| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 8, August 2025, pages 445-459
The Elimination Effect of Medical-Grade Honey on Pseudomonas aeruginosa Biofilms: A Systematic Review and Meta-Analysis
Hariyudo Hariyudoa, b, f, Hasan Rizky Benokrib, Yohanes Widodo Wirohadidjojoa, c, Camelia Herdinia, d, Arief Budiyantoa, c, Dhite Bayu Nugrohoe
aFaculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia
bDepartment of Otorhinolaryngology-Head and Neck Surgery, Banyumas Regional General Hospital, Banyumas, Indonesia
cDepartment of Dermatology and Venereology, Dr. Sardjito General Hospital, Yogyakarta, Indonesia
dDepartment of Otorhinolaryngology-Head and Neck Surgery, Dr. Sardjito General Hospital, Yogyakarta, Indonesia
eClinical Epidemiology and Biostatistics Unit, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia; Cochrane Indonesia
fCorresponding Author: Hariyudo Hariyudo, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia
Manuscript submitted June 30, 2025, accepted August 12, 2025, published online August 31, 2025
Short title: Effect of Honey on P. aeruginosa Biofilms
doi: https://doi.org/10.14740/jocmr6312
| Abstract | ▴Top |
Background: This systematic review aimed to evaluate the efficacy of medical-grade honey (MGH) in eliminating and inhibiting Pseudomonas aeruginosa (P. aeruginosa) biofilms, which are known for their resistance to conventional antibiotics and significant role in chronic infections.
Methods: Following Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines and registered in PROSPERO (CRD42024614542), a systematic search was conducted across PubMed, ProQuest, Scopus, and EBSCO using terms related to P. aeruginosa, biofilm, and MGH. Inclusion criteria encompassed in vitro studies assessing MGH’s effect on P. aeruginosa biofilms, with reported outcomes including biofilm inhibition and eradication. Data extraction and risk-of-bias assessment were performed independently by two reviewers using the Quality Assessment Tool for In Vitro Studies (QUIN) tool. Publication bias was estimated through forest plot.
Results: A total of 1,934 records were identified from four databases. After screening and full-text review, six in vitro studies met the inclusion criteria for qualitative synthesis, and five were eligible for meta-analysis. All studies evaluated the effect of MGH, including Manuka and Surgihoney, on P. aeruginosa biofilms using crystal violet staining and spectrophotometric analysis. Pooled results showed that MGH significantly reduced biofilm formation (standardized mean difference (SMD) = -4.98; 95% confidence interval (CI): -6.72 to -3.25) and effectively disrupted established biofilms (SMD = -4.44; 95% CI: -6.62 to -2.26). Subgroup analysis revealed stronger effects on American Type Culture Collection (ATCC) strains than clinical isolates, with low within-subgroup heterogeneity. MGH also demonstrated significant superiority compared to active biofilm controls, although sterility control comparisons showed high variability. Mechanistic analysis found that Medihoney outperformed sugar solutions and methylglyoxal (MGO) alone, suggesting that its antibiofilm activity results from synergistic bioactive compounds. Overall, these findings support MGH as a potent antibiofilm agent, warranting further research for clinical application against P. aeruginosa biofilm infections.
Conclusion: MGH exhibits consistent and substantial anti-biofilm activity against P. aeruginosa in vitro, affecting both biofilm formation and established biofilms. These findings support its potential application as a topical therapeutic agent in managing biofilm-related infections, particularly in chronic wounds. Future research should prioritize standardized application protocols and investigate synergistic effects with conventional antimicrobials through clinical trials.
Keywords: Biofilm; Medical-grade honey; Pseudomonas aeruginosa; Topical agent
| Introduction | ▴Top |
Pseudomonas aeruginosa (P. aeruginosa) is a widespread gram-negative, aerobic (growth in 21% oxygen) bacterium responsible for nosocomial infections and severe infections in immunocompromised individuals. It is also can be found in a wide range of environments, including moist areas, animal tissues, organic matter, plant soil, terrestrial habitats, and swimming pools [1, 2]. Its high adaptability to diverse conditions is due to its production of various functional enzymes, such as phospholipase, elastase, and siderophores, along with exceptional metabolic flexibility [3]. Recognized as one of the most dangerous pathogens, it has been classified by the World Health Organization (WHO) as a priority for antibiotic research and development. Its ability to form biofilms further complicates treatment, as biofilms shield the bacteria from environmental stress, prevent phagocytosis, and enhance their capacity for colonization and long-term survival [2]. Biofilm-related infections result in significant economic losses and contribute to over half a million deaths each year. The estimated annual cost of treating biofilm infections is approximately USD 94 billion. In 2007, the Centers for Disease Control and Prevention (CDC) reported that biofilms were responsible for around 1.7 million hospital-acquired infections [4].
Antibiotics are commonly used to treat biofilm-related infections, but their effectiveness is limited by challenges such as drug resistance, restricted penetration through the biofilm matrix, and complex drug-microbe interactions [5, 6]. Although antibiotic therapy remains the primary and most effective method for controlling bacterial infections, biofilms exhibit strong resistance to both antibiotics and the immune response [7]. Treating P. aeruginosa biofilms with a single antibiotic is particularly challenging. As a result, various strategies have been developed to overcome these issues, including increasing antibiotic concentrations or using combination therapies to enhance treatment efficacy and prevent resistance [6]. However, the use of antibiotics carries certain risks, including the potential for allergic reactions, with the most concerning being the rising incidence of bacterial resistance [8]. P. aeruginosa exhibits resistance to various antibiotics, including aminoglycosides, quinolones, and β-lactams. Its resistance mechanisms are generally classified into intrinsic, acquired, and adaptive resistance. Intrinsic resistance arises from low outer membrane permeability, the expression of efflux pumps that expel antibiotics, and the production of antibiotic-inactivating enzymes. Acquired resistance occurs through the horizontal transfer of resistance genes or genetic mutations. Adaptive resistance is associated with biofilm formation in the lungs of infected patients, where the biofilm acts as a diffusion barrier, restricting antibiotic penetration to bacterial cells [9].
The ineffectiveness of traditional oral antimicrobial treatments in eliminating bacteria present in biofilm formations has encouraged the exploration of topical therapies. Honey has been used for a long time as a topical antimicrobial treatment for wound infections, and licensed medical product appeared in the 1990s [10]. Honeybees produce honey after consuming flower nectar, blossoms, or floral secretions. These collected substances are combined with specific compounds from the bees and then stored in the wax honeycomb, where they undergo maturation over time. The composition of honey varies depending on the plant sources that the bees forage from [11]. The sugars present in honey have been associated with interference in quorum sensing in P. aeruginosa [12, 13]. Additionally, high concentrations of fructose in honey or sugar solutions have been shown to inhibit P. aeruginosa biofilm formation [14]. This sensitivity to sugar solutions may also be attributed to a deficiency in sugar transporters in P. aeruginosa [15]. Honey’s effectiveness against bacterial infections stems from two key mechanisms: the presence of bactericidal compounds (low pH, high osmolarity, hydrogen peroxide or methylglyoxal (MGO) production, and the presence of bioactive compounds such as flavonoids [16]) and its ability to inhibit quorum sensing [12]. Medihoney and Manuka honey have demonstrated in vivo efficacy and are effective in treating ulcers, infected wounds, and burns. Additionally, honey can be used as a treatment for peptic ulcers and gastritis. It may aid in the repair of damaged intestinal mucosa, promote new tissue growth, and act as an anti-inflammatory agent. Raw honey is rich in bioactive compounds, including flavonoids and polyphenols, which serve as antioxidants [17]. Solutions with high osmolarity, such as honey, glucose, and sugar pastes, have been found to inhibit microbial growth by binding water molecules, depriving bacteria of the moisture needed for growth. This high osmolarity plays a crucial role in infection treatment by preventing bacterial proliferation and promoting healing [18]. Honey can be applied topically for the treatment of wounds and burns due to its antibacterial properties and ability to promote wound healing [19]. MGO, the active compound in Manuka honey, is a potent antimicrobial agent effective against both planktonic bacteria and biofilms. Its high concentration is primarily responsible for honey’s antibacterial properties. MGO targets arginine residues in collagen, leading to collagen disruption and contributing to fibrosis in chronic tissue infections. Additionally, by altering the structure and function of key immunological proteins and enzymes, MGO can impair the efficiency of peripheral blood immune cell responses. However, it is important to note that bees feeding on different plant sources produce various types of honey, each with a unique composition. These compositional differences can impact the medicinal properties of honey [20]. Antimicrobial mechanisms of honey can be achieved in two ways: direct and indirect actions. The direct mechanism involves the elimination of bacteria through honey’s components, while the indirect mechanism enhances the body’s antibacterial response. Direct antibacterial factors include hydrogen peroxide, non-peroxide compounds, acidity, high osmolality, and phenols, all of which are toxic to pathogens. Indirect antimicrobial effects involve the stimulation of lymphocyte and antibody production, cytokine release, nitric oxide activity, and immunomodulation [21]. Conventional honey differs from medical-grade honey (MGH), which is required to meet high-quality standards, including being organically derived, free from contaminants and toxic substances, and sterilized using standardized gamma irradiation to eliminate pathogenic microorganisms. It must be proven safe for therapeutic use, adhere to strict production and storage protocols, and comply with applicable legal and safety regulations. In addition, it should fulfill essential physicochemical criteria required for its effectiveness in wound care applications. These requirements ensure that the product is both clinically reliable and suitable for medical use [22]. The behavior of a biofilm is a complex dance between two key forces: the microorganism’s intrinsic ability to colonize and the external environmental conditions it inhabits. First, the strain-surface attachment defines the foundation of the biofilm; this is determined by the specific bacterial strain’s characteristics, such as cell wall composition, motility, and its production of extracellular polymeric substance (EPS), as well as the physical properties of the surface itself. Simultaneously, the biofilm’s development is shaped by media flow conditions, which are the dynamic, external forces of the surrounding fluid. These conditions, including shear stress, nutrient availability, and flow rate, control the biofilm’s growth, detachment, and overall physical structure. While these two sets of variables are distinct in their origin, one being internal to the microbe and the other external to the environment, their constant interaction dictates the entire lifecycle, stability, and ultimate fate of the biofilm.
While numerous in vitro studies have demonstrated that MGH exhibits antibiofilm properties against P. aeruginosa, the existing evidence remains fragmented, variable in quality, and lacks comprehensive synthesis, particularly in relation to its effects on both biofilm formation and mature biofilms. To address this gap, the present systematic review and meta-analysis is the first to quantitatively evaluate the biofilm-disrupting efficacy of MGH across varying bacterial strains, concentrations, and stages of biofilm development. The primary objective of this study is to systematically compile and analyze current in vitro evidence on the capacity of MGH to eliminate P. aeruginosa biofilms.
This present study provides a focused, quantitative examination of the antibiofilm efficacy of MGH, thereby complementing existing reviews that have primarily offered a general overview of honey’s therapeutic properties. Previous comprehensive reviews, such as those by Mandal and Mandal [17] and Oryan et al [20], have extensively detailed honey’s benefits in wound healing, including its antibacterial compounds, anti-inflammatory effects, antioxidant activity, and ability to stimulate tissue growth. While Oryan et al [20] also included a meta-analysis supporting honey’s role in the healing of infected wounds, positioning it as a safe and cost-effective biomaterial, these studies lack a systematic, quantitative assessment of its specific impact on bacterial biofilms. To address this gap, our review utilizes a quantitative method based on the absorbance values (optical density) of bound crystal violet dye, which serves as a direct and objective measure of biofilm biomass.
| Materials and Methods | ▴Top |
This study followed the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines and was registered on the PROSPERO’s international prospective register of systematic reviews (CRD42024614542). This systematic review was carried out in accordance with the PRISMA guidelines [23].
Eligibility criteria
We included studies that reported the benefits of MGH in treating P. aeruginosa biofilms. This study was conducted in an in vitro setting to evaluate the advantage of honey while eliminating potential confounding factors, such as the host immune response. Interventions using medical grade honey, can be used alone or combined with other treatments, with comparators in the form of other treatments (for example antibiotics) or without treatment. For the outcome, we examined the effects of MGH on P. aeruginosa biofilms, focusing on its role in biofilm elimination and/or prevention. Studies that reported the minimum inhibitory concentration (MIC) and minimum biofilm eradication concentration (MBEC) of MGH against P. aeruginosa biofilms were considered preferable. Only studies published in English were considered for inclusion.
Literature search
A comprehensive literature search was conducted across PubMed, ProQuest, Scopus, and EBSCO databases using a combination of controlled vocabulary (e.g., MeSH terms) and free-text terms, including “Pseudomonas aeruginosa”, “bacterial”, “medical-grade honey”, ‘honey”, “manuka”, “biofilm”, “eradication”, “elimination”, and “disruption”. To enhance the sensitivity and specificity of the search, Boolean operators (AND, OR) were strategically applied to combine search terms, while truncation and wildcards were used where appropriate to include multiple word endings and synonyms.
Selection of studies
The retrieved search results were imported into Rayyan, a web-based platform designed to facilitate collaboration among reviewers during the systematic review process. The review team developed and applied structured screening questions based on predefined inclusion and exclusion criteria. Titles, abstracts, and full-text articles were uploaded into the platform for independent evaluation. The inclusion criteria comprised: 1) full-text availability in English; 2) in vitro study design; 3) investigation of P. aeruginosa biofilms; 4) use of MGH as the primary intervention; and 5) reported outcome measures including the type of MGH, MIC/MBEC values, and spectrophotometric analyses. Studies published prior to the year 2000 were excluded. Each reviewer independently assessed the relevance of the identified records based on title and abstract screening. Full texts were retrieved for studies that met the inclusion criteria or in cases of uncertainty. Subsequently, reviewer pairs evaluated the full-text articles for eligibility, with any discrepancies resolved through discussion and consensus.
Data extraction
Data extraction was independently performed by two reviewers. For each study included in the systematic review, comprehensive information was collected, including: sample and sample size; methodological details such as biofilm growth model, treatment approach, and duration; biofilm characteristics (model system, bacterial strain, and biofilm maturity); and intervention specifics, including the type and concentration of honey, method and duration of application, control conditions, degree of biofilm reduction, assay techniques employed (for crystal violet assays, we extracted details on: 1) fixation method, 2) washing cycles, and 3) destaining solvent), spectrophotometric outcomes, and any reported secondary endpoints. We extracted the numerical data for our graphs and figures by “digitizing” or “reverse-engineering” the plots from their source articles using the software tool WebPlotDigitizer [24]. This process involved uploading a high-resolution image of each graph, calibrating the axes, and then extracting the data points either manually or automatically. The extracted data were subsequently exported as a comma separated value (CSV) file for analysis. Although all included studies utilized spectrophotometry to quantify optical density as an indirect measure of biofilm biomass, the specific wavelengths applied were not standardized. To address this methodological variability and enable a more robust quantitative synthesis, the reported values were transformed into standardized mean differences (SMDs) for the meta-analysis.
Quality assessment
Although no universally accepted standard exists for evaluating the quality of in vitro studies, this review employed the Quality Assessment Tool for In Vitro Studies (QUIN) (Terna Dental College, Mumbai, India) [25], a framework originally developed in the field of prosthetic dentistry. In a systematic review of quality assessment tool in in vitro systematic review, Tran et al [26] state that no single tool adequately covers all critical aspects of in vitro systematic reviews. It suggests that a comprehensive guide should be developed to address the significant concerns and aspects of in vitro systematic reviews. The QUIN tool assesses risk of bias using 12 predefined criteria specifically designed for in vitro research contexts, including the clarity and blinding of the intervention and control groups, the reporting of outcome measures, the completeness of data reporting, and the appropriateness of statistical analysis [25]. Each criterion is scored as follows: adequately specified = 2 points, inadequately specified = 1 point, not specified = 0 points, and not applicable = excluded from the calculation. The scores are summed to generate a total score for the study, which is then used to categorize the risk of bias: low risk (> 70%), medium risk (50% to 70%), and high risk (< 50%). Its content validity was established by a Delphi panel and confirmed by a survey of 60 experts, who rated all 12 criteria with a high average score (greater than 6 out of 7). This strong consensus demonstrates the tool’s relevance and comprehensiveness for assessing in vitro methodological quality. The reliability of the QUIN framework was evaluated using Cronbach’s Alpha. This analysis demonstrated good overall inter-reviewer reliability (0.862 ± 0.071) and intra-reviewer repeatability (0.861 ± 0.079). Cronbach’s Alpha values for each criterion generally ranged from 0.71 to 0.94. These strong scores confirm consistent application of the tool by multiple reviewers and strong internal consistency across its components [25]. QUIN was adapted due to its comprehensive in vitro criteria, though we acknowledge its primary dental validation.
Statistical analysis
Meta-analysis was performed using the R statistical software 4.3.2 (R Foundation, Vienna, Austria) with the “meta” and “metafor” packages to calculate pooled effect sizes and assess heterogeneity. The analysis followed a format consistent with Review Manager 5.4 (The Cochrane Collaboration, Copenhagen, Denmark), including forest plot presentation and heterogeneity reporting using the I2 statistic. A fixed-effect model was applied, assuming that the included studies estimate a common true effect due to their methodological similarity. Effect sizes were expressed as SMDs with 95% confidence intervals (CIs). Statistical significance was determined with P value less than 0.05.
| Results | ▴Top |
Study selection
An initial search across PubMed, ProQuest, Scopus, and EBSCO databases retrieved a total of 1,934 records. Following the removal of duplicates, titles and abstracts were screened for relevance based on predefined eligibility criteria. This screening process resulted in 33 articles selected for full-text review, of which six studies met the inclusion criteria for qualitative synthesis. Among these, five studies provided sufficient data to be included in the meta-analysis (Fig. 1). Publication bias was assessed visually using funnel plots (Fig. 2).
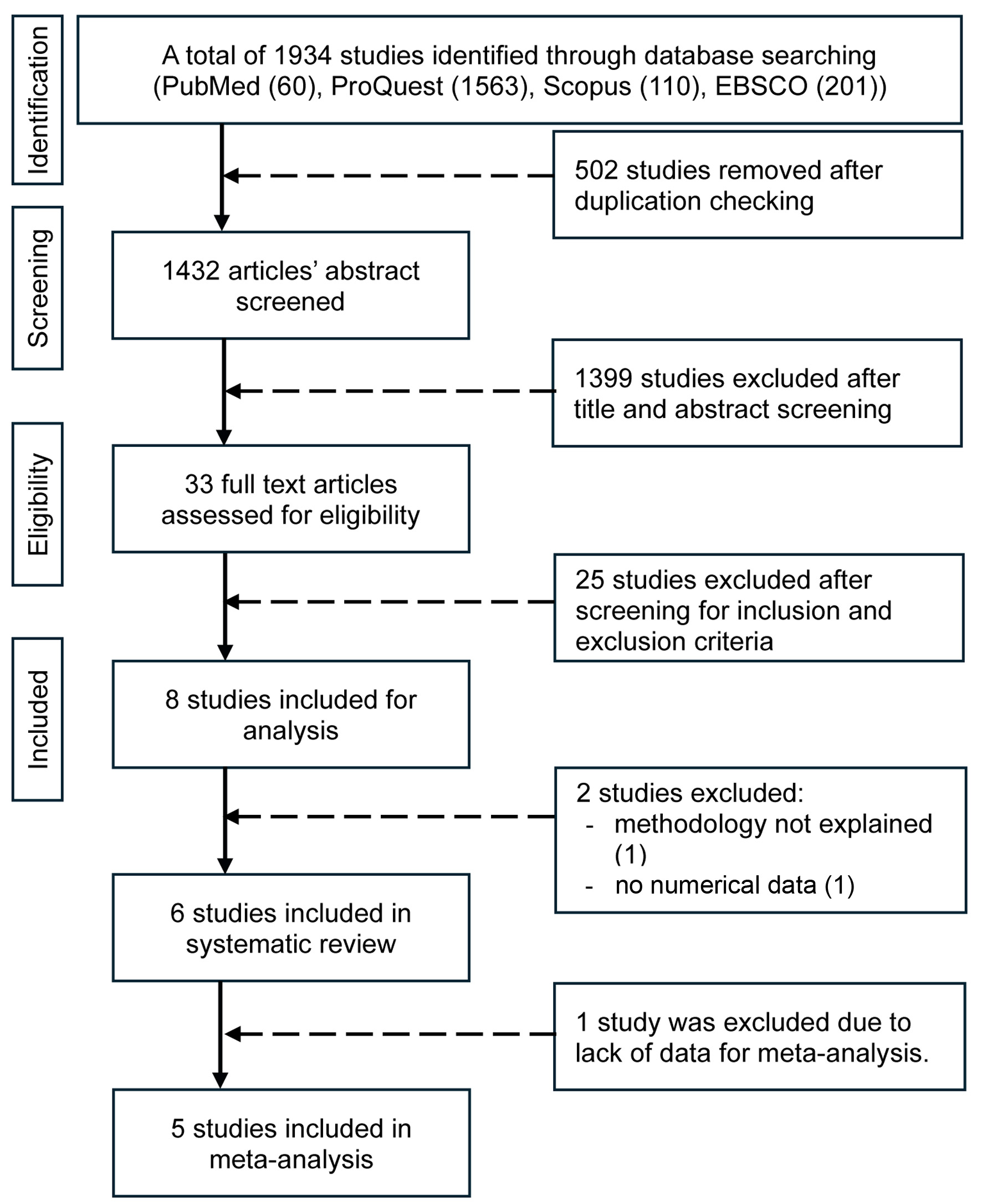 Click for large image | Figure 1. Flow diagram of the search process. |
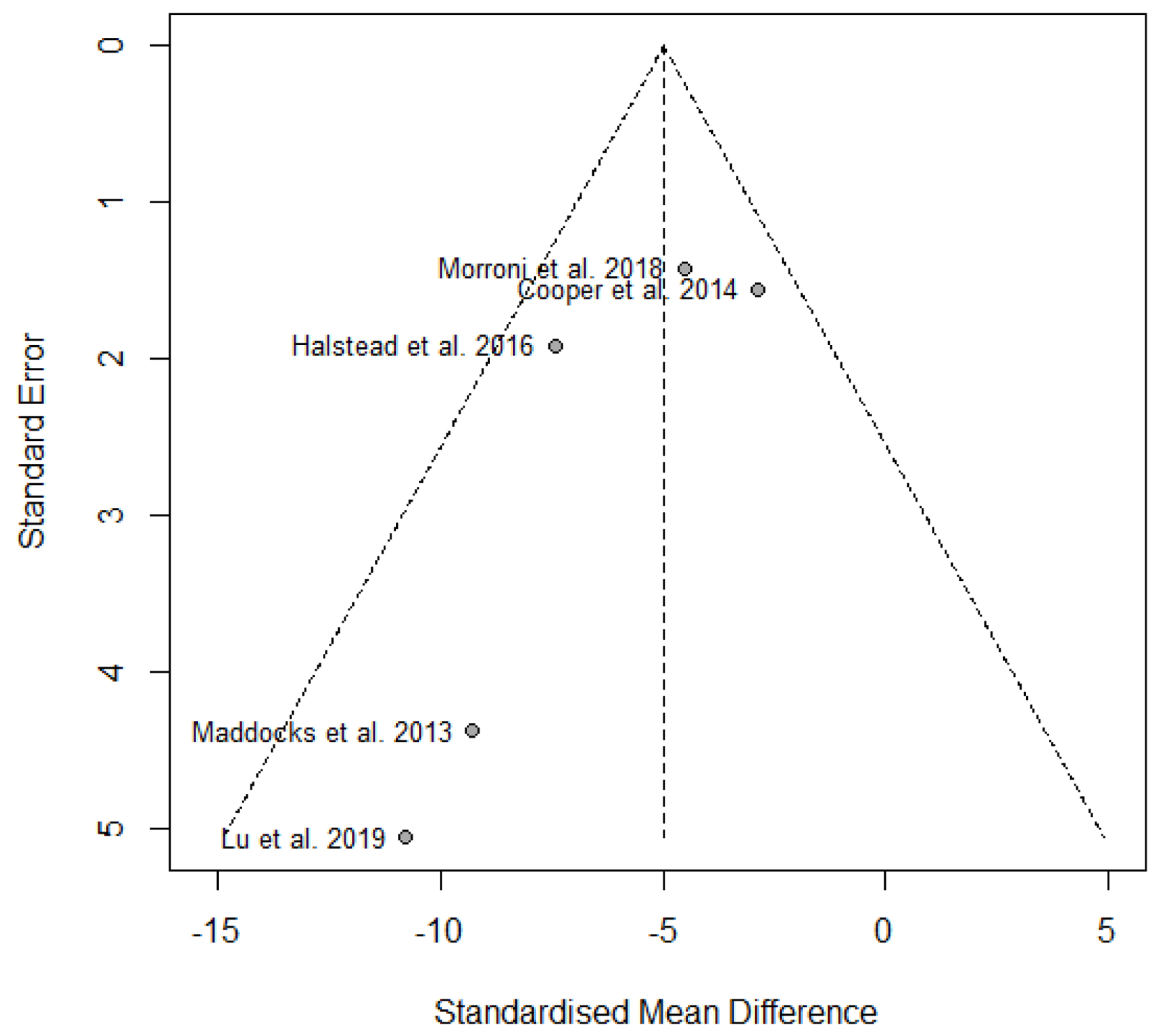 Click for large image | Figure 2. Funnel plot of included studies assessing the effect of medical-grade honey on P. aeruginosa biofilm. |
Study characteristics
The studies included in this review were published between 2013 and 2019. Four of the studies were conducted in the United Kingdom [10, 27-29], one in Italy [30], and one in Australia [13]. All studies employed in vitro experimental designs. Three studies utilized both standard bacterial strains and clinical isolates, two relied solely on clinical isolates, and one focused exclusively on a reference bacterial strain. MGH was tested across all studies, either in its natural form or as an engineered product. Control groups included negative controls and positive controls, which involved comparisons with other honey types or established antimicrobial therapies. Study characteristics of the included studies are summarized in Table 1 [10, 13, 27-30].
 Click to view | Table 1. Descriptive Summary of Included Studies |
Quality assessment
The majority of the included studies provided adequate information for critical appraisal, encompassing clearly defined study objectives, detailed methodologies, comparator groups, outcome measures, statistical analyses, and presentation of results. However, none of the studies reported on key methodological features such as randomization procedures, blinding of investigators, or the roles and qualifications of operators and outcome assessors. Based on evaluation using the QUIN tool, all studies achieved scores exceeding 80%, signifying a low risk of bias. A breakdown of the specific criteria assessed for each study is presented in the corresponding summary table (Table 2).
 Click to view | Table 2. Quality Assessment for Included Studies Using QUIN Tool |
Extraction outcome
All studies included in this review shared a common objective: to evaluate the effect of MGH on the formation and structural integrity of P. aeruginosa biofilms. The primary honey interventions consisted of Manuka honey at concentrations of 80% and 100%, as well as engineered formulations such as Surgihoney. Comparative interventions included other MGH and non-MGH types, as well as alternative standardized antimicrobial agents. All honey products used were commercially available. The P. aeruginosa strains employed originated from recognized sources, including the American Type Culture Collection (ATCC), the National Collection of Type Cultures (NCTC), and the University of California Berkeley Plant Pathology (UCBPP). Some studies also incorporated clinical isolates derived from chronic wound swabs and chronic rhinosinusitis specimens. The bacterial strains used across the included studies comprised both standardized ATCC strains and patient-derived clinical isolates, a distinction that critically impacts the consistency, generalizability, and clinical relevance of the findings. ATCC strains are advantageous for their high reproducibility, yet they may not fully reflect the virulence and resistance profiles of bacteria from actual infections. In contrast, while clinical isolates exhibit greater variability, they offer a more accurate representation of the pathogens encountered in clinical settings [31, 32].
A subset of studies included additional bacterial species to broaden the evaluation of honey’s antibiofilm properties. Sample replication was predominantly performed in triplicate, although some studies utilized duplicate or quadruplicate replication formats. All experiments were conducted in vitro using microtiter plate-based biofilm assays. Biofilms were stained with crystal violet and quantified spectrophotometrically through optical density (OD) measurements, enabling objective comparison of total biofilm biomass. Most studies provided detailed descriptions of honey dilution procedures and biofilm formation protocols. The duration of biofilm development prior to treatment ranged from 24 to 72 h, with a median of 24 h. A variety of culture media were used, including Mueller-Hinton broth (MHB), cation-adjusted Mueller-Hinton broth (CAMHB), tryptone soya broth (TSB), and nutrient broth (NB).
Honey effects on biofilm formation
A meta-analysis encompassing five in vitro studies was performed to evaluate the efficacy of MGH in inhibiting P. aeruginosa biofilm formation. SMDs were utilized to normalize variations in outcome measurement scales across the included studies. The aggregated analysis revealed a statistically significant reduction in biofilm metrics among honey-treated groups compared to controls, with a pooled SMD of -4.98 (95% CI: -6.72 to -3.25, P < 0.0001), indicating a pronounced anti-biofilm effect. Among the individual studies, Halstead et al [28] reported a robust inhibitory effect (SMD = -7.44; 95% CI: -11.20 to -3.68), while Maddocks et al [29] demonstrated an even larger effect size (SMD = -9.28; 95% CI: -17.85 to -0.70). Morroni et al [30] also found a significant reduction (SMD = -4.51; 95% CI: -7.29 to -1.72), and Lu et al [13] reported a considerable effect (SMD = -10.78; 95% CI: -20.70 to -0.86), though the wide CI likely reflects a limited sample size. In contrast, Cooper et al [27] observed a non-significant trend toward biofilm reduction (SMD = -2.85; 95% CI: -5.90 to 0.19), as the CI encompassed the null value. Analysis of heterogeneity yielded an I2 statistic of 32.3%, a between-study variance (Tau2) of 2.3897, and a non-significant Cochran’s Q test (P = 0.2064), suggesting moderate but not substantial variability in effect sizes across studies. The 95% prediction interval (-10.82 to 0.09) highlights the potential range of true effects in future research, indicating that although the overall effect favors honey, some subsequent studies may report attenuated or non-significant outcomes (Fig. 3).
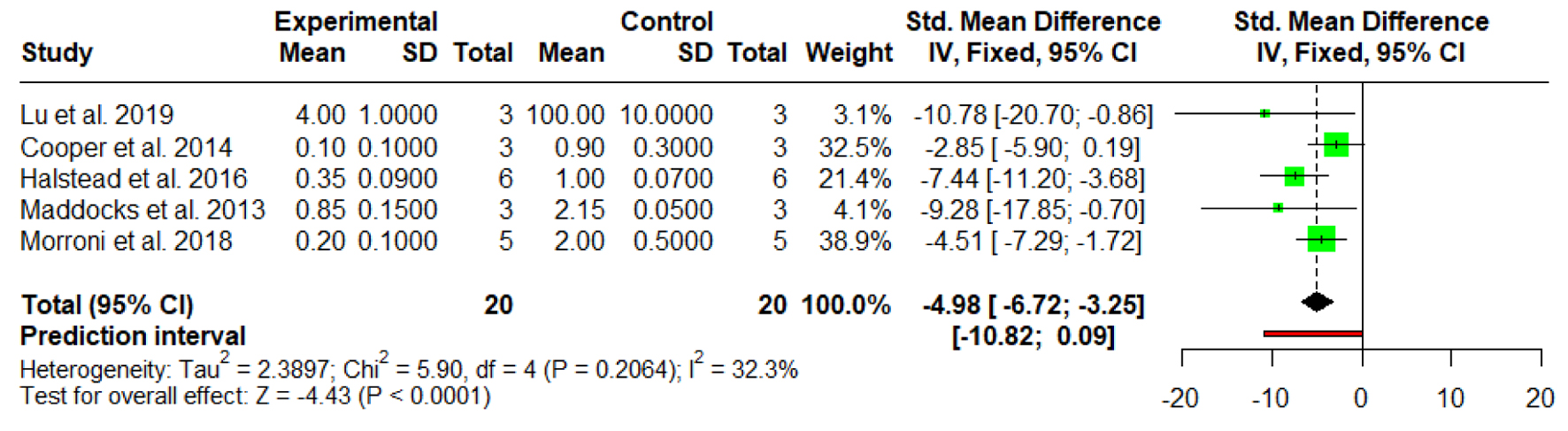 Click for large image | Figure 3. Forest plot illustrating the effect of medical-grade honey on biofilm formation. Forest plots represent static biofilm models only. |
Honey effects on established biofilm
A fixed-effect meta-analysis of three in vitro investigations was conducted to examine the impact of MGH on P. aeruginosa biofilms. The combined SMD was -4.44 (95% CI: -6.62 to -2.26, P < 0.0001), indicating a statistically significant and notable inhibitory effect of honey treatment compared to the control groups. Lu et al [13] reported the most substantial effect (SMD = -12.83; 95% CI: -24.59 to -1.07), although the broad CI reflects the limited sample size (n = 3). Cooper et al [27] also demonstrated a significant reduction (SMD = -5.35; 95% CI: -10.47 to -0.24), while the study by Morroni et al [30], which carried the greatest analytical weight (78.3%), showed a moderate but statistically meaningful decrease in biofilm parameters (SMD = -3.86; 95% CI: -6.33 to -1.39). Heterogeneity analysis revealed low variability across studies, with I2 = 12.7%, a non-significant Cochran’s Q (χ2 = 2.29, P = 0.3181), and negligible between-study variance (Tau2 < 0.0001). The 95% prediction interval (-9.24 to 0.35) indicates that, although the overall evidence supports a strong anti-biofilm effect of honey, future studies may report smaller or potentially non-significant outcomes (Fig. 4).
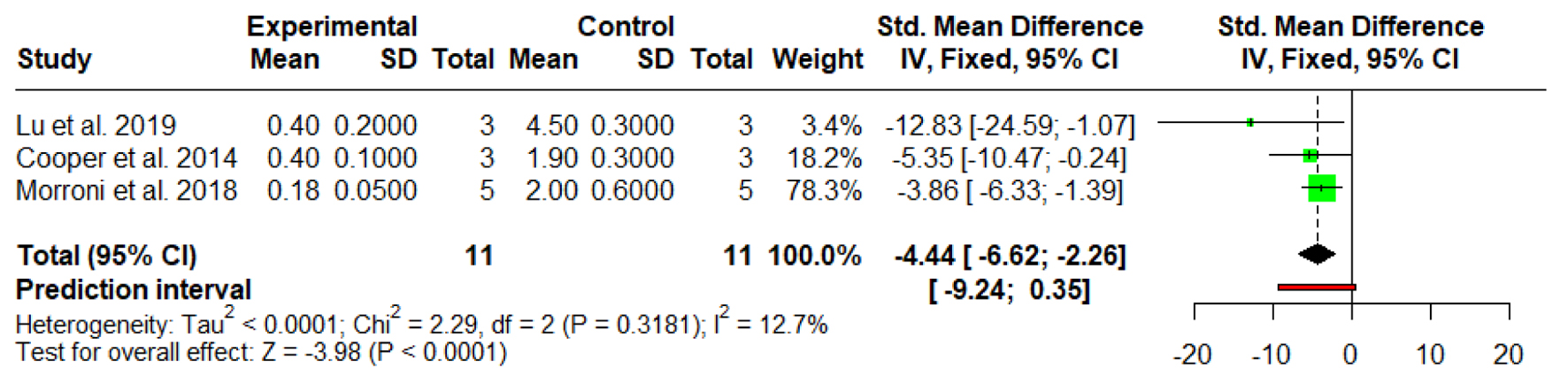 Click for large image | Figure 4. Forest plot illustrating the effect of medical-grade honey on established biofilm. Forest plots represent static biofilm models only. |
Honey effects on subgroups
1) ATCC and clinical isolate strain
Three studies utilizing ATCC strains demonstrated a markedly strong inhibitory effect of MGH. The pooled SMD was 31.79 (95% CI: -42.60 to -20.98), indicating a large and statistically significant reduction in biofilm biomass compared to control treatments. Individual effect sizes ranged from 29.52 to -43.38. No heterogeneity was detected (I2 = 0%), suggesting consistency across ATCC-based assays. Two studies using clinical P. aeruginosa isolates also reported significant biofilm inhibition, with a pooled SMD of -4.35 (95% CI: -6.40 to -2.29). While the effect size was smaller than that observed in ATCC strains, it remained statistically significant. Heterogeneity in this subgroup was also negligible (I2 = 0%), indicating methodological consistency within this subgroup.
The combined analysis yielded an overall pooled effect size of -18.84 (95% CI: -33.85 to -3.83), confirming a significant antibiofilm activity of MGH across all strains. However, the prediction interval ranged from -65.89 to 28.20, suggesting potential variability in future studies, particularly under differing experimental conditions. Notably, the test for subgroup differences was statistically significant (χ2 = 23.90, P < 0.0001), highlighting that the antibiofilm effect was substantially greater in ATCC strains than in clinical isolates. Considerable between-study heterogeneity was observed overall (I2 = 84.0%), although within-subgroup heterogeneity remained low (Fig. 5).
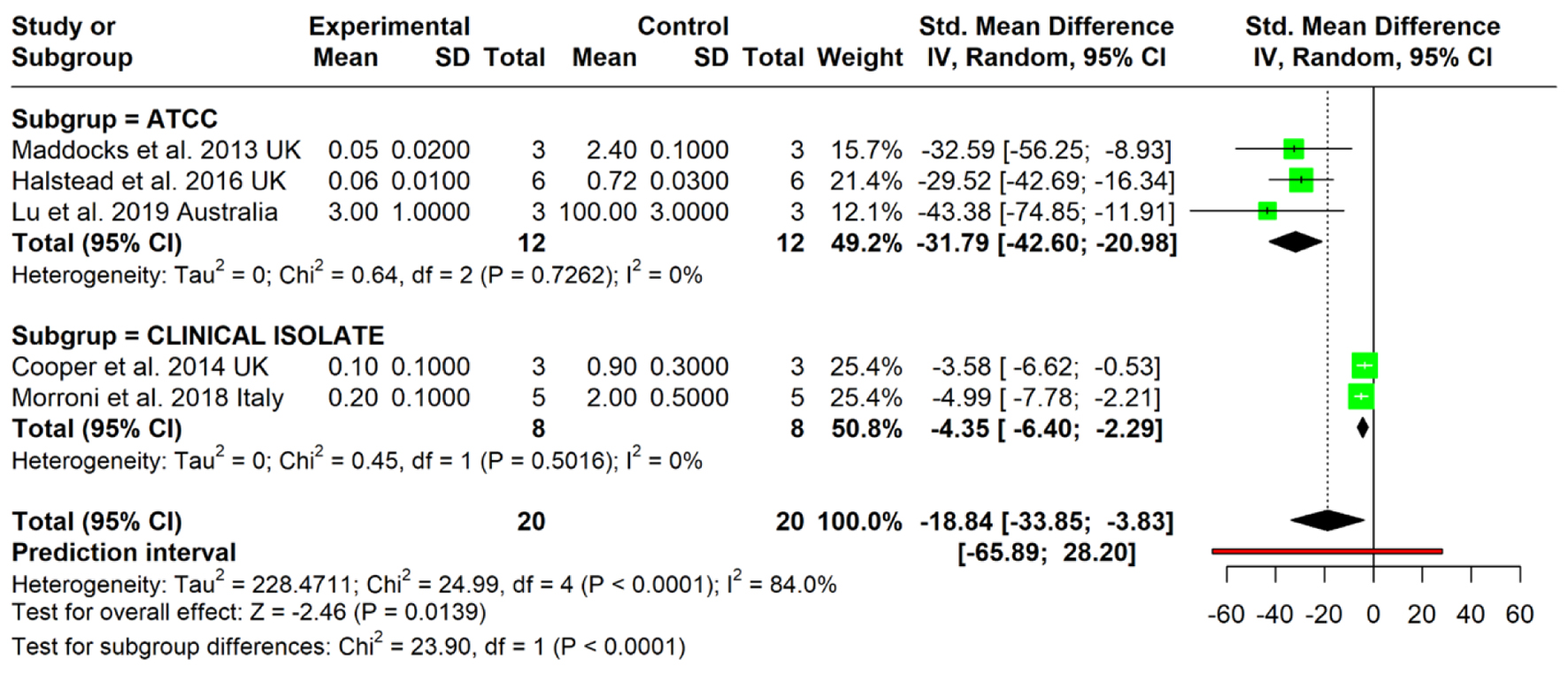 Click for large image | Figure 5. Forest plot illustrating the effect of medical-grade honey subgroup division based on bacterial strains used in the study. Forest plots represent static biofilm models only. |
2) Positive and negative control
To evaluate honey’s effect, we categorized the control groups into three subgroups: two quantitative and one qualitative. The first, the sterility control (SC), compares the honey-treated group to a sample containing only the growth medium, ensuring no bacterial contamination; this was used by Cooper et al [27] and Halstead et al [28]. The second subgroup is the active biofilm control (ABC), which provides a baseline of untreated, active bacterial biofilms. This was used by Cooper et al [27], Halstead et al [28], Maddocks et al [29], and Morroni et al [30]. Finally, the third subgroup is the mechanistic control, which is a more exploratory, qualitative analysis that examines whether honey’s effects are due to specific components like sugar or MGO. This was only used by Lu et al [13].
Two studies compared the intervention with an SC. The pooled SMD was -14.83 (95% CI: -42.01 to 12.34), which was not statistically significant. This subgroup exhibited very high heterogeneity (I2 = 94%), suggesting major inconsistencies in study design or outcome reporting. Notably, one study (Halstead et al [28]) reported a strong effect (SMD = -29.52), while the other (Cooper et al [27]) showed minimal effect (SMD = -1.74), indicating potential strain- or model-specific differences in response to the intervention. Four studies evaluated the intervention compared to biofilm-producing bacteria controls. The pooled SMD was -5.80 (95% CI: -8.26 to -3.35), showing a statistically significant inhibition of biofilm formation in favor of the intervention. Heterogeneity was moderate (I2 = 44.5%), reflecting more consistent findings across studies. Individual study results consistently favored the intervention, with SMDs ranging from -3.58 to -11.63.
The overall pooled estimate across all studies was SMD = -8.02 (95% CI: -13.87 to -2.17), indicating a statistically significant antibiofilm effect of the intervention. However, the prediction interval ranged widely from -26.82 to 10.78, suggesting that future studies may yield variable results. The overall heterogeneity was substantial (I2 = 81.6%), driven mainly by inconsistency within the SC subgroup. The test for subgroup differences was not statistically significant (χ2 = 0.42, P = 0.5166), indicating that the intervention’s effect did not significantly differ whether it was compared with sterility or antibiofilm controls (Fig. 6).
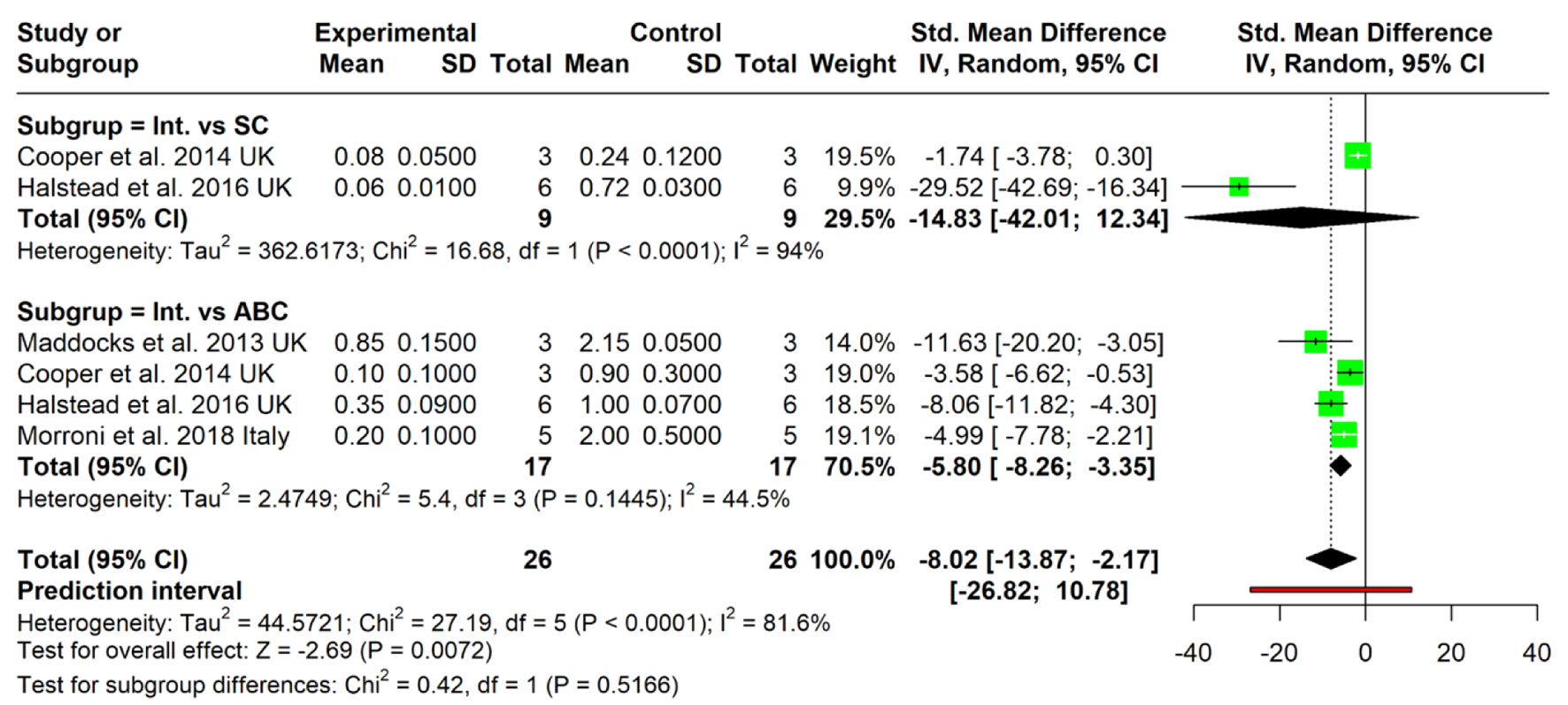 Click for large image | Figure 6. Forest plot illustrating the effect of medical-grade honey on subgroup division based on control used in the study. Forest plots represent static biofilm models only. ABC: active biofilm control; Int.: intervention; SC: sterility control. |
The findings reported by Lu et al [13] provide important insight into the multifactorial nature of Medihoney’s antibiofilm efficacy. Their study demonstrated that Medihoney effectively inhibited biofilm formation and disrupted established P. aeruginosa biofilms at relatively low concentrations (16-32%) across both PAO1 and PA14 strains. Although a synthetic sugar solution showed partial inhibitory effects, particularly at higher concentrations, it failed to match the potency of Medihoney, underscoring the role of non-sugar components. MGO, a well-characterized antibacterial constituent of Manuka honey, showed moderate biofilm inhibition when tested alone or in combination with sugar. However, its effects were significantly weaker than those of the complete honey, suggesting that MGO alone is insufficient to account for the observed biofilm disruption. These results support the hypothesis that Medihoney’s activity stems from a synergistic interplay of multiple bioactive compounds, rather than from any single constituent.
| Discussion | ▴Top |
This systematic review and meta-analysis aimed to comprehensively evaluate the in vitro efficacy of MGH in eliminating and inhibiting P. aeruginosa biofilms. Methodological quality assessment using the QUIN tool indicated a low risk of bias for all included studies, with scores consistently exceeding 80%. The findings provide robust evidence supporting MGH’s significant anti-biofilm activity, offering promising avenues for its application in clinical settings.
Key findings and explanation
A meta-analysis of five studies found that MGH significantly reduces biofilm formation compared to controls, with a pooled SMD of -4.98 (P < 0.0001), indicating that honey effectively prevents the initial microbial colonization and organization into a biofilm structure. This is a critical finding, as biofilm formation is a primary mechanism of chronic wound infection and a major cause of antibiotic treatment failure. The analysis showed a moderate but not substantial variability across studies (I2 = 32.3%). Three studies found that honey has a statistically significant inhibitory effect on established P. aeruginosa biofilms. The combined SMD was -4.44, with low variability among the studies (I2 = 12.7%). Mature biofilms present a greater challenge to eradication due to their complex architecture and protective matrix [6]. The ability of MGH to significantly reduce these established structures, as evidenced by studies like Lu et al [13] and Cooper et al [27] utilizing crystal violet staining and epifluorescence, highlights its potential as a therapeutic agent in chronic infections where mature biofilms are prevalent.
A significant outcome of this analysis is the comparison between the anti-biofilm effects of honey on ATCC reference strains versus clinical isolates. The effect on ATCC strains was found to be substantially stronger (SMD = -31.79) compared to clinical isolates (SMD = -4.35). This difference is statistically significant (χ2 = 23.90, P < 0.0001). The observed differences between ATCC strains and clinical isolates also merit consideration. ATCC strains, being standardized and laboratory-adapted, generally exhibit higher sensitivity to antimicrobial agents and more consistent responses. Clinical isolates, on the other hand, may possess enhanced resistance mechanisms, such as efflux pumps, altered membrane permeability, or enhanced stress response pathways [32], contributing to relatively lower, yet still significant, susceptibility to MGH. These findings highlight the clinical relevance of using both strain types in experimental designs to balance reproducibility and applicability. Careful consideration is needed when extrapolating in vitro findings from laboratory strains to the clinical setting.
The comparison of honey-treated groups to a sterile growth medium was not statistically significant, with a pooled SMD of -14.83. This subgroup also showed very high heterogeneity (I2 = 94%), indicating major inconsistencies between the two studies that used this control. This suggests that methodological variations, such as different concentrations of honey, bacterial strains, or biofilm assay protocols, may influence the outcomes. When compared to an active, untreated biofilm, MGH showed a statistically significant inhibition of biofilm formation. The pooled SMD was -5.80, with moderate heterogeneity across the four included studies. The overall pooled estimate across all studies and control types was a statistically significant antibiofilm effect, with an SMD of -8.02. However, the overall heterogeneity was substantial (I2 = 81.6%), primarily driven by the inconsistency within the SC subgroup. The qualitative analysis from Lu et al [13] (the mechanistic control) found that a synthetic sugar solution and MGO alone were less potent than the complete honey. These findings suggest that the anti-biofilm properties of honey are not solely attributable to its high osmolarity or a single active compound like MGO. Instead, its efficacy likely stems from the synergistic interplay of multiple bioactive components (enzymatic content, phytochemicals, and reactive oxygen species production), an aspect that makes it particularly difficult for bacteria to develop resistance to. Lu et al [13] showed that honey concentrations as low as 4% w/v could reduce biomass by over 90%. Lu et al [13] and Cooper et al [27] further confirmed that honey exposure led to visible disruption in mature biofilm architecture. These observations align with the idea that honey, through both direct bactericidal action and biofilm-modulating effects, can overcome the defense advantages conferred by the biofilm state. Taken together, the molecular mechanisms elucidated by Wang et al [33] illustrate how biofilms protect P. aeruginosa through multiple coordinated layers of defense, while evidence from in vitro studies supports the use of honey as a multi-targeted strategy to compromise these defenses. The combination of osmotic stress, acidic pH, hydrogen peroxide release, and quorum sensing inhibition makes honey a unique natural agent capable of acting both preventively, by blocking biofilm formation, and therapeutically, by degrading pre-formed biofilms.
Furthermore, honey’s interference with quorum sensing mechanisms may prevent the expression of genes essential for biofilm initiation, maturation, and antibiotic resistance. This is a key advantage of honey over traditional single-target antibiotics, where resistance is a growing concern. Bioactive compounds in MGH, such as flavonoids and phenolic acids, can inhibit quorum sensing by mimicking acyl-homoserine lactone (AHL) autoinducers and blocking quorum sensing receptors like LasR and rhamnolipid locus regulator (RhlR). Additionally, compounds like MGO may suppress AHL synthesis by disrupting key metabolic precursors, while peroxide can induce oxidative stress. These actions collectively impair quorum sensing signaling and biofilm development in P. aeruginosa [34].
Maddocks et al [29] reported that Manuka honey can inhibit bacterial adhesion in vitro by preventing bacterial binding to host proteins present at wound sites and on keratinocyte surfaces, thereby, in some instances, blocking bacterial invasion. This mechanism is closely linked to the dynamics of biofilm formation, whose structural integrity and behavior are primarily governed by two key factors. The first is the intrinsic characteristics of the microbial strain, particularly its capacity for strain-surface attachment, which influences adhesion potential, extracellular matrix production, and intercellular signaling. The second is the extrinsic media flow conditions, such as shear stress, nutrient supply, and flow rate, which significantly affect biofilm development and stability. Although these factors interact, each constitutes an independent determinant shaping the biofilm lifecycle. The antibiofilm action of MGH in this context appears to operate primarily through modulation of the intrinsic characteristics of bacterial strains [35].
Clinical implication
The consistent and substantial in vitro anti-biofilm activity of MGH against P. aeruginosa holds significant clinical implications, particularly for the management of chronic wound infections. P. aeruginosa biofilms are a major impediment to healing in conditions such as diabetic foot ulcers, pressure ulcers, and burn wounds, often leading to persistent infection and treatment failure with conventional antibiotics [4]. Our findings suggest that MGH, already available in various medical-grade formulations (e.g., Medihoney™, Surgihoney™) and widely used in wound care, could serve as an effective topical therapeutic agent. Its ability to disrupt both biofilm formation and established biofilms makes it a valuable tool in preventing and treating these recalcitrant infections, potentially reducing the reliance on systemic antibiotics and mitigating the risk of further antimicrobial resistance [8].
The non-specific mechanisms of action of honey (osmotic stress, acidity, hydrogen peroxide, MGO) are less likely to induce resistance compared to targeted antibiotics, offering a promising alternative or adjunct therapy against multidrug-resistant P. aeruginosa strains common in healthcare settings. The observed large effect sizes (SMD values) indicate a clinically meaningful impact on biofilm burden, suggesting that even relatively low concentrations of honey can achieve significant reductions, which is relevant for patient comfort and cost-effectiveness. Furthermore, the diversity of methodologies used across studies, including optical density measurements, biomass quantification, and viable cell counts, reflects the flexibility of MGH’s mechanism of action. However, the prediction interval observed in the meta-analysis on biofilm formation (-10.82 to 0.09) highlights variability in response and suggests that clinical outcomes may differ depending on factors such as the type of honey, wound environment, bacterial strain, and mode of application. This variability reinforces the importance of standardizing honey formulations and delivery protocols in clinical practice.
Strength and limitation of study
This systematic review and meta-analysis possesses several strengths. To our knowledge, it is the first to quantitatively synthesize in vitro evidence on the elimination effect of MGH on P. aeruginosa biofilms, addressing a critical gap in the literature. The rigorous methodology, including adherence to PRISMA guidelines and PROSPERO registration, enhances the transparency and reproducibility of our findings. The application of the QUIN tool for risk of bias assessment, specifically adapted for in vitro studies, allowed for a comprehensive evaluation of methodological quality, with all included studies demonstrating a low risk of bias. Our subgroup approach to control groups (e.g., distinguishing between “no honey” active biofilm controls and “just medium, no bacteria” SCs, as well as mechanistic controls) allowed for a more nuanced and accurate interpretation of honey’s efficacy, directly addressing the methodological heterogeneity identified. The inclusion of studies using diverse P. aeruginosa strains (reference and clinical isolates) and various honey types (Manuka, Surgihoney) further strengthens the generalizability of the in vitro findings.
Despite these strengths, certain limitations must be acknowledged. A significant constraint is the in vitro nature of all included studies. While these static models are invaluable for controlled investigation of mechanisms, they do not fully replicate the complex physiological conditions of a living host, such as the host immune response, tissue penetration, or the dynamic composition of wound exudate. Dynamic models, like flow-cells, offer more clinically relevant results by creating robust, durable biofilms that better mimic those found in vivo. As a result, findings from simplified static systems may not accurately reflect the true therapeutic potential of a treatment in a real-world wound environment [36]. Therefore, direct extrapolation of these findings to in vivo clinical efficacy should be approached with caution. Furthermore, despite our efforts with subgroup analysis, the inherent variability in control group methodologies across studies introduced some level of complexity. A truly standardized control would strengthen future research in this area. The meta-analysis on established biofilms included only three studies, which, despite showing a strong effect, limits the statistical power and precision of this specific pooled estimate. The wide range of honey concentrations and varying exposure durations across studies could also influence the magnitude of the observed effects, making it challenging to pinpoint an optimal in vitro treatment protocol. MGH formulations varied in viscosity (e.g., Surgihoney RO vs. raw Manuka), which may influence biofilm penetration. Future studies should standardize delivery methods (e.g., hydrogel carriers). Finally, consistent with the quality assessment, none of the included studies reported on key methodological details such as randomization procedures, blinding of investigators, or detailed operator/assessor qualifications. While this is a common reporting gap in in vitro literature, it represents a limitation in fully assessing potential performance or detection bias.
Recommendation for future research
Based on the findings and limitations of this review, several recommendations for future research emerge. First, inconsistency in the use of standardized ATCC strains versus patient-derived clinical isolates limits reproducibility, generalizability, and clinical relevance. ATCC strains provide consistency but may not reflect the virulence and resistance of real-world pathogens, whereas clinical isolates are more representative yet introduce variability. Future work should justify strain selection and evaluate its impact on outcomes. Secondly, future in vitro studies should prioritize the standardization of experimental protocols, particularly regarding biofilm models, honey concentrations, exposure times, and the precise definition and application of control groups. This standardization would facilitate more robust meta-analyses and allow for clearer comparisons across studies. Thirdly, while biomass reduction is important, future in vitro research should increasingly incorporate measures of viable cell count within biofilms (e.g., CFU/mL), as this is more directly indicative of bacterial elimination and clinical impact. Fourthly, given the rising challenge of antimicrobial resistance, future research should investigate the synergistic effects of MGH when combined with conventional antibiotics or other anti-biofilm agents. This could lead to novel combination therapies that enhance efficacy and mitigate resistance development.
Finally, the promising in vitro findings strongly warrant further translational research. This includes well-designed in vivo animal models of chronic P. aeruginosa biofilm infections and, ultimately, rigorous randomized controlled clinical trials to validate the efficacy and safety of MGH as a topical therapeutic agent in human patients. Concurrently, continued mechanistic studies are needed to fully elucidate the molecular pathways by which honey components interact with P. aeruginosa biofilms, particularly focusing on quorum sensing inhibition, EPS degradation, and resistance modulation.
Conclusion
This systematic review emphasizes the anti-biofilm effects of MGH, particularly against P. aeruginosa. The analyzed studies consistently show that MGH effectively prevents biofilm formation and reduces existing biofilms. It holds promise as an alternative or complementary treatment in wound care, especially for infections involving biofilm-producing bacteria. Although MGH exhibits significant anti-biofilm activity against P. aeruginosa, its effectiveness depends on various factors, including the type and concentration of honey, biofilm development stage, and bacterial strain differences. Investigating combination therapies, such as MGH with antibiotics, could enhance its antimicrobial properties while mitigating bacterial resistance. These findings highlight its potential for treating biofilm-associated infections but also stress the importance of optimizing treatment protocols to maximize benefits while minimizing resistance. Future studies should aim to refine application methods, explore synergistic approaches, and conduct clinical trials to validate its effectiveness, particularly its long-term antimicrobial effectiveness, in real-world medical settings.
Acknowledgments
We would like to thank Dr. Dani Esti Novia, Director of Banyumas Regional General Hospital, for the support of this research and Mr. Murti Prasetyo for his support in statistical analysis.
Financial Disclosure
The authors received no financial support for the research or publication of this manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest in the publication of this manuscript.
Informed Consent
Not applicable.
Author Contributions
HH and HRB were involved in conceptualizing and designing the study, conducting the systematic literature search, analyzing the data, performing statistical analyses, and drafting the initial manuscript. HH and DBN participated in the critical review and revision of the manuscript. YWW, CH, AB, and DBN supervised the project and offered substantial intellectual contributions during the revision process. All authors reviewed and approved the final version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ABC: active biofilm control; AHLs: acyl-homoserine lactones; ATCC: American Type Culture Collection; c-di-GMP: cyclic dimeric guanosine monophosphate; CAMHB: cation-adjusted Mueller-Hinton broth; CDC: Centers for Disease Control and Prevention; CFU/mL: colony-forming units per milliliter; CSV: comma separated value; CI: confidence interval; DNA: deoxyribonucleic acid; e.g.: exempli gratia (English, for example); eDNA: environmental deoxyribonucleic acid; EPS: extracellular polymeric substance; MBEC: minimum biofilm eradication concentration; MBIC: minimum biofilm inhibitory concentration; MeSH: Medical Subject Headings; MGH: medical-grade honey; MGO: methylglyoxal; MHB: Mueller-Hinton broth; MIC: minimum inhibitory concentration; NB: nutrient broth; NCTC: National Collection of Type Cultures; OD: optical density; P. aeruginosa: Pseudomonas aeruginosa; PBS: phosphat-buffered saline; Pel: pellicle; PQS: Pseudomonas quinolone signal; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analysis; PRISMA-P: Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols; PROSPERO: International Prospective Register of Systematic Reviews; Psl: polysaccharide synthesis locus; QUIN: Quality Assessment Tool for In Vitro Studies; Rhl: rhamnolipid-homoserine lactone; RhlR: rhamnolipid locus regulator; SC: sterility control; SD: standard deviation; SMD: standardized mean difference; TSB: tryptone soya broth; UCBPP: University of California Berkeley Plant Pathology; USD: United States Dollar; WHO: World Health Organization
| References | ▴Top |
- Silby MW, Winstanley C, Godfrey SA, Levy SB, Jackson RW. Pseudomonas genomes: diverse and adaptable. FEMS Microbiol Rev. 2011;35(4):652-680.
doi pubmed - Thi MTT, Wibowo D, Rehm BHA. Pseudomonas aeruginosa biofilms. Int J Mol Sci. 2020;21(22):8671.
- Streeter K, Katouli M. Pseudomonas aeruginosa: a review of their pathogenesis and prevalence in clinical settings and the environment. Infect Epidemiol Microbiol. 2016;2(1):25-32.
- Darvishi S, Tavakoli S, Kharaziha M, Girault HH, Kaminski CF, Mela I. Advances in the sensing and treatment of wound biofilms. Angew Chem Int Ed Engl. 2022;61(13):e202112218.
doi pubmed - Kamaruzzaman NF, Tan LP, Mat Yazid KA, Saeed SI, Hamdan RH, Choong SS, Wong WK, et al. Targeting the bacterial protective Armour; challenges and novel strategies in the treatment of microbial biofilm. Materials (Basel). 2018;11(9):1705.
doi pubmed - Yin R, Cheng J, Wang J, Li P, Lin J. Treatment of pseudomonas aeruginosa infectious biofilms: challenges and strategies. Front Microbiol. 2022;13:955286.
doi pubmed - Srinivasan R, Santhakumari S, Poonguzhali P, Geetha M, Dyavaiah M, Xiangmin L. Bacterial biofilm inhibition: a focused review on recent therapeutic strategies for combating the biofilm mediated infections. Front Microbiol. 2021;12:676458.
doi pubmed - Carmeli Y, Troillet N, Eliopoulos GM, Samore MH. Emergence of antibiotic-resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents. Antimicrob Agents Chemother. 1999;43(6):1379-1382.
doi pubmed - Pang Z, Raudonis R, Glick BR, Lin TJ, Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv. 2019;37(1):177-192.
doi pubmed - Camplin AL, Maddocks SE. Manuka honey treatment of biofilms of Pseudomonas aeruginosa results in the emergence of isolates with increased honey resistance. Ann Clin Microbiol Antimicrob. 2014;13:19.
doi pubmed - Almasaudi S. The antibacterial activities of honey. Saudi J Biol Sci. 2021;28(4):2188-2196.
doi pubmed - Wang R, Starkey M, Hazan R, Rahme LG. Honey's ability to counter bacterial infections arises from both bactericidal compounds and QS inhibition. Front Microbiol. 2012;3:144.
doi pubmed - Lu J, Cokcetin NN, Burke CM, Turnbull L, Liu M, Carter DA, Whitchurch CB, et al. Honey can inhibit and eliminate biofilms produced by Pseudomonas aeruginosa. Sci Rep. 2019;9(1):18160.
doi pubmed - Lerrer B, Zinger-Yosovich KD, Avrahami B, Gilboa-Garber N. Honey and royal jelly, like human milk, abrogate lectin-dependent infection-preceding Pseudomonas aeruginosa adhesion. ISME J. 2007;1(2):149-155.
doi pubmed - Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406(6799):959-964.
doi pubmed - Pleeging CCF, Coenye T, Mossialos D, de Rooster H, Chrysostomou D, Wagener F, Cremers NAJ. Synergistic antimicrobial activity of supplemented medical-grade honey against pseudomonas aeruginosa biofilm formation and eradication. Antibiotics (Basel). 2020;9(12):1-16.
doi pubmed - Mandal MD, Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed. 2011;1(2):154-160.
doi pubmed - Al-Waili N, Salom K, Al-Ghamdi AA. Honey for wound healing, ulcers, and burns; data supporting its use in clinical practice. ScientificWorldJournal. 2011;11:766-787.
doi pubmed - Yaghoobi R, Kazerouni A, Kazerouni O. Evidence for clinical use of honey in wound healing as an anti-bacterial, anti-inflammatory anti-oxidant and anti-viral agent: a review. Jundishapur J Nat Pharm Prod. 2013;8(3):100-104.
doi pubmed - Oryan A, Alemzadeh E, Moshiri A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J Tissue Viability. 2016;25(2):98-118.
doi pubmed - Al-Waili NS, Salom K, Butler G, Al Ghamdi AA. Honey and microbial infections: a review supporting the use of honey for microbial control. J Med Food. 2011;14(10):1079-1096.
doi pubmed - Hermanns R, Mateescu C, Thrasyvoulou A, Tananaki C, Wagener FADTG, Cremers NAJ. Defining the standards for medical grade honey. J Apic Res. 2020;59(2):1-11.
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
doi pubmed - Marin F, Rohatgi A, Charlot S. WebPlotDigitizer, a polyvalent and free software to extract spectra from old astronomical publications: application to ultraviolet spectropolarimetry. arXiv. 2017:1708.02025.
- Sheth VH, Shah NP, Jain R, Bhanushali N, Bhatnagar V. Development and validation of a risk-of-bias tool for assessing in vitro studies conducted in dentistry: The QUIN. J Prosthet Dent. 2024;131(6):1038-1042.
doi pubmed - Tran L, Tam DNH, Elshafay A, Dang T, Hirayama K, Huy NT. Quality assessment tools used in systematic reviews of in vitro studies: A systematic review. BMC Med Res Methodol. 2021;21(1):101.
doi pubmed - Cooper R, Jenkins L, Hooper S. Inhibition of biofilms of Pseudomonas aeruginosa by Medihoney in vitro. J Wound Care. 2014;23(3):93-104.
doi pubmed - Halstead FD, Webber MA, Rauf M, Burt R, Dryden M, Oppenheim BA. In vitro activity of an engineered honey, medical-grade honeys, and antimicrobial wound dressings against biofilm-producing clinical bacterial isolates. J Wound Care. 2016;25(2):93-102.
doi pubmed - Maddocks SE, Jenkins RE, Rowlands RS, Purdy KJ, Cooper RA. Manuka honey inhibits adhesion and invasion of medically important wound bacteria in vitro. Future Microbiol. 2013;8(12):1523-1536.
doi pubmed - Morroni G, Alvarez-Suarez JM, Brenciani A, Simoni S, Fioriti S, Pugnaloni A, Giampieri F, et al. Comparison of the antimicrobial activities of four honeys from three countries (New Zealand, Cuba, and Kenya). Front Microbiol. 2018;9:1378.
doi pubmed - Alnuaimi AD, O'Brien-Simpson NM, Reynolds EC, McCullough MJ. Clinical isolates and laboratory reference Candida species and strains have varying abilities to form biofilms. FEMS Yeast Res. 2013;13(7):689-699.
doi pubmed - Del Mar Cendra M, Torrents E. Differential adaptability between reference strains and clinical isolates of Pseudomonas aeruginosa into the lung epithelium intracellular lifestyle. Virulence. 2020;11(1):862-876.
doi pubmed - Wang X, Liu M, Yu C, Li J, Zhou X. Biofilm formation: mechanistic insights and therapeutic targets. Mol Biomed. 2023;4(1):49.
doi pubmed - Ng WJ, Matejczuk K, Ee KY, Boutrou I, Hew PS, Szweda P, Mossialos D. Honey bee and stingless bee products: effects on microbial virulence factors and pathogenicity mechanisms. J Funct Foods. 2025;132:1-31.
- Sanchez MC, Alonso-Espanol A, Ribeiro-Vidal H, Alonso B, Herrera D, Sanz M. Relevance of biofilm models in periodontal research: from static to dynamic systems. Microorganisms. 2021;9(2):428.
doi pubmed - Ramachandra SS, Wright P, Han P, Abdal-Hay A, Lee RSB, Ivanovski S. Evaluating models and assessment techniques for understanding oral biofilm complexity. Microbiologyopen. 2023;12(4):e1377.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.