| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 9, October 2025, pages 529-535
Predictors of a Paroxysm of Atrial Fibrillation Developing in Patients With Exacerbated Chronic Obstructive Pulmonary Disease
Aida I. Tarzimanovaa , Anna E. Braginaa
, Evgeniya V. Kazantsevaa
, Anna E. Pokrovskayaa, c
, Natalia A. Dragomiretskayaa
, Tatiana A. Safronovaa
, Tatiana S. Varginaa
, Irakli Zh. Loriyaa
, Igor V. Cherkesovb
, Liubov A. Ponomarevaa
, Daria D. Vaninaa
, Valeri F. Valitovaa
, Valery I. Podzolkova
a2nd Internal Medicine (2nd Faculty Therapy) Department, N.V. Sklifosovskiy Institute of Clinical Medicine, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
bDepartment of Plastic Surgery, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
cCorresponding Author: Anna E. Pokrovskaya, Department of Faculty Therapy No. 2, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
Manuscript submitted July 17, 2025, accepted September 24, 2025, published online October 15, 2025
Short title: Predictors of a Paroxysm of Atrial Fibrillation
doi: https://doi.org/10.14740/jocmr6323
| Abstract | ▴Top |
Background: Chronic obstructive pulmonary disease (COPD) is a frequent cause of atrial fibrillation (A-fib). A paroxysm of A-fib will protract the patients’ stay at hospital and increase their lethality risk considerably. Despite extensive research done in this field, the predictors of A-fib recurrence in COPD patients have not been definitively studied.
Methods: Our single-institution prospective study involved 569 patients hospitalized for COPD exacerbation. Depending on whether they developed an A-fib paroxysm while in hospital, we divided the patients into two groups: group I (the main one) was composed of 111 COPD patients whose electrocardiogram (ECG) showed an A-fib paroxysm during hospitalization, and group II (the comparison group) comprised 458 COPD patients who had no A-fib paroxysm in their entire hospitalization period. All the patients were given a comprehensive clinical, laboratory, and instrumental examination that included a complete blood count and blood chemistry test, coagulogram, room air oxygen saturation test, external respiration test, standard ECG, echocardiogram, Holter daily ECG monitoring, and a chest computed tomography (CT) scan.
Results: In exacerbated COPD patients, arterial hypertension (AH) would increase their risk of developing an A-fib paroxysm by a factor of 3.74 (confidence interval (CI) 2.78 - 4.19). Arrhythmic patients’ Charlson Comorbidity Index was veritably higher (5 (3 - 6) points) than in the comparison group (3 (2 - 5) points) (P = 0.004). A-fib patients had considerably lower oxygen saturation on room air (SpO2) (91.8% (89-95%)) than non-arrhythmic patients (95.2% (91-97%)). Right atrial area enlargement would increase exacerbated COPD patients’ risk of developing an A-fib paroxysm by a factor of 1.34 (1.16 - 1.56). A-fib patients were found to have a veritably raised level of C-reactive protein to 21.0 mg/L (5.65 - 35.2 mg/L), compared to non-arrhythmic patients with 8.3 mg/L (1.31 - 16.4 mg/L).
Conclusion: The development of an A-fib paroxysm in patients hospitalized for exacerbated COPD is predicted by Charlson Comorbidity Index above 4, right atrial area enlargement, and heightened C-reactive protein.
Keywords: Atrial fibrillation; Chronic obstructive pulmonary disease; Predictors
| Introduction | ▴Top |
Atrial fibrillation (A-fib) is one of the most common heart rhythm disorders. The onset of such arrhythmia leads to a worsened quality of life, shortened life expectancy, and increased risks of thromboembolic complications and cardiovascular mortality.
Most A-fib patients have a “secondary” form of the arrhythmia that develops in the presence of cardiac pathology or internal diseases. Chronic obstructive pulmonary disease (COPD) is a frequent cause of A-fib development [1]. A-fib incidence is much higher in COPD patients than among the general population and reaches 18.2% in exacerbated cases of COPD [2]. As shown by Mullerova et al, in the first month after a COPD exacerbation, the risk of cardiovascular diseases that require hospitalization is higher than for patients who had no exacerbation [3]. Hirayama et al showed the risk of A-fib paroxysms to treble within 30 days after a COPD exacerbation [4].
On the other hand, the onset of arrhythmia in COPD has a negative effect on the patients’ prognosis. A paroxysm of A-fib will protract the patients’ stay in hospital and increase their lethality risk considerably. Abdullah et al showed A-fib to be an independent predictor of mortality (odds ratio (OR): 1.44; confidence interval (CI): 133 - 156, P < 0.001), longer stay at hospital (OR: 1.63; CI: 1.57 - 1.69, P < 0.001), and more costly treatment (OR: 1.45; CI: 1.40 - 1.49, P < 0.001) [5] for patients hospitalized with exacerbated COPD.
A number of mechanisms behind A-fib development in COPD are now under discussion. A study by the Malmo Preventive Project assessed the significance of bronchial obstructive syndrome in arrhythmia development. The authors showed forced expiratory volume in one second (FEV1) to be inversely related to first A-fib onset incidence [6]. Some studies showed hypercapnia and hypoxia to be significant in arrhythmia development in COPD [7]. Altered blood gases lead to increased pulmonary arterial pressure and tricuspid regurgitation that overload the right atrium. Hypercapnia makes atrial myocardium more refractory and significantly less conductive. In an experimental study on porcine models, Linz et al showed intrathoracic pressure fluctuations during bronchial obstruction to temporarily shorten the right atrium’s effective refractory period and to increase the probability of A-fib development [8]. Terzano et al showed an effect of chronic systemic inflammation in COPD patients on the development of A-fib paroxysms [9], and the significance of oxidative stress in the development of arrhythmia is also under discussion [10, 11].
Despite numerous studies in this field, the predictors of A-fib recurrence in COPD patients have not been definitively studied. The identification of generally comprehensible predictors of A-fib will improve both clinical status and prognosis for COPD patients.
The purpose of the study was to identify predictors of an A-fib paroxysm in patients hospitalized for COPD exacerbation.
| Materials and Methods | ▴Top |
Study design
Our single-institution prospective study involved 569 patients admitted to the University Clinical Hospital No. 4 of the Sechenov University with COPD exacerbation. Depending on whether they developed an A-fib paroxysm while in hospital, we divided the patients into two groups: group I (the main one) was composed of 111 COPD patients whose electrocardiogram (ECG) showed an A-fib paroxysm during hospitalization, and group II (the comparison group) comprised 458 COPD patients who had no A-fib paroxysm in their entire hospitalization period. The average duration of their in-patient treatment was 12 ± 2.7 days. An A-fib paroxysm was confirmed on ECG or daily ECG monitoring that attending doctors prescribed to all the patients on admission and/or if they complained of heart palpitations or interruptions.
The patient exclusion criteria were: bronchial asthma, acute coronary syndrome, inflammatory heart diseases, heart defects, chronic heart failure (CHF) with reduced left ventricular ejection fraction (LVEF less than 50%), grave renal or hepatic pathology, anemia, thyroid diseases, cancer, pregnancy, mental disorders, and/or alcohol abuse, long-standing or persistent A-fib.
All the patients were given a comprehensive clinical, laboratory, and instrumental examination that included a complete blood count and blood chemistry test, coagulogram, room air oxygen saturation test, external respiration test, standard ECG, echocardiogram, Holter daily ECG monitoring, and a chest computed tomography (CT) scan.
Ethical approval
The study was approved by the Local Ethics Committee of the I.M. Sechenov First Moscow State Medical University (Sechenov University), as per meeting minutes No.19-23 of October 26, 2023. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Statistical data analysis
We used Jamovi 2.3.13 software for statistical data processing. Veritably normal indicator distribution was tested with the Kolmogorov-Smirnov test. The quantitative data are presented as the median and interquartile range (Me (Q1 - Q3)). Binomial and categorical variables are presented in terms of both absolute number and percentage. Continuous variables were compared using the non-parametric Mann-Whitney U test. Categorical variables were compared using Pearson’s χ2 test. To explore the prognostic value of clinical, laboratory, and echocardiographic parameters, univariate logistic regression analysis was performed with calculation of OR and 95% CI. Predictors demonstrating statistical significance in the univariate analysis (P < 0.05) were subsequently entered into a multivariate logistic regression model. The results of the multivariate analysis are presented as adjusted OR with 95% CI, allowing identification of independent predictors of paroxysmal A-fib. The classification model’s cut-off point threshold value was established using receiver operating characteristic (ROC) analysis with area under curve (AUC) calculation. Variations with P < 0.05 were considered statistically significant.
| Results | ▴Top |
Patients’ clinical profile
Group I patients were older and presented with more marked bronchial obstruction and respiratory distress than group II patients. The incidence of arterial hypertension (AH), ischemic heart disease (IHD), CHF, and obesity was higher in group I than in group II (Table 1). All the patients included in our study had long (10 to 35 years) histories of cigarette smoking; their smoker index was 26.3 (12 - 29) pack-years in group I and 24.9 (10 - 30) pack-years in group II, without any veritable difference. Arrhythmic patients’ Charlson Comorbidity Index was veritably higher than in the comparison group (P = 0.004).
 Click to view | Table 1. The Patients’ Clinical Profile |
In group I and group II patients, 109 (98.1%) and 432 (94.3%) were on inhaled bronchodilators, 96 (86.5%) and 256 (55.9%) on inhaled corticosteroids, 67 (60.3%) and 259 (56.6%) on antihypertensive therapy with angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers, 32 (28.8%) and 46 (10%) on selective beta-blockers, and 29 (26.1%) and 104 (22.7%) on glucose lowering drugs. Ongoing therapy data were included in our regression analysis to assess their prognostic significance. An A-fib paroxysm in hospital setting would be arrested by intravenous drip administration of amiodarone, 5 mg/kg body weight.
Echocardiography
All the patients included in our study had a preserved LVEF, with no veritable difference found between left ventricular end-diastolic and end-systolic volume (EDV and ESV). Transthoracic echocardiography showed group I patients to have LV myocardial mass, left atrial (LA) volume, right atrial (RA) area, and right ventricular (RV) size veritably raised as compared to group II patients (P < 0.05) (Table 2).
 Click to view | Table 2. Transthoracic Echocardiography Findings in the Groups Under Review |
COPD and paroxysmal A-fib patients presented with veritably higher systolic pulmonary artery pressure (sPAP) compared to non-arrhythmic COPD patients.
Laboratory value changes in the groups under review
Hemoglobin level and absolute erythrocyte, platelet, and leukocyte counts did not differ veritably between the groups. Group I patients presented with higher neutrophil count and erythrocyte sedimentation rate (ESR) than group II patients (Table 3).
 Click to view | Table 3. Comprehensive Blood Count in the Groups Under Review |
Blood chemistry tests showed no statistically veritable difference between the groups. Paroxysmal A-fib patients presented with C-reactive protein veritably raised to 21.0 mg/L (5.65 - 35.2 mg/L), compared to A-fib free patients with 8.3 mg/L (1.31 - 16.4 mg/L) (Fig. 1).
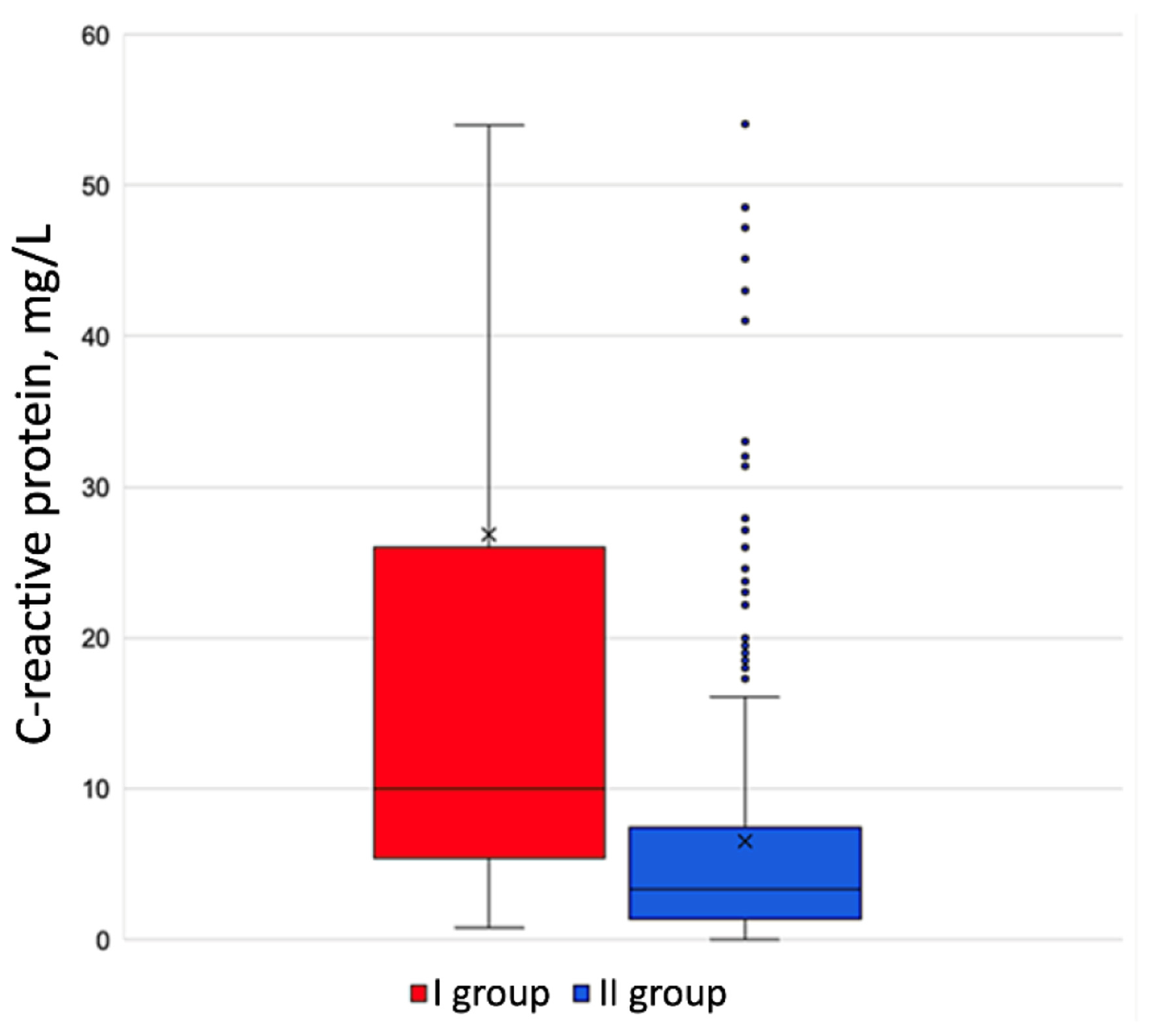 Click for large image | Figure 1. C-reactive protein level in the groups under review. |
Predictors of A-fib development in COPD exacerbation patients
Our research culminated in the development of a model for assessing the probability of COPD patients developing an A-fib paroxysm. Selected as potential predictors were those parameters that had made a statistically veritable difference as we conducted univariate regression analysis. Multivariate logistic regression identified Charlson Comorbidity Index > 4, right atrial area, and C-reactive protein as independent predictors of paroxysmal A-fib. Adjusted ORs with 95% CIs are shown in Table 4.
 Click to view | Table 4. Predictors of an A-fib Paroxysm Developing in Exacerbated COPD Patients |
To assess the risk of exacerbated COPD patients developing an A-fib paroxysm, we designed a mathematical model. The correlation observed is described by the equation:
The threshold value of the p logistic function was 27%: with р ≥ 27%, exacerbated patients’ risk of developing an A-fib paroxysm was established as high, and with р < 27%, as low. With this threshold value, the model was 87% sensitive and 85% specific, and ROC AUC was 0.932 (Fig. 2).
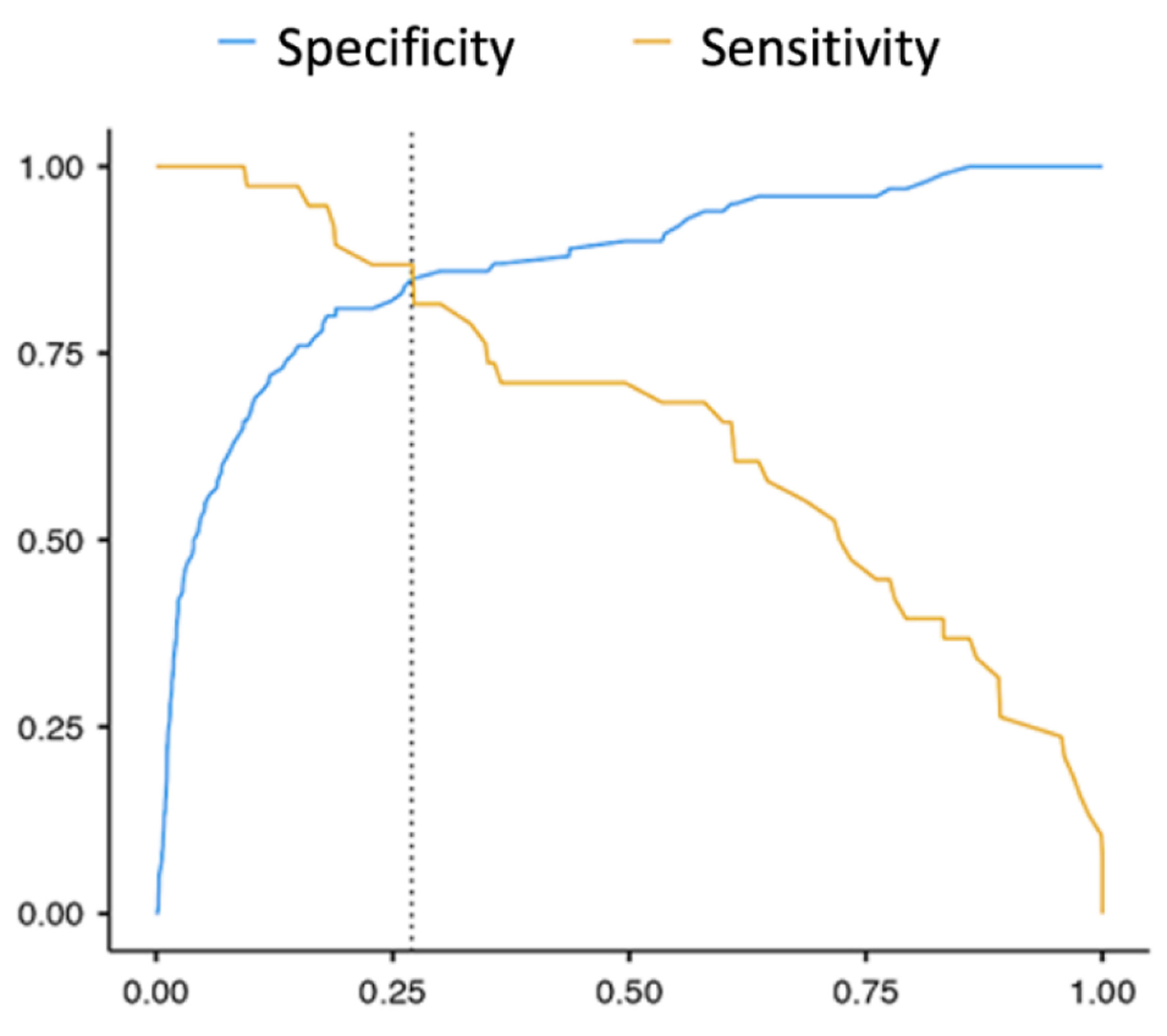 Click for large image | Figure 2. The sensitivity and specificity ROC curve of the mathematical model for assessing the probability of A-fib paroxysm recurrence in exacerbated COPD patients. A-fib: atrial fibrillation; COPD: chronic obstructive pulmonary disease; ROC: receiver operating characteristic. |
| Discussion | ▴Top |
Our study has shown the incidence of cardiovascular diseases among group I patients to be considerably higher than in group II. Among independent predictors of COPD patients developing an A-fib paroxysm, AH was the top contributor (OR: 3.74; CI: 2.78 - 4.19, P < 0.05). AH is known to predispose to A-fib development and persistence due to its adverse hemodynamic effect and excessive activation of the renin-angiotensin-aldosterone system. AH significance in A-fib development has been demonstrated in studies entitled Standardized Treatment of Pulmonary Exacerbations II study (STOP-2), The Captopril Prevention Project (CAPPP), and Lifestyle Interventions and Independence for Elders study (LIFE) [12-14]. A high degree of AH diagnosed in group I patients leads to left ventricular hypertrophy, disrupted LV diastolic function, and left atrial distension.
Ninety-five (85.6%) group I patients were diagnosed with three to six comorbidities; a Charlson Comorbidity Index above 4 was an independent risk factor for an A-fib paroxysm developing in COPD patients (1.61; CI: 1.27 - 2.04, P < 0.001). The patients’ polymorbidity in the group under review included COPD combined with cardiovascular diseases such as AH, ischemic heart disease (IHD), CHF, and obesity. High activity of the renin-angiotensin-aldosterone system, leading to fibroblast proliferation, altered electro-physiological characteristics of atrial cardiomyocytes, and formation of an arrhythmogenic substrate, is also considered an important pathogenetic component behind cardiovascular complications in polymorbid patients [11].
Right heart dilation is an important contributor to structural myocardial remodeling in COPD patients. Our study showed an expanded right atrial area to heighten the risk of an A-fib paroxysm developing in exacerbated COPD by a factor of 1.34 (CI: 1.16 - 1.56). Xie et al refer to myocardial MRI to convincingly show that an expanded right atrium is an independent risk factor for the development of first diagnosed A-fib in the general population [15].
Hypoxia is considered a significant factor behind arrhythmia development in COPD patients. Our study showed SpO2 falling below 92% to increase the risk of an A-fib paroxysm by a factor of 1.96 (CI 1.86 - 2.13, P < 0.001). Respiratory distress induces a faster heart rate and greater excitability of atrial tissue. An important consequence of lower tissue oxygenation in COPD is a reduced amount of superoxide dismutase and glutathione peroxidase [16], resulting in accumulation of free radicals that affect the condition of lipid membranes as they promote their peroxidation.
The pathogenetic role of inflammation in the A-fib development and persistence is actively being discussed in the recent years [17, 18]. Increased plasma levels of C-reactive protein are a simple and highly sensitive marker of systemic inflammation. Our study showed high concentrations of C-reactive protein to be independent predictors of an A-fib paroxysm developing in COPD exacerbation patients. Pro-inflammatory cytokines are known to have an arrhythmogenic effect as they affect the function of ion channels directly [19]. Electrophysiological remodeling occurs, which includes reduction of re-polarizing potassium currents, expression of connexins, and disrupted metabolism of intra-cellular calcium. Through activation of the system of metalloproteinases, pro-inflammatory cytokines foster the development of endothelial dysfunction, which leads to death of cardiomyocytes amid hypoxia and ultimately to heart rhythm disturbances.
Conclusions
The independent predictors of an A-fib paroxysm developing in patients hospitalized for an exacerbation of COPD thus include: Charlson Comorbidity Index above 4 points (OR: 1.53; CI: 1.11 - 2.12), right atrial area enlargement (OR: 1.21, CI: 1.02 - 1.44), and a raised level of C-reactive protein (OR: 1.07, CI: 1.01 - 1.14).
Acknowledgments
None to declare.
Financial Disclosure
The authors hereby state that this study received no financial support.
Conflict of Interest
All the authors hereby declare no potential conflict of interest to be disclosed in this article.
Informed Consent
All the patients were informed and gave their written and informed consent.
Author Contributions
AT contributed to methodology; EK contributed to software; AT, AB, AP, ND, TS, TV, and VP contributed to validation; LP, DV, IC, IL, and VV contributed to formal analysis; AT and EK contributed to investigation; EK contributed to data curation; AT and EK contributed to the writing of the original draft; AT, AB, AP, ND, TS, TV, and VP contributed to draft editing and reviewing.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Nguyen HL, Nguyen TD, Phan PT. Prevalence and associated factors of paroxysmal atrial fibrillation and atrial arrhythmias during hospitalizations for exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2024;19:1989-2000.
doi pubmed - Xiao X, Han H, Wu C, He Q, Ruan Y, Zhai Y, Gao Y, et al. Prevalence of Atrial Fibrillation in hospital encounters with end-stage COPD on home oxygen: national trends in the United States. Chest. 2019;155(5):918-927.
doi pubmed - Abdullah AS, Eigbire G, Ali M, Awadalla M, Wahab A, Ibrahim H, Salama A, et al. Relationship of atrial fibrillation to outcomes in patients hospitalized for chronic obstructive pulmonary disease exacerbation. J Atr Fibrillation. 2019;12(2):2117.
doi pubmed - Mullerova H, Marshall J, de Nigris E, Varghese P, Pooley N, Embleton N, Nordon C, et al. Association of COPD exacerbations and acute cardiovascular events: a systematic review and meta-analysis. Ther Adv Respir Dis. 2022;16:17534666221113647.
doi pubmed - Hirayama A, Goto T, Shimada YJ, Faridi MK, Camargo CA, Jr., Hasegawa K. Acute exacerbation of chronic obstructive pulmonary disease and subsequent risk of emergency department visits and hospitalizations for atrial fibrillation. Circ Arrhythm Electrophysiol. 2018;11(9):e006322.
doi pubmed - Johnson LS, Juhlin T, Engstrom G, Nilsson PM. Reduced forced expiratory volume is associated with increased incidence of atrial fibrillation: the Malmo Preventive Project. Europace. 2014;16(2):182-188.
doi pubmed - Simons SO, Elliott A, Sastry M, Hendriks JM, Arzt M, Rienstra M, Kalman JM, et al. Chronic obstructive pulmonary disease and atrial fibrillation: an interdisciplinary perspective. Eur Heart J. 2021;42(5):532-540.
doi pubmed - Linz D, Hohl M, Ukena C, Mahfoud F, Wirth K, Neuberger HR, Bohm M. Obstructive respiratory events and premature atrial contractions after cardioversion. Eur Respir J. 2015;45(5):1332-1340.
doi pubmed - Terzano C, Romani S, Conti V, Paone G, Oriolo F, Vitarelli A. Atrial fibrillation in the acute, hypercapnic exacerbations of COPD. Eur Rev Med Pharmacol Sci. 2014;18(19):2908-2917.
pubmed - Chen X, Lin M, Wang W. The progression in atrial fibrillation patients with COPD: a systematic review and meta-analysis. Oncotarget. 2017;8(60):102420-102427.
doi pubmed - Goudis CA. Chronic obstructive pulmonary disease and atrial fibrillation: an unknown relationship. J Cardiol. 2017;69(5):699-705.
doi pubmed - Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies C. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903-1913.
doi pubmed - Healey JS, Connolly SJ. Atrial fibrillation: Hypertension as a causative agent, risk factor for complications, and potential therapeutic target. Am J Cardiol. 2003;91(10 Suppl. 1):9-14.
doi - Apostolakis S, Sullivan R. Left ventricular geometry and outcomes in patients with atrial fibrillation: the AFFIRM Trial. Int J Cardiol. 2014;170(3):3038-3063.
doi - Xie E, Yu R, Ambale-Venkatesh B, Bakhshi H, Heckbert SR, Soliman EZ, Bluemke DA, et al. Association of right atrial structure with incident atrial fibrillation: a longitudinal cohort cardiovascular magnetic resonance study from the Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Magn Reson. 2020;22(1):36.
doi pubmed - Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes NA, 3rd, Page RL, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57(2):223-242.
doi pubmed - Padfield GJ, Steinberg C, Swampillai J, Qian H, Connolly SJ, Dorian P, Green MS, et al. Progression of paroxysmal to persistent atrial fibrillation: 10-year follow-up in the Canadian Registry of Atrial Fibrillation. Heart Rhythm. 2017;14(6):801-807.
doi pubmed - Laghi F, Adiguzel N, Tobin MJ. Endocrinological derangements in COPD. Eur Respir J. 2009;34(4):975-996.
doi pubmed - Lazzerini PE, Capecchi PL, El-Sherif N, Laghi-Pasini F, Boutjdir M. Emerging arrhythmic risk of autoimmune and inflammatory cardiac channelopathies. J Am Heart Assoc. 2018;7(22):e010595.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.