res Public Health. 2022;19(4):2424.
doi pubmed - Hudgins AF, McDonald B, Bush PA, Harding JL, Kolailat I, Martinez M, et al. Assessing the prevalence of anemia post COVID-19 infection in adult members of a southeastern U.S. integrated healthcare system. Preprint. Research Square. 2023.
doi - Abdel-Aziz T, Nabil Abd El Aziz G, Fawzy M, Ibrahim EL Dosoky N, Iraqi Ahmed Hegab H, Dahshan I. Hematological impact of COVID-19: anemia in post-COVID. Benha Med J. 2024;41(2):1220131.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.