| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Review
Volume 17, Number 8, August 2025, pages 409-422
Research on the Impact of Thyroid Disorders on Reproductive Function: A Narrative Review
Jian Tana, b, e, Yu Ying Yangc, e, De Ying Yind, Qun Xinb, Xian Cai Geb, f
aDepartment of Medical Genetics, Naval Medical University, Shanghai, China
bDepartment of General Surgery, The Navy 971 Hospital of the People’s Liberation Army, Qingdao, China
cDepartment of Burns and Plastic Surgery, The Navy 971 Hospital of the People’s Liberation Army, Qingdao, China
dThe Second Department of Health Care, The Navy 971 Hospital of the People’s Liberation Army, Qingdao, China
eThese authors contributed equally to this article.
fCorresponding Author: Xian Cai Ge, Department of General Surgery, The Navy 971 Hospital of the People’s Liberation Army, Qingdao, China
Manuscript submitted July 4, 2025, accepted August 20, 2025, published online August 31, 2025
Short title: Thyroid Disorders and Reproductive Function
doi: https://doi.org/10.14740/jocmr6315
- Abstract
- Introduction
- Methods
- Hypothyroidism and Female Infertility
- Hyperthyroidism and Female Infertility
- Hypothyroidism and Male Infertility
- Hyperthyroidism and Male Infertility
- Subclinical Thyroid Dysfunction and Infertility
- TAI and Infertility
- Potential Therapies for Thyroid Dysfunction-Related Infertility
- Conclusions and Future Directions
- References
| Abstract | ▴Top |
Infertility, characterized by the failure to achieve clinical pregnancy after 12 months or more of regular unprotected sexual intercourse, has emerged as a substantial global reproductive health challenge. According to recent epidemiological studies, this condition affects approximately 15-20% of couples within the reproductive age group worldwide. Notably, thyroid dysfunction has been identified as a significant contributing factor, being present in 5-10% of infertile couples. Although the well-established association between thyroid dysfunction and human infertility has been extensively documented in the literature, the precise underlying mechanisms remain incompletely understood and continue to be a subject of ongoing scientific investigation. In this review, we comprehensively examine the current literature regarding the impact of abnormal thyroid function on reproductive health and explore potential therapeutic interventions for thyroid dysfunction-associated infertility. This analysis aims to identify promising research directions and advance treatment strategies for managing infertility related to thyroid disorders.
Keywords: Hypothyroidism; Hyperthyroidism; Subclinical thyroid dysfunction; Thyroid autoimmunity; Female infertility; Male infertility
| Introduction | ▴Top |
The failure to achieve a clinical pregnancy following 1 year of consistent, unprotected sexual activity or as a result of a person’s inability to reproduce, either alone or with a partner, is known as infertility. It is thought to impact over 15% of couples in their reproductive years globally, and it has grown to be a significant medical and societal issue [1]. About 37% of infertility is caused by female factors, 8% by male factors, 35% by bilateral factors, and 5% by immunological and unexplained causes [2]. There are several known reasons of male infertility, including idiopathic causes, sperm transportation issues, endocrine disorders (often brought on by hypogonadism), and primary testicular anomalies [3]. It has been shown that endocrine metabolic dysfunction, genetic mutations, and congenital reproductive disorders all contribute to male infertility and subfertility. In females, reasons of infertility include uterine and tubal factors, ovarian reserve, ovulatory dysfunction, obesity, and hormone-related illnesses. Endocrine metabolic abnormalities are the most common causes of male and female infertility.
Thyroid dysfunction, one of the most prevalent endocrine problems, mainly encompasses subclinical and overt hypo- and hyperthyroidism, with a prevalence ranging from 8% to 10% in female and from 1% to 2% in male [4, 5]. In particular, hyperthyroidism affects 1-2% of women of childbearing age, overt hypothyroidism involves 0.3%, and subclinical hypothyroidism affects up to 15% [6]. The majority of hypothyroidism cases are categorized as primary hypothyroidism, which results from insufficient thyroid hormone production by the thyroid gland. Thyroid-stimulating hormone (TSH) levels above, and free thyroxine (FT4) levels below, the laboratory standard range are both considered indicators of overt hypothyroidism. TSH levels above the reference range, and FT4 levels within the reference range, are indicative of subclinical hypothyroidism [5]. Biochemically, overt hyperthyroidism is characterized by elevated levels of FT4, total, or free triiodothyronine (FT3), and decreased levels of TSH in the serum. Subclinical thyroid dysfunction, which includes subclinical hypothyroidism (SCH) and subclinical hyperthyroidism (SC-Hyper), is characterized by serum TSH levels that are either higher or lower than the reference range with normal thyroxine concentrations. Thyroid hormone receptors can be found in both male and female genitalia, such as the testis, corpora cavernosa, ovary, and vagina [7]. Although the close relationship and interaction between thyroid dysfunction and human infertility have been well documented, underlying potential processes are still up for discussion and lack a clear understanding. The primary objective of this review is to systematically evaluate the impact of various thyroid disorders - including hyperthyroidism, hypothyroidism, subclinical thyroid dysfunction, and thyroid autoimmunity (TAI) - on human reproductive function and fertility outcomes. Furthermore, this review aims to examine current thyroid-targeted interventions and their efficacy in addressing infertility.
| Methods | ▴Top |
This narrative review was conducted through a systematic literature search in PubMed using key terms including “hypothyroidism”, “hyperthyroidism”, “subclinical thyroid dysfunction”, “thyroid autoimmunity”, “female infertility”, and “male infertility”. A total of 67 relevant English-language articles published between 2020 and 2025 were selected as primary references based on their scientific merit and relevance to the research topic.
| Hypothyroidism and Female Infertility | ▴Top |
Untreated hypothyroidism is closely associated with a higher chance of infertility and pregnancy problems. According to recent research, women with hypothyroidism might have an infertility rate of up to 30%, while women with normal thyroid function usually have an infertility rate of 10-15% [8]. Hypothyroidism contributes to infertility by disrupting multiple reproductive processes, including follicular development, fertilization, embryo implantation, and hormonal regulation. This endocrine disorder affects the secretion of key reproductive hormones, notably thyrotropin-releasing hormone (TRH), prolactin (PRL), gonadotropin-releasing hormone (GnRH), follicle-stimulating hormone (FSH), and luteinizing hormone (LH), thereby compromising normal reproductive function. A study has shown that hypothyroidism in adult female rats may have a negative impact on fertility because it causes a significant reduction in the number of primordial, primary, and preantral follicles, as well as a downward trend in the numbers of antral follicles and corpora lutea [9]. Mechanistically, hypothyroidism activates the apoptotic signaling pathway by downregulating glucose-regulated protein 78 (GRP78) expression and concurrently upregulating C/EBP homologous protein (CHOP) and cleaved caspase-3 levels, thereby suppressing follicular development [10]. In addition, hypothyroidism alters the level of triacylglycerol (TAG), total cholesterol (TC), oxidized lipids, glycogen, and immune cell infiltration into the ovary and uterus, leading to infertility, tubal abnormalities, and ectopic pregnancy [11]. Accordingly, a study demonstrated that hypothyroidism affects follicular development and reduces ovulation rates through raising oxidative stress, hence affecting reproductive health in women [12]. Hypothyroidism has also been shown to affect embryo implantation by lowering E2 levels and reducing the expression of osteopontin and homeobox A10 (HOXA10), two uterine-receptivity factors. Moreover, hypothyroidism markedly suppresses uterine prostaglandin levels and downregulates critical components of the prostaglandin F2-alpha (PGF2α), prostaglandin I2 (PGI2), and prostaglandin E2 (PGE2) signaling pathways. Given the well-established role of prostaglandins in critical uterine functions - including decidualization and blastocyst implantation - these findings highlight a potential mechanistic link between thyroid dysfunction and reproductive processes [13]. In hypothyroidism, integrin β3, integrin αvβ3, leukemia inhibitory factor (LIF), and HOXA10 immune reaction intensities were found to be low, whereas MUC1 immunoreactivity was high. This resulted in morphological abnormalities, vacuolization, epithelial degeneration, and uterine gland hypertrophy in the endometrial layer [14]. Similarly, a recent study discovered that hypothyroidism affected female fertility by promoting uterine hyperplasia and inflammation through increased expression of vascular endothelial growth factor A (VEGF-A), progesterone receptor (PR), estrogen receptor (ER), and thyroid hormone receptors (TRs) and decreased uterine content of perilipin A (PLIN-A), TAG, and TC [15]. Additionally, by altering hormone secretion, hypothyroidism causes anovulation and reduces luteal phase function, which results in infertility [16]. Hypothyroidism also stimulates excessive TRH production, leading to hyperprolactinemia. The elevated PBL levels subsequently disrupt the pulsatile secretion of GnRH, thereby suppressing the production of FSH and LH. Moreover, hyperprolactinemia exerts direct inhibitory effects on ovarian follicle endocrine function [9]. Approximately 80% of women diagnosed with hypothyroidism exhibit menstrual disturbances or irregularities, which can significantly impact female reproductive function and fertility [17]. Women with hypothyroidism frequently experience weight gain due to impaired metabolic function. For young women of reproductive age, this obesity can lead to significant reproductive consequences, including reduced fertility, increased risk of recurrent pregnancy loss, higher incidence of preeclampsia, and impaired fetal growth [18, 19]. To sum up, these results indicate that hypothyroidism plays a crucial and intricate role in female infertility, although the hidden mechanisms remain poorly known and require additional research.
| Hyperthyroidism and Female Infertility | ▴Top |
According to prospective studies, 5.8% of women with hyperthyroidism experience primary or secondary infertility. Hyperthyroidism promotes female fertility mostly through hormonal imbalances, ovarian dysfunction, and intrauterine problems. It has been reported that hyperthyroidism causes hormonal changes, including increased sex hormone-binding globulin (SHBG) levels, which, together with reduced estradiol metabolism, increase testosterone production, and enhance conversion of testosterone to estradiol, thereby leading to elevated estradiol levels. Additionally, hyperthyroidism results in increased production of androgens such as androstenedione and dehydroepiandrosterone sulfate, as well as a higher conversion of testosterone to estrone. As a result, excessively high estrogen levels impair female reproductive capacity [9]. Another study discovered that in hyperthyroidism, thyroid hormones caused an estrogen shortage that hindered nitric oxide synthase (NOS) activity, which in turn decreased NO synthesis and inhibited the growth of large antral follicles [20]. However, how hyperthyroidism leads to estrogen deficiency remains unclear. Similarly, one study found that hyperthyroidism modifies NOS expression profiles, which play crucial roles in female reproductive physiology [21]. Furthermore, elevated T3 levels in hyperthyroidism disrupt ovarian steroidogenesis, which is thought to be the reason for impaired ovulation and folliculogenesis [22]. Elevated T3 levels in hyperthyroidism consistently decrease FSH-stimulated aromatase activity in granulosa cells, which prevents the follicular cavity from forming and hampers the mice’s follicle development. Additionally, a recent study demonstrated that maternal hyperthyroidism affects fertility by causing an imbalance in the dolichos biflorus agglutinin-positive (DBA+) uterine natural killer (uNK) cell population and the inflammatory cytokine profile in decidua [23]. Taken together, these findings suggest that hyperthyroidism contributes significantly to female infertility by affecting ovarian dysfunction, hormonal abnormalities, and intrauterine issues. However, further research is necessary to comprehend the mechanisms and therapeutic potentials of hyperthyroidism in female infertility due to controversies and unclear aspects.
| Hypothyroidism and Male Infertility | ▴Top |
According to a few studies, the infertility rate in males with hypothyroidism ranges from 30% to 50%, which is much higher than the 10-15% rate in normal men [24]. Hypothyroidism inhibits male reproductive function by influencing sex hormone levels, sperm quality, metabolism, and psychological well-being. Hypothyroidism impairs male reproductive function primarily through direct detrimental effects on testicular Leydig cells. These effects include: 1) disruption of gonadotropin-mediated Leydig cell and Sertoli cell (SC) functions; 2) testicular atrophy (reduced testicular weight); 3) depletion of germ cells; 4) degeneration of seminiferous tubules; and 5) impairment of testosterone synthesis [25]. The PI3K/AKT signaling pathway has been established as the principal mediator of thyroid and leptin hormone actions on male reproductive function. In the context of obesity-associated hypothyroidism, impairment of PI3K/AKT signaling may represent a key pathogenic mechanism contributing to male reproductive dysfunction [26]. Hypothyroidism can also cause a decline in baseline levels of FSH and LH in men at the posttranscriptional level, resulting in male infertility [27]. According to a study, hypothyroidism may cause hypogonadotropic hypogonadism by impairing the pulsatile production of GnRH through an increase in PRL [28]. Moreover, studies have found that hypothyroidism can suppress the testicular Kiss1/Kiss1r signaling pathway while upregulating hypothalamic Pdyn expression, ultimately leading to the inhibition of the hypothalamic-pituitary-gonadal (HPG) axis in male rats and consequent infertility [29]. Extensive research has demonstrated that hypothyroidism can lead to reduced testicular weight, a decreased number of germ cells, smaller and fewer seminiferous tubules, and impaired sperm parameters. First of all, hypothyroidism significantly downregulates the expression of genes associated with sperm viability, capacitation, fertilization, oxidative stress defense, and energetic metabolism in the seminal vesicle (SV), while concurrently upregulating the expression of proteins related to tissue damage [30]. More specifically, Santos et al demonstrated that hypothyroidism disrupts the unfolded protein response (UPR) pathway in testicular tissue and induces oxidative stress [31]. GoMEZ-ZunIGA et al established that hypothyroidism induces alterations in the functional state of SCs, which may have led to a deficiency in the synthesis and/or release of molecules necessary for gonocyte differentiation, as well as disorders in the process of meiosis that result in sperm absence [32]. El-Shaer et al demonstrated that hypothyroidism induces testicular dysfunction through dual mechanisms: 1) centrally via disruption of the hypothalamic-pituitary-testicular axis, characterized by nesfatin-1 downregulation and subsequent inhibition of the MAPK/ERK signaling pathway; and 2) peripherally through direct impairment of testicular metabolic homeostasis and redox balance [33]. Hypothyroidism similarly causes testicular degeneration and functional impairment through multiple mechanisms, including elevation of plasma total homocysteine, NO metabolites, and malondialdehyde levels; disturbance of the oxidized glutathione to reduced glutathione (GSSG/GSH) ratio; and alteration of the intramitochondrial thiol redox state. These pathological changes ultimately result in spermatogenesis impairment [34]. Hypothyroidism can also cause erectile dysfunction (ED) in men, impacting male fertility to some degree [35]. Additionally, males with hypothyroidism usually have weight gain and fat storage due to a drop in their basal metabolic rate, which in turn impacts male fertility [36]. Although several studies have demonstrated that hypothyroidism can impair male reproductive function in a variety of ways, the processes by which hypothyroidism influences male hormone levels and spermatogenesis still require more detailed examination.
| Hyperthyroidism and Male Infertility | ▴Top |
Although robust epidemiological data on infertility rates in male hyperthyroidism patients are not yet available, some studies have indicated a potential elevation in infertility risk among this population compared to healthy individuals. In a manner analogous to hypothyroidism, hyperthyroidism can lead to male infertility by disrupting hormonal homeostasis, compromising sperm parameters, and inducing sexual dysfunction. According to research, hyperthyroidism-induced increased T3 and T4 levels suppress the HPG axis, lowering gonadotropin release (including LH and FSH). This, in turn, impacts testosterone production and ultimately results in male infertility [37]. Hyperthyroidism also encourages the liver to synthesize more SHBG, which binds to testosterone more, resulting in a drop in free testosterone levels (biologically active testosterone), which further impacts male reproductive function. Additionally, hyperthyroidism may upregulate aromatase activity, thereby accelerating the conversion of testosterone to estrogen, which subsequently exerts additional inhibitory effects on the HPG axis and exacerbates testosterone deficiency [38]. Hyperthyroidism not only diminishes sperm quality by suppressing testosterone levels but also induces structural and genetic damage to spermatozoa. The condition promotes excessive free radical generation, leading to oxidative stress that compromises sperm membrane integrity and causes DNA damage. These pathological changes manifest as impaired sperm motility, morphological abnormalities, and significantly increased DNA fragmentation rates [39]. Moreover, hyperthyroidism-induced elevation of basal metabolic rate may lead to increased scrotal temperatures in males, potentially resulting in impaired spermatogenesis characterized by reduced sperm count and decreased sperm motility [40]. The precise molecular mechanisms underlying the impact of hyperthyroidism on male sperm quality remain incompletely understood. Further comprehensive investigations are warranted to elucidate these pathological pathways, which will facilitate the development of targeted therapeutic strategies for improving spermatogenesis and sperm quality in hyperthyroid male patients.
| Subclinical Thyroid Dysfunction and Infertility | ▴Top |
Emerging evidence suggests that subclinical thyroid dysfunction may have a subtle but clinically relevant impact on human fertility potential. This section specifically examines the implications of subclinical hypothyroidism on female and male reproductive function. According to reports, the prevalence of SCH in women between the ages of 20 and 45 ranges from 5% to 7%; however, it is predicted that the prevalence among infertile women is between 11% and 43% [9]. A recent study revealed that SCH may exacerbate lipid and glucose metabolic dysregulation in patients with polycystic ovary syndrome (PCOS), potentially impairing female reproductive function [41]. Apart from the metabolic abnormalities, SCH impacts ovulation by inducing hyperprolactinemia, resulting in infertility in these women [42]. Furthermore, Zhang et al found that SCH may function as an independent factor influencing ovarian reserve and may be linked to a decrease in ovarian reserve among infertile women [43]. In addition, increased levels of TSH generated by SCH upregulated the LIF/STAT3 signaling pathway, ultimately leading to decreased endometrial receptivity [44]. Accordingly, Kakita-Kobayashi et al demonstrated that subclinical hypothyroidism contributes to female infertility through the impairment of decidualization [45]. SCH also exerts a non-negligible impact on male reproductive function, potentially compromising fertility through multiple pathways. Zhao et al demonstrated a significant association between SCH and elevated risk of abnormal sperm DNA fragmentation index (DFI) [46]. In addition, oxidative stress levels were significantly elevated in patients with SCH, thereby adversely affecting male reproductive function [47]. In the meanwhile, SCH has been linked to ED, which can exacerbate fertility problems by impairing sexual function and conception [7]. In addition to SCH, SC-Hyper has been reported to adversely affect semen quality, yet the mechanisms by which SC-Hyper influences sperm quality are not yet fully understood [48]. Recent studies have demonstrated that both SC-Hyper and hypothyroidism may progress to overt thyroid dysfunction in infertile women undergoing ovarian stimulation, adversely affecting their fertility treatment outcomes [49, 50]. Nevertheless, the precise mechanisms underlying this progression remain elusive. More clinical research is required in the future to elucidate the relationship between SC-Hyper and human infertility, as there are currently few studies on the effects of this condition on male and female reproductive function.
| TAI and Infertility | ▴Top |
TAI is primarily characterized by the presence of circulating thyroid-specific autoantibodies, including thyroid peroxidase antibodies (TPOAbs) and thyroglobulin antibodies (TgAbs), along with the infiltration of autoreactive lymphocytes into thyroid tissue, ultimately leading to thyrocyte destruction and the development of hypothyroidism. In this section, we will specifically examine the impact of TAI, as indicated by elevated concentrations of TPOAb or TgAb in the absence of thyroid dysfunction, on human fertility. TAI has been reported to affect 8-14% of women of reproductive age, with TPOAbs being detected in 9.5% of women experiencing previous pregnancy loss or subfertility [51]. Recent studies have demonstrated that TAI can initiate an inflammatory cascade mediated by the interferon-γ (IFNγ)-induced CXCL9/10/11-mediated recruitment of CXCR3+ T lymphocyte axis within the follicular microenvironment, which may subsequently impair ovarian angiogenesis and consequently inhibit follicular development [52]. Additionally, TAI is frequently associated with various autoimmune disorders, encompassing both organ-specific conditions, such as autoimmune polyglandular syndrome types 1-4, characterized by anti-ovarian antibodies, and systemic autoimmune diseases, including systemic lupus erythematosus, Sjogren’s syndrome, and rheumatoid arthritis, which are typically accompanied by the presence of antinuclear antibodies, antiphospholipid antibodies, and anti-laminin-1 antibodies (aLN-1). These immunological markers have been well documented to exert adverse effects on fertility and pregnancy outcomes [53]. Notably, approximately 20% of women with TAI exhibit non-organ-specific antibodies capable of interacting with trophoblastic/placental tissues, potentially triggering a cascade of pathological events, including prothrombotic state induction, cytokine dysregulation, and complement system activation [54]. Furthermore, TAI can disrupt the systemic balance of helper lymphocyte subsets, including Th1, Th2, Th17, and regulatory T cells (Tregs). This immunological dysregulation leads to elevated secretion of inflammatory cytokines such as interleukin (IL)-2, IL-17, and IFNγ, which are well-established contributors to implantation failure and pregnancy loss [55]. Additionally, TAI promotes excessive activation and cytotoxicity of natural killer (NK) cells in both peripheral blood and uterine tissue, along with the upregulation of NKT-like cells [53]. A recent study has suggested a potential association between TAI and diminished ovarian reserve or premature ovarian insufficiency (POI) [56]; however, the underlying mechanisms remain poorly understood. In addition to TAI per se, both TPOAbs and TgAbs have been demonstrated to exert direct impacts on human fertility. Research has indicated that TPOAbs can induce thyrocyte death through two distinct mechanisms: antibody-dependent cellular cytotoxicity (ADCC) and C3 complement-mediated cytotoxicity. Similarly, TgAb is hypothesized to exert its effects primarily through ADCC. Importantly, the potential damage may extend beyond the thyroid gland, as reproductive organs expressing TPO and Tg could also be affected by these autoimmune processes [57]. Additionally, the cross-reactivity between TPOAbs and human chorionic gonadotropin (hCG) receptors within the zona pellucida has been suggested as an alternative mechanism contributing to infertility [55]. Several studies have demonstrated that TPOAbs are significantly associated with both diminished ovarian reserve and impaired embryo quality [58]. Nevertheless, there is ongoing debate as some research indicates that TAI might not be linked to infertility in both female and male populations [59]. The impact of TAI on human reproductive function remains a subject of significant controversy, primarily due to the paucity of robust clinical evidence establishing a definitive correlation between TAI and impaired male reproductive capacity. While extensive research has been conducted to elucidate the mechanisms through which TAI may induce fertility complications in females, the precise pathophysiological processes underlying TAI-associated premature ovarian failure and diminished ovarian function remain incompletely understood. Currently, there is a lack of sufficient research to determine whether TAI impacts male reproductive ability. Consequently, there is an imperative need for more comprehensive and rigorous investigations to clarify the effects of TAI on human reproductive function across both genders.
| Potential Therapies for Thyroid Dysfunction-Related Infertility | ▴Top |
Accumulating clinical and experimental evidence has consistently demonstrated a strong association between thyroid dysfunction and human infertility, suggesting that thyroid-targeted therapeutic approaches may represent a promising strategy for infertility management. Thyroxine supplementation improves reproductive outcomes in infertile women with clinical and subclinical hypothyroidism [60]. The early administration of levothyroxine replacement therapy for subclinical hypothyroidism during pregnancy may decrease the incidence of pregnancy loss [16]. L-thyroxine supplementation was demonstrated to significantly mitigate the detrimental impact of hypothyroidism on endometrial receptivity [14]. It should be noted that L-thyroxine supplementation must maintain serum thyroxine levels within the normal range to ensure thyroid hormone replacement therapy has no adverse effects on fertility. Selenium (Se), a crucial constituent of selenoenzymes that possess both catalytic and antioxidant properties, has been reported to potentially serve as a non-hormonal therapeutic option for managing male infertility associated with chronic autoimmune thyroiditis (AT) [61]. Mechanistically, Se supplementation significantly reduced serum levels of TPOAb and TgAb, while concurrently improving thyroid gland echogenicity on ultrasonographic examination [62]. Additionally, studies have demonstrated that melatonin supplementation can effectively ameliorate gonadal dysfunction associated with hypothyroidism through multiple mechanisms. The therapeutic effects are primarily mediated by its antioxidant and anti-inflammatory properties, which lead to significant improvements in semen parameters, particularly enhancing sperm motility and viability. Furthermore, melatonin has been shown to stimulate testosterone production, thereby contributing to the restoration of normal gonadal function in hypothyroid conditions [63]. Meanwhile, exogenous melatonin treatment in female rats boosted T4 levels and stabilized T3 serum levels, reversing hypothyroidism-induced ovarian follicle loss [64]. Similarly, alpha-lipoic acid (ALA), a potent antioxidant, has been demonstrated to effectively mitigate the toxic effects of hypothyroidism on spermatogenesis in rat models. This therapeutic agent not only enhances the structural integrity and physiological functions of testicular tissues in hypothyroidism-induced rats but also provides significant protection against oxidative damage in the testicular environment [65]. Although further research is required to fully elucidate the underlying mechanisms, recent studies have demonstrated that both carbimazole (CBZ) monotherapy and its combination with vitamin E (VE) effectively attenuate hyperthyroidism-induced testicular injury. The therapeutic efficacy appears to be mediated through VE’s protective effects against oxidative stress-induced cellular damage [66]. Furthermore, intratesticular platelet-rich plasma (PRP) injection has been proposed as a promising therapeutic approach for male infertility associated with hypothyroidism, based on its demonstrated efficacy in enhancing testicular morphology and function. This treatment modality has shown significant improvements in key testicular parameters, including the volumetric proportions of seminiferous tubules and interstitial tissue, as well as promoting germ cell proliferation in rat models of hypothyroidism-induced infertility [67]. Given the inhibitory effects of hypothyroidism on the HPG axis, primarily mediated through the suppression of the testicular Kiss1/Kiss1r signaling pathway, kisspeptin analogs have emerged as a promising therapeutic strategy for managing male reproductive dysfunction associated with hypothyroid conditions [29]. Similarly, treatment with kisspeptin-10 (Kp10) effectively ameliorated hypothyroidism-induced ovarian dysfunction by restoring estrogen regularity, normalizing plasma LH levels, improving ovarian and uterine morphology, and upregulating mRNA expression of Cyp11a1, 3β-Hsd, and 20α-Hsd in the corpora lutea, independent of hyperprolactinemia status [68]. According to recent research findings, Elettaria cardamomum extract (ECE) has demonstrated significant therapeutic potential in addressing male reproductive dysfunction associated with hypothyroidism. The study revealed that ECE effectively counteracts the inhibitory effects of hypothyroidism on testicular tissue, enhances spermatogenesis by increasing the number of germ cells, and stimulates testosterone secretion [69]. Notably, TAI represents a significant immunological factor contributing to infertility. In this context, emerging cell and gene therapies have shown considerable therapeutic potential for managing TAI-associated infertility [70], However, extensive clinical research and rigorous validation are still essential to ensure their successful translation into clinical practice. In conclusion, elucidating the molecular mechanisms underlying thyroid dysfunction-induced infertility is imperative for the development of more efficacious and targeted therapeutic interventions.
| Conclusions and Future Directions | ▴Top |
Infertility has emerged as a critical medical and social challenge, affecting more than 15% of reproductive-aged couples across the world. Particularly, thyroid dysfunction has been recognized as a significant contributing factor, occurring in 5-10% of infertile couples. The well-documented association between thyroid dysfunction and infertility has been extensively established in clinical literature [9, 34], highlighting the critical need to elucidate the underlying mechanisms and explore their potential therapeutic implications for infertility management. Our present review comprehensively summarizes the latest understanding of the mechanisms, and potential clinical significance of thyroid dysfunction in infertility (Table 1) [5, 7-17, 19-41, 44-47, 49, 50]. However, the underlying mechanisms of thyroid dysfunction contributing to infertility are complex and not yet fully elucidated. For instance, both overt hypothyroidism and hyperthyroidism have been demonstrated to significantly disrupt sex hormone homeostasis [27], but the precise molecular mechanisms underlying these endocrine alterations remain poorly understood. Additionally, the mechanisms of TAI causing diminished ovarian reserve are poorly known. There is ongoing controversy, as some evidence indicates that TAI may not be associated with infertility in both male and female populations [59]. Another point of contradiction is that a study has found that hyperthyroidism increases estrogen levels in females [9], while another research has observed decreased estrogen levels in females with hyperthyroidism [20]. The apparent contradictions regarding the relationship between hyperthyroidism and estrogen levels may stem from differences in the experimental models used. The first set of data was derived from clinical observations in female hyperthyroidism patients, whereas the second set was obtained from a hyperthyroid rat model induced by exogenous thyroxine administration. Additionally, both hyperthyroidism and hypothyroidism can affect male fertility by inhibiting the HPG axis [29, 37]. Although the mechanisms differ, the fact that these two opposite thyroid dysfunction states exert similar effects on the HPG axis warrants further in-depth research. The intricate relationship between thyroid dysfunction and infertility has demonstrated considerable complexity and multifactorial involvement (Figs. 1, 2), necessitating further comprehensive research and extensive clinical data to fully elucidate the underlying pathophysiological mechanisms.
 Click to view | Table 1. Multiple Pathogenic Mechanisms Underlie Thyroid Disorder-Induced Infertility |
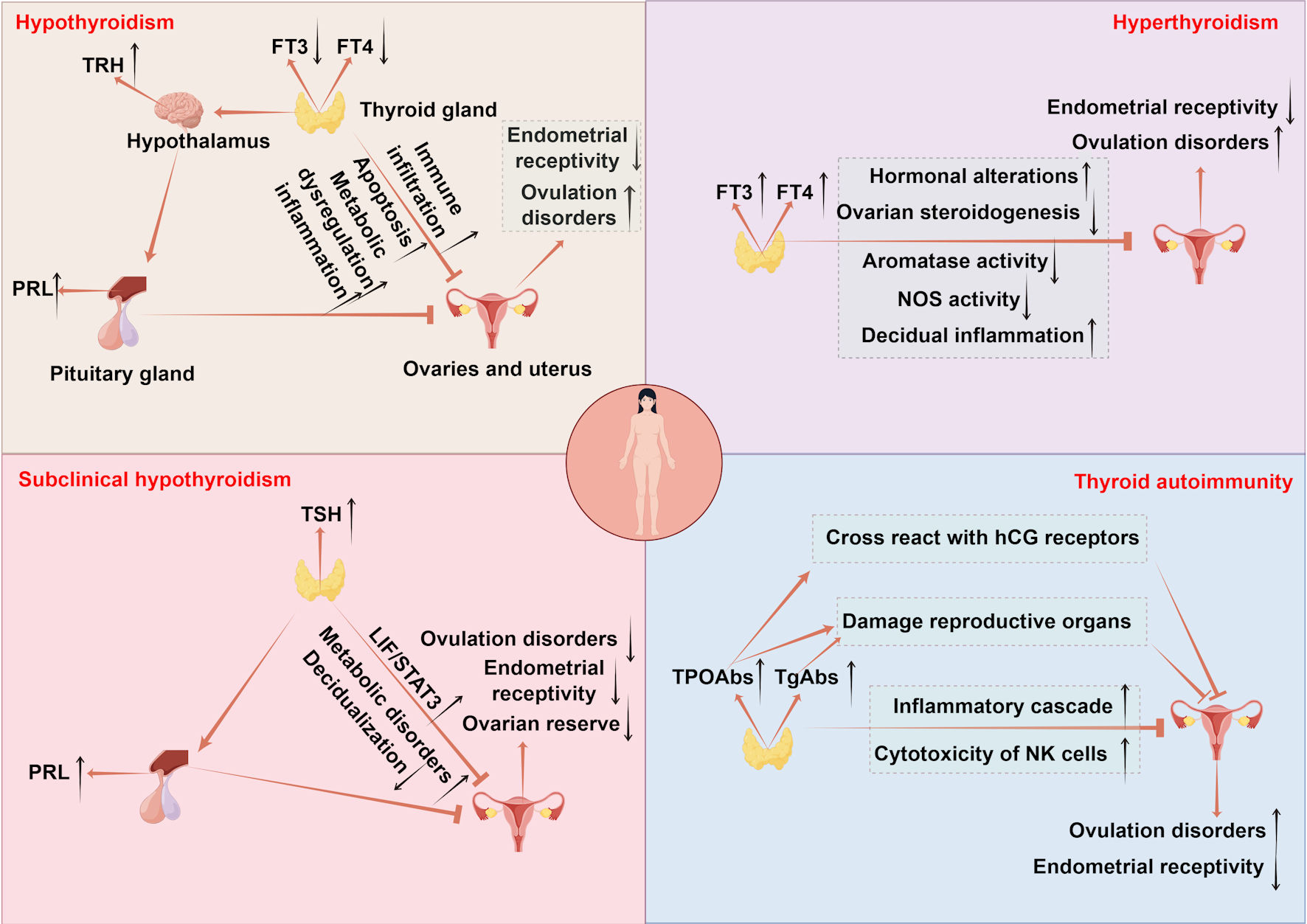 Click for large image | Figure 1. The mechanisms of thyroid disorders affecting female fertility. Hypothyroidism disrupts follicular development via CHOP and caspase3-mediated apoptosis and causes metabolic imbalance in lipids and glucose accompanied by immune infiltration, impairing ovarian and uterine function. Endometrial receptivity is diminished due to low E2 osteopontin and HOXA10, while uterine gland function is impaired with reduced integrins and LIF and increased MUC1. Uterine hyperplasia and inflammation occur due to elevated VEGF-A, alongside decreased PLINA, TAG and TC. Hyperprolactinemia from excessive TRH and menstrual irregularities further reduce fertility. Hyperthyroidism leads to infertility by elevating SHBG and estradiol which disrupts folliculogenesis through impaired antral follicle growth and dysregulated NOS expression. It also suppresses FSH stimulated aromatase activity in ovarian steroidogenesis and causes decidual imbalance with elevated DBA-positive uNK cells and abnormal cytokine production. SCH contributes to infertility by promoting metabolic dysregulation in lipids and glucose and hyperprolactinemia reducing ovarian reserve and upregulating LIF/STAT3 signaling, which collectively impair endometrial receptivity and decidualization. TAI drives follicular inflammation through IFNγ-induced CXCL9/10/11-mediated recruitment of CXCR3+ T cells and enhances NK cell cytotoxicity. Non organ specific antibodies cross react with trophoblasts and disrupt Th1/Th2 balance promoting implantation failure and pregnancy loss. TPOAbs cross reactivity with hCG receptors on the zona pellucida, direct damage by TPOAb and TgAb to reproductive tissues, and reduced ovarian reserve and embryo quality further contribute to reproductive impairment. TSH: thyroid-stimulating hormone; FT4: free thyroxine; FT3: free triiodothyronine; CHOP: C/EBP homologous protein; TAG: triacylglycerol; HOXA10: homeobox A10; LIF: leukemia inhibitory factor; MUC1: mucin 1; VEGF-A: vascular endothelial growth factor A; PLIN-A: perilipin A; DBA: dolichos biflorus agglutinin; uNK: uterine natural killer; IFNγ: interferon-γ; CXCL9/10/11: CXC chemokine ligands 9; CXCR3: CXC chemokine receptor 3; TRH: thyrotropin-releasing hormone; FSH: follicle-stimulating hormone; SHBG: sex hormone-binding globulin; NOS: nitric oxide synthase; PRL: prolactin; TPOAbs: thyroid peroxidase antibodies; TgAbs: thyroglobulin antibodies; hCG: human chorionic gonadotropin; SCH: subclinical hypothyroidism; TAI: thyroid autoimmunity. |
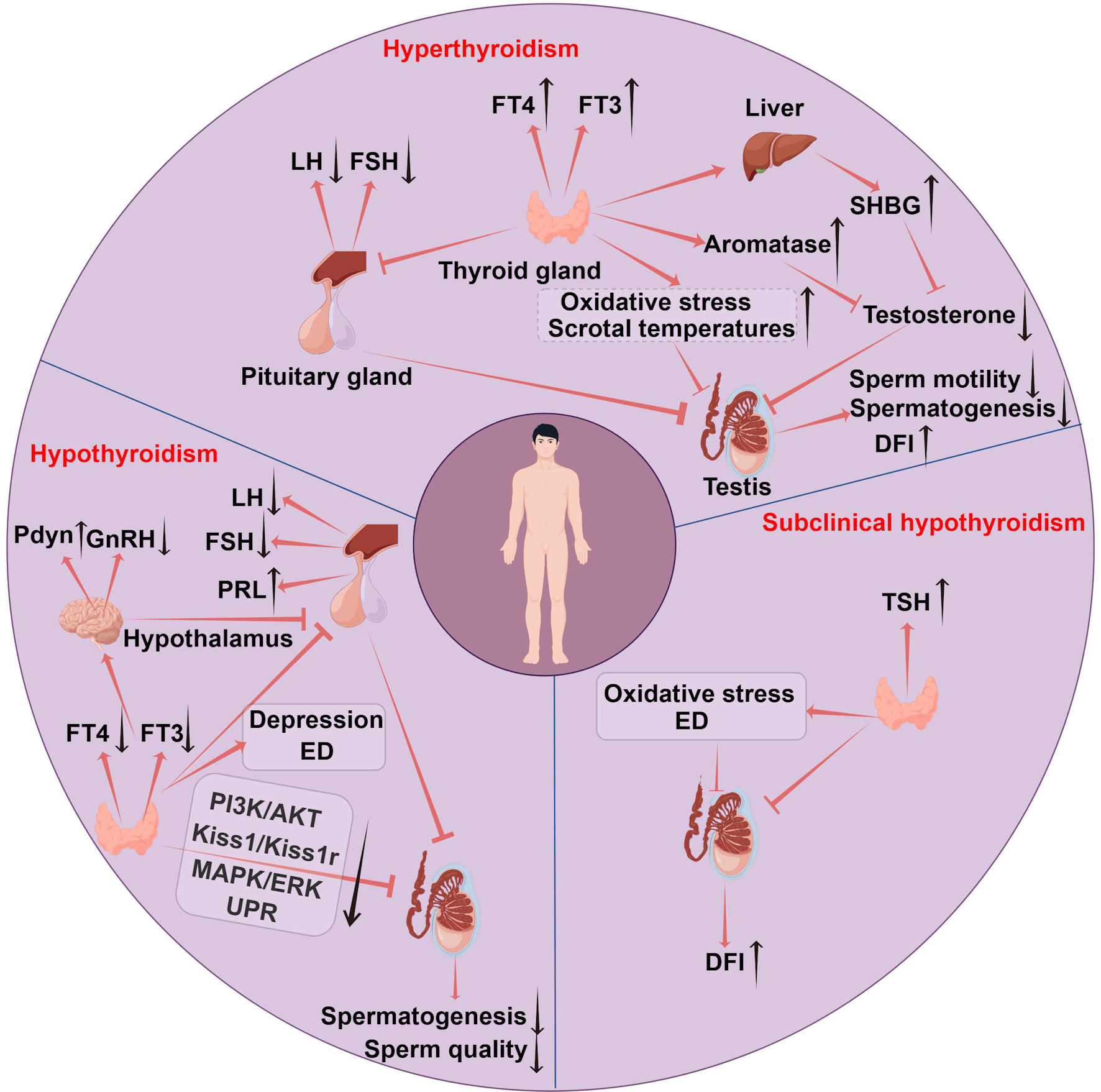 Click for large image | Figure 2. The mechanisms of thyroid disorders affecting male fertility. Hyperthyroidism impairs male fertility through multiple mechanisms: suppression of the HPG axis leads to decreased LH and FSH levels; increased SHBG reduces free testosterone; elevated aromatase activity enhances the conversion of testosterone to estrogen; oxidative stress causes sperm membrane and DNA damage; and elevated scrotal temperature impairs spermatogenesis. Hypothyroidism affects fertility via increased hypothalamic Pdyn, which suppresses GnRH release resulting in hypogonadism; reduced PI3K/AKT signaling disrupts thyroid-leptin crosstalk; decreased MAPK/ERK activity contributes to HPG axis suppression; impaired unfolded protein response in the testes; Leydig cell cytotoxicity; and secondary effects such as depression and erectile dysfunction. Subclinical hypothyroidism contributes to infertility through erectile dysfunction, oxidative stress, and increased sperm DFI. TSH: thyroid-stimulating hormone; FT4: free thyroxine; FT3: free triiodothyronine; GnRH: gonadotropin-releasing hormone; FSH: follicle-stimulating hormone; LH: luteinizing hormone; SHBG: sex hormone-binding globulin; PRL: prolactin; HPG: hypothalamic-pituitary-gonadal; UPR: unfolded protein response; ED: erectile dysfunction; DFI: DNA fragmentation index. |
Currently, several therapeutic agents have been developed to target the mechanisms underlying infertility caused by thyroid dysfunction (Table 2) [10, 12, 23, 54-61]. Among these interventions, thyroxine supplementation therapy is widely used to correct thyroxine deficiency in hypothyroidism [16]. Se supplementation therapy has demonstrated specific efficacy in reducing TPOAbs and TgAbs levels [62]. Melatonin has been shown to enhance testosterone production, thereby promoting the restoration of normal gonadal function in hypothyroid conditions [63]. Furthermore, Kp10 has been shown to ameliorate ovarian dysfunction associated with hypothyroidism [68]. However, the efficacy and safety of these drugs remain controversial. Research has shown that while women with infertility and high-normal TSH levels treated with levothyroxine exhibited an increased conception rate, they paradoxically experienced a lower live birth rate compared to their untreated counterparts [71]. Moreover, a recent study demonstrated that levothyroxine treatment did not result in higher live birth rates in women with recurrent pregnancy loss, who were positive for TPOAbs [72]. Additionally, research indicates that melatonin might be ineffective in mitigating hyperthyroidism-induced reproductive disorders [37]. Iodine plays a crucial role in human physiology, primarily in the synthesis of thyroid hormones, including T3 and T4. The relationship between iodine intake and fertility follows a U-shaped curve: while iodine deficiency is associated with diminished fertility, excessive iodine consumption can also impair reproductive health. In males, high iodine levels have been linked to reduced sperm count, impaired motility, and abnormal morphology. In females, excess iodine may contribute to maternal thyroid dysfunction and increase the risk of congenital hypothyroidism in offspring. Further research is needed to establish safe and effective dosing guidelines for iodine supplementation, including optimal preparation methods and routes of administration [73, 74]. In conclusion, further large-scale, well-designed clinical trials are imperative to comprehensively evaluate both the therapeutic efficacy and safety profile of thyroid-targeted pharmacological interventions in the management of infertility disorders. Meanwhile, understanding the gene expression profiles associated with thyroid dysfunction-induced infertility and elucidating their underlying molecular mechanisms are crucial for developing safe and effective therapeutic strategies to address infertility.
 Click to view | Table 2. Thyroid-Targeted Therapeutic Interventions for Infertility |
Acknowledgments
None to declare.
Financial Disclosure
This research was conducted without financial support from any funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
No conflict of interest exists in the submission, and the manuscript is approved by all authors for publication.
Author Contributions
Jian Tan conceived and drafted the manuscript. Yu Ying Yang and Xian Cai Ge contributed to the conceptualization and critical revision of the manuscript. All authors participated in literature collection and analysis.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
TSH: thyroid-stimulating hormone; FT4: free thyroxine; FT3: free triiodothyronine; SCH: subclinical hypothyroidism; SC-Hyper: subclinical hyperthyroidism; GRP78: glucose-regulated protein 78; CHOP: C/EBP homologous protein; TAG: triacylglycerol; TC: total cholesterol; HOXA10: homeobox A10; TRH: thyrotropin-releasing hormone; GnRH: gonadotropin-releasing hormone; FSH: follicle-stimulating hormone; LH: luteinizing hormone; SHBG: sex hormone-binding globulin; NOS: nitric oxide synthase; PRL: prolactin; HPG: hypothalamic-pituitary-gonadal; SV: seminal vesicle; UPR: unfolded protein response; SCs: Sertoli cells; ED: erectile dysfunction; PCOS: polycystic ovary syndrome; DFI: DNA fragmentation index; TAI: thyroid autoimmunity; TPOAbs: thyroid peroxidase antibodies; TgAbs: thyroglobulin antibodies; hCG: human chorionic gonadotropin; POI: premature ovarian insufficiency; Se: selenium; ALA: alpha-lipoic acid; CBZ: carbimazole; VE: vitamin E; PRP: platelet-rich plasma; Kp10: kisspeptin-10; ECE: Elettaria cardamomum extract
| References | ▴Top |
- Awonuga AO, Camp OG, Biernat MM, Abu-Soud HM. Overview of infertility. Syst Biol Reprod Med. 2025;71(1):116-142.
doi pubmed - Faso C. Infertility. Prim Care. 2025;52(2):307-316.
doi pubmed - Andrabi SW, Ara A, Saharan A, Jaffar M, Gugnani N, Esteves SC. Sperm DNA Fragmentation: causes, evaluation and management in male infertility. JBRA Assist Reprod. 2024;28(2):306-319.
doi pubmed - Chaker L, Cooper DS, Walsh JP, Peeters RP. Hyperthyroidism. Lancet. 2024;403(10428):768-780.
doi pubmed - Taylor PN, Medici MM, Hubalewska-Dydejczyk A, Boelaert K. Hypothyroidism. Lancet. 2024;404(10460):1347-1364.
doi pubmed - Hubalewska-Dydejczyk A, Gietka-Czernel M, Trofimiuk-Muldner M, Zgliczynski W, Ruchala M, Lewinski A, Bednarczuk T, et al. Thyroid diseases and fertility disorders - Guidelines of the Polish Society of Endocrinology [Choroby tarczycy a zaburzenia plodnosci - rekomendacje Polskiego Towarzystwa Endokrynologicznego]. Endokrynol Pol. 2022;73(4):645-679.
doi pubmed - Bates JN, Kohn TP, Pastuszak AW. Effect of thyroid hormone derangements on sexual function in men and women. Sex Med Rev. 2020;8(2):217-230.
doi pubmed - van der Ham K, Stekelenburg KJ, Louwers YV, van Dorp W, Schreurs MWJ, van der Wal R, Steegers-Theunissen RPM, et al. The prevalence of thyroid dysfunction and hyperprolactinemia in women with PCOS. Front Endocrinol (Lausanne). 2023;14:1245106.
doi pubmed - Concepcion-Zavaleta MJ, Coronado-Arroyo JC, Quiroz-Aldave JE, Concepcion-Urteaga LA, Paz-Ibarra J. Thyroid dysfunction and female infertility. A comprehensive review. Diabetes Metab Syndr. 2023;17(11):102876.
doi pubmed - Wang Q, Ma X, Zhang C. Effects of thyroid hormone on ovarian cell apoptosis in the rat. Reprod Fertil Dev. 2020;32(12):1060-1066.
doi pubmed - Mendez-Tepepa M, Zepeda-Perez D, Espindola-Lozano M, Rodriguez-Castelan J, Arroyo-Helguera O, Pacheco P, Nicolas-Toledo L, et al. Hypothyroidism modifies differentially the content of lipids and glycogen, lipid receptors, and intraepithelial lymphocytes among oviductal regions of rabbits. Reprod Biol. 2020;20(2):247-253.
doi pubmed - Li S, Zhang L, Li W, Qin J, Qi L, Xiao X, Xue Z, et al. Adult-onset hypothyroidism induces granulosa cell apoptosis and affects ovarian follicle development in rats. Front Cell Dev Biol. 2025;13:1610694.
doi pubmed - Kowalczyk-Zieba I, Staszkiewicz-Chodor J, Boruszewska D, Lukaszuk K, Jaworska J, Woclawek-Potocka I. Hypothyroidism affects uterine function via the modulation of prostaglandin signaling. Animals (Basel). 2021;11(9).
doi pubmed - Erbas E, Gedikli S. Investigation of the endometrial receptivity status in experimental hypothyroid-induced female rats. Iran J Basic Med Sci. 2022;25(9):1077-1083.
doi pubmed - Rodriguez-Castelan J, Del Moral-Morales A, Pina-Medina AG, Zepeda-Perez D, Castillo-Romano M, Mendez-Tepepa M, Espindola-Lozano M, et al. Hypothyroidism induces uterine hyperplasia and inflammation related to sex hormone receptors expression in virgin rabbits. Life Sci. 2019;230:111-120.
doi pubmed - Unuane D, Velkeniers B. Impact of thyroid disease on fertility and assisted conception. Best Pract Res Clin Endocrinol Metab. 2020;34(4):101378.
doi pubmed - Mazzilli R, Medenica S, Di Tommaso AM, Fabozzi G, Zamponi V, Cimadomo D, Rienzi L, et al. The role of thyroid function in female and male infertility: a narrative review. J Endocrinol Invest. 2023;46(1):15-26.
doi pubmed - Feldt-Rasmussen U, Effraimidis G, Bliddal S, Klose M. Consequences of undertreatment of hypothyroidism. Endocrine. 2024;84(2):301-308.
doi pubmed - Gitsi E, Livadas S, Argyrakopoulou G. Nutritional and exercise interventions to improve conception in women suffering from obesity and distinct nosological entities. Front Endocrinol (Lausanne). 2024;15:1426542.
doi pubmed - Zheng K, Sulieman FJ, Li J, Wei Q, Xu M, Shi F. Nitric oxide and thyroid hormone receptor alpha 1 contribute to ovarian follicular development in immature hyper- and hypo-thyroid rats. Reprod Biol. 2015;15(1):27-33.
doi pubmed - Xu K, Tian Y, Weng X, Hu X, Heng D, Xia G, Zhang C. Effect of thyroid dysfunction on NOS expression in the female rat. Cell Tissue Res. 2020;379(2):291-300.
doi pubmed - Colella M, Cuomo D, Giacco A, Mallardo M, De Felice M, Ambrosino C. Thyroid hormones and functional ovarian reserve: systemic vs. peripheral dysfunctions. J Clin Med. 2020;9(6).
doi pubmed - Santos LC, de Souza CA, Silva JF, Ocarino NM, Serakides R. Maternal hyperthyroidism alters the immunological mediators profile and population of natural killers cells in decidua of rats. Acta Histochem. 2023;125(3):152026.
doi pubmed - Hussein R. Thyroid hormones and their role in male infertility: a comprehensive review. International Journal of Biomedicine. 2024;14(2):231-234.
- Sengupta P, Dutta S, Karkada IR, Chinni SV. Endocrinopathies and male infertility. Life (Basel). 2021;12(1).
doi pubmed - Barman M, Giribabu N, Salleh N. Roles of thyroid and leptin hormones and their crosstalk in male reproductive functions: an updated review. Endocrine. 2025;87(3):891-906.
doi pubmed - Babatunde EM, Emokpae MA. A comprehensive review of thyroid dysfunction: hidden player in male infertility. Fertility & Reproduction. 2024;06(04):166-175.
- Coskuner ER, Ozkan B. Premature ejaculation and endocrine disorders: a literature review. World J Mens Health. 2022;40(1):38-51.
doi pubmed - Santos LC, Dos Anjos Cordeiro JM, da Silva Santana L, Barbosa EM, Santos BR, Mendonca LD, Cunha M, et al. Kisspeptin treatment reverses high prolactin levels and improves gonadal function in hypothyroid male rats. Sci Rep. 2023;13(1):16819.
doi pubmed - Correa D, Bargi-Souza P, Oliveira IM, Razera A, Oliveira CA, Romano MA, Romano RM. Quantitative proteomic profile analysis of thyroid dysfunction effects on seminal vesicles and repercussions on male fertility. Mol Cell Endocrinol. 2023;578:112048.
doi pubmed - Santos LC, Dos Anjos Cordeiro JM, Cunha M, Santos BR, Oliveira LS, da Silva AL, Barbosa EM, et al. Kisspeptin-10 improves testicular redox status but does not alter the unfolded protein response (UPR) that is downregulated by hypothyroidism in a rat model. Int J Mol Sci. 2024;25(3).
doi pubmed - Gomez-Zuniga A, Landero-Huerta DA, Rojas-Castaneda JC, Sanchez-Huerta K, Contreras-Garcia IJ, Reynoso-Robles R, Arteaga-Silva M, et al. Methimazole-induced congenital hypothyroidism affects gonocytes differentiation and arrests meiosis: role of Sertoli cells. Front Cell Dev Biol. 2024;12:1493872.
doi pubmed - El-Shaer RAA, Ibrahim S, Hewady PM, Atef MM, El-Deeb OS, Hafez YM, Amer RS, et al. Selenium protects against nesfatin-1 modulation of the hypothalamic-pituitary-testicular axis during hypothyroidism in male rats. Physiol Rep. 2024;12(2):e15923.
doi pubmed - Anelli V, Gatta E, Pirola I, Delbarba A, Rotondi M, Cappelli C. Thyroid impairment and male fertility: a narrative review of literature. Aging Male. 2024;27(1):2310303.
doi pubmed - Korkmaz FN, Yilmaz-Oral D, Asker H, Guven B, Turkcan D, Kirlangic OF, Oztekin CV, et al. Combined levothyroxine and testosterone treatment for restoring erectile dysfunction in propylthiouracil-induced hypothyroid rats. J Sex Med. 2023;20(6):732-741.
doi pubmed - Dharia A, Desai D, Desai K. Exploring the link between thyroid disorders and obesity: mechanisms, impacts, and clinical implications. Endocr Pract. 2025;31(5):660-667.
doi pubmed - Ramadan HM, Taha NA, Ahmed HH. Melatonin enhances antioxidant defenses but could not ameliorate the reproductive disorders in induced hyperthyroidism model in male rats. Environ Sci Pollut Res Int. 2021;28(4):4790-4804.
doi pubmed - Concepcion-Zavaleta M, Paz Ibarra JL, Ramos-Yataco A, Coronado-Arroyo J, Concepcion-Urteaga L, Roseboom PJ, Williams CA. Assessment of hormonal status in male infertility. An update. Diabetes Metab Syndr. 2022;16(3):102447.
doi pubmed - Bahtiyar N, Yoldas A, Abbak Y, Dariyerli N, Toplan S. Erythroid microRNA and oxidant status alterations in l-thyroxine-induced hyperthyroid rats: effects of selenium supplementation. Minerva Endocrinol (Torino). 2021;46(1):107-115.
doi pubmed - Yau WW, Yen PM. Thermogenesis in adipose tissue activated by thyroid hormone. Int J Mol Sci. 2020;21(8).
doi pubmed - Fan H, Ren Q, Sheng Z, Deng G, Li L. The role of the thyroid in polycystic ovary syndrome. Front Endocrinol (Lausanne). 2023;14:1242050.
doi pubmed - Peddemul A, Tejovath S, Hassan D, K KP, Sikandar R, Kahlon SS, Nair S, et al. Influence of subclinical hypothyroidism on women with polycystic ovary syndrome: a literature review. Cureus. 2022;14(8):e28468.
doi pubmed - Zhang H, Qiu H, Liu Z, Wu Y, Liu W, Huang C. Subclinical/overt hypothyroidism may be associated with diminished ovarian reserve in infertile women independent of thyroid autoimmunity. Front Endocrinol (Lausanne). 2024;15:1477665.
doi pubmed - Shan L, Zhou Y, Peng S, Wang X, Shan Z, Teng W. Implantation failure in rats with subclinical hypothyroidism is associated with LIF/STAT3 signaling. Endocr Connect. 2019;8(6):718-727.
doi pubmed - Kakita-Kobayashi M, Murata H, Nishigaki A, Hashimoto Y, Komiya S, Tsubokura H, Kido T, et al. Thyroid hormone facilitates in vitro decidualization of human endometrial stromal cells via thyroid hormone receptors. Endocrinology. 2020;161(6).
doi pubmed - Zhao S, Tang L, Fu J, Yang Z, Su C, Rao M. Subclinical hypothyroidism and sperm DNA fragmentation: a cross-sectional study of 5401 men seeking infertility care. J Clin Endocrinol Metab. 2022;107(10):e4027-e4036.
doi pubmed - Ma X, Wang F, Zhen X, Zhao L, Fang L, Dong Z, Chen W, et al. gp91(phox), a novel biomarker evaluating oxidative stress, is elevated in subclinical hypothyroidism. Int J Endocrinol. 2020;2020:3161730.
doi pubmed - Bahreiny SS, Ahangarpour A, Rajaei E, Sharifani MS, Aghaei M. Meta-analytical and meta-regression evaluation of subclinical hyperthyroidism's effect on male reproductive health: hormonal and seminal perspectives. Reprod Sci. 2024;31(10):2957-2971.
doi pubmed - Vassallo A, Di Filippo L, Frara S, Bertoli M, Pagani M, Presciuttini B. New onset of Graves' disease after controlled ovarian stimulation: A case report and brief literature review. Int J Gynaecol Obstet. 2025;168(3):913-918.
doi pubmed - Dunbar S, Dhillon-Smith R, Maheshwari A. Thyroid function testing prior to fertility treatment: will we ever agree? Hum Reprod. 2025;40(7):1243-1248.
doi pubmed - Venables A, Wong W, Way M, Homer HA. Thyroid autoimmunity and IVF/ICSI outcomes in euthyroid women: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2020;18(1):120.
doi pubmed - Huang N, Liu D, Lian Y, Chi H, Qiao J. Immunological microenvironment alterations in follicles of patients with autoimmune thyroiditis. Front Immunol. 2021;12:770852.
doi pubmed - Bucci I, Giuliani C, Di Dalmazi G, Formoso G, Napolitano G. Thyroid autoimmunity in female infertility and assisted reproductive technology outcome. Front Endocrinol (Lausanne). 2022;13:768363.
doi pubmed - Szeliga A, Calik-Ksepka A, Maciejewska-Jeske M, Grymowicz M, Smolarczyk K, Kostrzak A, Smolarczyk R, et al. Autoimmune diseases in patients with premature ovarian insufficiency-our current state of knowledge. Int J Mol Sci. 2021;22(5).
doi pubmed - Tanska K, Gietka-Czernel M, Glinicki P, Kozakowski J. Thyroid autoimmunity and its negative impact on female fertility and maternal pregnancy outcomes. Front Endocrinol (Lausanne). 2022;13:1049665.
doi pubmed - Li Z, Xu S, Luo W, Hu J, Zhang T, Jiao X, Qin Y. Association between thyroid autoimmunity and the decline of ovarian reserve in euthyroid women. Reprod Biomed Online. 2022;45(3):615-622.
doi pubmed - Boguslawska J, Godlewska M, Gajda E, Piekielko-Witkowska A. Cellular and molecular basis of thyroid autoimmunity. Eur Thyroid J. 2022;11(1).
doi pubmed - Wei SX, Wang L, Liu YB, Fan QL, Fan Y, Qiao K. TPOAb positivity can impact ovarian reserve, embryo quality, and IVF/ICSI outcomes in euthyroid infertile women. Gynecol Endocrinol. 2023;39(1):2266504.
doi pubmed - Birjandi B, Ramezani Tehrani F, Amouzegar A, Tohidi M, Bidhendi Yarandi R, Azizi F. The association between subclinical hypothyroidism and TPOAb positivity with infertility in a population-based study: Tehran thyroid study (TTS). BMC Endocr Disord. 2021;21(1):108.
doi pubmed - Wadhwa L, Marghret KM, Arora S. Evaluation of reproductive outcome in infertile hypothyroid women on thyroxine therapy. J Hum Reprod Sci. 2020;13(4):272-276.
doi pubmed - Cannarella R, Condorelli RA, Calogero AE, Bagnara V, Aversa A, Greco EA, Brunetti A, et al. Effects of selenium supplementation on sperm parameters and DNA-fragmentation rate in patients with chronic autoimmune thyroiditis. J Clin Med. 2021;10(16).
doi pubmed - Peng B, Wang W, Gu Q, Wang P, Teng W, Shan Z. Effects of different supplements on Hashimoto's thyroiditis: a systematic review and network meta-analysis. Front Endocrinol (Lausanne). 2024;15:1445878.
doi pubmed - Ezzat W, Abdelbasset WK, Hussien RS, Azab AR, Sulieman A, Yousry S. Effect of melatonin on reproductive function in propylthiouracil induced hypothyroidism in adult male rats. Eur Rev Med Pharmacol Sci. 2024;28(5):1920-1930.
doi pubmed - Albuquerque YML, Silva WED, Souza FAL, Teixeira VW, Teixeira AAC. Melatonin on hypothyroidism and gonadal development in rats: a review. JBRA Assist Reprod. 2020;24(4):498-506.
doi pubmed - Ibrahim AA, Mohammed NA, Eid KA, Abomughaid MM, Abdelazim AM, Aboregela AM. Hypothyroidism: morphological and metabolic changes in the testis of adult albino rat and the amelioration by alpha-lipoic acid. Folia Morphol (Warsz). 2021;80(2):352-362.
doi pubmed - Hussein RS, Eyada MM, Mostafa RM, Elaidy SM, Elsayed SH, Saad HM. Impact of carbimazole combined with vitamin E on testicular injury induced by experimental hyperthyroidism in adult albino rats: oxidative/inflammatory/apoptotic pathways. Asian J Androl. 2024;26(4):396-401.
doi pubmed - Bordbar H, Sattar-Shamsabadi M, Dehghani F, Karimi F. Protective effect of platelet-rich plasma against structural and functional changes of the adult rat testis in carbimazole-induced hypothyroidism. Clin Exp Reprod Med. 2024;51(3):225-235.
doi pubmed - de Oliveira LS, da Silva TQM, Barbosa EM, Dos Anjos Cordeiro JM, Santos LC, Henriques PC, Santos BR, et al. Kisspeptin treatment restores ovarian function in rats with hypothyroidism. Thyroid. 2022;32(12):1568-1579.
doi pubmed - Atabaki B, Mirazi N, Hosseini A, Sarihi A, Izadi Z, Nourian A. Effect of Elettaria cardamomum L. on hormonal changes and spermatogenesis in the propylthiouracil-induced hypothyroidism male BALB/c mice. Endocrinol Diabetes Metab. 2023;6(5):e438.
doi pubmed - Medenica S, Abazovic D, Ljubic A, Vukovic J, Begovic A, Cucinella G, Zaami S, et al. The role of cell and gene therapies in the treatment of infertility in patients with thyroid autoimmunity. Int J Endocrinol. 2022;2022:4842316.
doi pubmed - Galbiati F, Jokar TO, Howell LM, Li R, Fourman LT, Lee H, Jeong JH, et al. Levothyroxine for a high-normal TSH in unexplained infertility. Clin Endocrinol (Oxf). 2024;100(2):192-198.
doi pubmed - van Dijk MM, Vissenberg R, Fliers E, van der Post JAM, van der Hoorn MP, de Weerd S, Kuchenbecker WK, et al. Levothyroxine in euthyroid thyroid peroxidase antibody positive women with recurrent pregnancy loss (T4LIFE trial): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10(5):322-329.
doi pubmed - Khudair A, Khudair A, Niinuma SA, Habib H, Butler AE. From deficiency to excess: the impact of iodine excess on reproductive health. Front Endocrinol (Lausanne). 2025;16:1568059.
doi pubmed - Mathews DM, Johnson NP, Sim RG, O'Sullivan S, Peart JM, Hofman PL. Iodine and fertility: do we know enough? Hum Reprod. 2021;36(2):265-274.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.