| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 000, Number 000, October 2025, pages 000-000
Pericardial Fat Tissue as a Predictor of the Severity of Acute Coronavirus Infection COVID-19
Anna Braginaa, b , Aida Tarzimanovaa
, Yulia Rodionovaa, b, d
, Konstantin Osadchiya
, Tatiana Ishinaa
, Ivan Medvedeva
, Lubov Vasilevaa
, Natalia Druzhininaa, b
, Karina Umbetovac
, Olga Belayac
, Maria Kutushevaa
, Zlata Nefedovaa
, Valery Podzolkova
aDepartment of Internal Medicine No. 2, Institute of Clinical Medicine, I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenovskiy University), Moscow, Russia
bInstitute of Personalized Cardiology of The Center “Digital Biodesign and Personalized Healthcare” of Biomedical Science and Technology Park, Federal State Autonomous Educational Institution of Higher Education I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenovskiy University) , Moscow, Russia
cDepartment of Infectious Diseases, Institute of Clinical Medicine, I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenovskiy University), Moscow, Russia
dCorresponding Author: Yulia Rodionova, Department of Internal Medicine No. 2, Institute of Clinical Medicine, I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenovskiy University), Moscow 119991, Russian Federation
Manuscript submitted July 11, 2025, accepted October 2, 2025, published online October 17, 2025
Short title: Pericardial Fat Tissue and COVID-19
doi: https://doi.org/10.14740/jocmr6324
| Abstract | ▴Top |
Background: Coronavirus disease 2019 (COVID-19) and obesity remain pressing global health concerns. Identifying predictors of severe disease is of particular importance. Pericardial fat tissue (PFT) is a known source of metainflammation due to its secretion of adipocytokines and inflammatory mediators. Moreover, cytokine storm plays a major role in COVID-19-related mortality. This study aimed to investigate the association between preexisting PFT volume and inflammatory markers in patients with COVID-19.
Methods: The study included 290 hospitalized patients with confirmed COVID-19 infection. Based on PFT volume (above or below 3.45 cm3), patients were divided into two groups: with and without pericardial obesity (PO), consisting of 132 and 158 individuals, respectively. Clinical, laboratory, and imaging data were analyzed. Statistical analysis was performed in Statistica 12.0.
Results: Significant intergroup differences were observed in the PO group for the following variables: male sex (P < 0.001), body mass index (BMI) (P < 0.001), obesity (P < 0.001), and history of diabetes mellitus (P = 0.003). No significant differences were found in lung computed tomography (CT) severity scores. However, patients with PO showed significantly lower oxygen saturation (SpO2) levels (P = 0.014) and a higher frequency of SpO2 ≤ 93% (P = 0.012). Ferritin levels were significantly higher in the PO group (median 440 (274.00 - 552.70) vs. 292.55 (156.00 - 521.50), P = 0.010). Linear correlation analysis revealed a positive association between PFT volume and age, BMI, glucose, ferritin, C-reactive protein, and D-dimer levels, and a negative correlation with oxygen saturation. Multivariate logistic regression confirmed an independent association between PFT volume and SpO2 ≤ 93%. Receiver operating characteristic (ROC) analysis identified a threshold PFT volume of 3.45 cm3 for predicting increased risk of severe COVID-19 (SpO2 ≤ 93%), with sensitivity of 66.7%, specificity of 65.0%, and area under the curve (AUC) of 0.710 (95% confidence interval (CI): 0.551 - 0.868, P < 0.001).
Conclusions: Our data suggest that a PFT volume greater than 3.45 cm3 is a potential predictor of severe COVID-19.
Keywords: COVID-19; SARS-CoV-2 infection; Obesity; Pericardial fat tissue; Inflammation
| Introduction | ▴Top |
Obesity is one of the most common non-communicable diseases and a major risk factor for cardiovascular disease and metabolic disorders. More than one billion people > 5 years old are obese, 16% of adults aged ≥ 18 years are obese, and 43% are overweight [1]. The population of most countries tends to increase their body weight [2]. Subjects with obesity are at increased risk of development and severe course of various infectious diseases [3], which is confirmed by the coronavirus pandemic of 2020 - 2023 (coronavirus disease 2019 (COVID-19)), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Several meta-analyses have demonstrated that obesity is associated with a more severe course of COVID-19 and increased mortality [4]. A meta-analysis by Singh et al, which included 167 studies and a total of 3,140,413 patients, demonstrated that obesity is a significant predictor of severe COVID-19 and related mortality. Its impact was comparable to other major risk factors such as chronic pulmonary diseases, diabetes mellitus (DM), older age, arterial hypertension (AH), and cardiovascular diseases (CVDs) [5].
Several authors have shown that obesity is linked to both CVD and COVID-19 through metainflammation [6, 7]. Pathological adipose tissue remodeling in obesity results in activation of adipocytokines and pro-inflammatory cytokines secretions, such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1), C-reactive protein (CRP), serum amyloid A protein, and others. In subjects with obesity, metainflammation also intensifies dysregulation of the renin-angiotensin-aldosterone system, endothelial dysfunction, and hypercoagulation, which are key mechanisms for COVID-19 development [7].
Along with general obesity, the role of ectopic adipose tissues has been actively studied in recent years. Visceral fat deposition can be observed even in the absence of general obesity [8]. Visceral adipose tissue (VAT) around the heart (epicardial fat tissue (EFT) and pericardial fat tissue (PFT)) plays an important role in the development of cardiometabolic disorders [9, 10], CVD [11, 12], and systemic inflammation [13]. Thus, a meta-analysis of 83 studies including 73,934 patients showed a significant influence of PFT on the development of coronary artery disease, ventricular dysfunction, heart failure, atrial fibrillation, and major adverse cardiac events [12].
The role of ectopic adipose tissue in COVID-19 is poorly understood. In a cross-sectional study of 364 patients with COVID-19, Su et al described that EFT contributes to the risk of myocardial injury [14]. The association of EFT with severe COVID-19 and mortality was identified in a Mexican cohort study by Mehta et al, independent of age, sex, and BMI [15].
Nowadays, the clinical picture of COVID-19 has undergone significant changes. It has become less severe. However, the disease is still a threat, as evidenced by the number of hospitalizations associated with its severe course. According to the World Health Organization, the worldwide number of deaths from COVID-19 continues to grow, with a total of 7 million 100 thousand recorded by April 2025 [16]. The timely assessment of the severity and prognosis of COVID-19 remains crucial, as it is vital for choosing an appropriate treatment strategy for patients. Furthermore, COVID-19 can be considered a special model of the acute infectious process caused by various pathogens. Pandemic data on general biological patterns of acute inflammatory processes can then be used for further prediction of the host and the microorganism interactions. Thus, the objective of our study was to investigate the association between the preexisting PFT volume and the severity of the COVID-19, as well as to evaluate its potential use as a predictor of adverse COVID-19 outcomes in the Russian population.
| Materials and Methods | ▴Top |
A retrospective cohort single-center study was conducted on patients with COVID-19 at the COVID hospital and the Department of Internal Diseases of the University Clinical Hospital No. 4 of Sechenov University from April 2020 to April 2022.
This study was conducted in accordance with the principles of the Declaration of Helsinki and the requirements of the Federal Law “On Personal Data” of 27.07.2006 No. 152-FL. All the patients signed a written informed consent to their participation in the study and further analysis of the data obtained during the study. The study was approved by the local ethical committee of Sechenov University (protocol number 01-22 dated 20.01.2022).
The inclusion criteria were age ≥ 18 years with COVID-19 and a signed informed consent to participate in the study. The diagnosis was verified by detection of SARS-CoV-2 RNA in nasopharyngeal swabs by polymerase chain reaction (PCR) and based on lung changes on multi-slice spiral computed tomography (CT). Exclusion criteria were acute coronary syndrome, acute cerebrovascular accident, pulmonary embolism, severe liver or kidney pathology (glomerular filtration rate (GFR) < 15 mL/min/1.73 m2), severe anemia, and cancer.
History of the present illness, comorbid conditions and medications, clinical symptoms, physical examination, laboratory, and instrumental data were analyzed. The data were obtained at the time of initial assessment at hospital admission. Patients included in our study did not take glucagon-like peptide 1 (GLP-1) agonist and pioglitazone. The severity of the SARS-CoV-2 was assessed in accordance with the temporary guidelines of the Russian Ministry of Health. Criteria for severe COVID-19 included respiratory rate (RR) > 30/min, blood oxygen saturation (SpO2) ≤ 93%, signs of unstable hemodynamics with systolic blood pressure (SBP) < 90 and/or diastolic blood pressure (DBP) < 60 mm Hg [17], which meet international criteria for the severity of COVID-19 [18].
PFT volume was assessed by CT according to the visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. This method is considered one of the tools for assessing ectopic chest depots [8]. The chest CT was performed in spiral mode on a Toshiba Aquilion Prime CT scanner using a standardized protocol. The tube voltage was set to 120 kV and the automatic tube current selection in the range from 100 to 500 mA, with 1 mm slice thickness. The PFT volume was calculated semi-automatically on a single slice at the level of the anterior segment of the fifth intercostal space with contouring images of adipose tissue around the heart and further calculation using specialized software - QCT Pro Tissue Composition Module (Mindways Software, Inc., USA). The scanner was calibrated by a special phantom. The extent of pulmonary impairment was evaluated based on the established categorization according to the results of multi-slice spiral CT of the chest: CT0 denotes the nonexistence of inflammation foci and infiltrates; CT1 signifies the manifestation of viral pneumonia symptoms, involving up to a quarter of the pulmonary tissue; CT2 corresponds to the breadth of lung impairment ranging from 25% to 50%; CT3 relates to the pulmonary tissue damage spanning from 50% to 75%; CT4 represents injury to over 75% of the pulmonary tissue.
Statistical processing was carried out using Statistica 12.0 (StatSoft Inc.) and SPSS 20.0 (IBM). The continuous variable was examined for normality of distribution using the Kolmogorov-Smirnov test. The data are expressed as mean and standard deviation (М ± σ) in case of normal distribution, as median, and interquartile range (Me (Q25 - Q75)) in nonparametric distribution, and as percentage for categorical and qualitative variables. The comparison of continuous variables was performed using parametric (Student’s t-criterion) and nonparametric (Mann-Whitney) criteria. To compare the categorical variables of two independent groups, the χ2 test was used. The relationship between the variables was evaluated in all participants using Spearman’s rank correlation coefficient (r). To assess the diagnostic significance of indicators for severe course of COVID-19, regression analysis was performed with further construction of receiver operating characteristic (ROC) curves. The area under the ROC curve was used to determine the predictive ability of the factors, with calculation of the area under the curve (AUC) with 95% confidence interval (CI), sensitivity, specificity (using Youden’s index), and determination of the optimal classification threshold (cut-off point). The prognostic value of the marker was considered statistically significant if the lower bound of the 95% CI for the AUC exceeded 0.5 and the P-value was < 0.05.
| Results | ▴Top |
A total of 296 patients were enrolled in the study: 141 men and 155 women, with a mean age of 61.07 ± 14.04 years. Based on the severity of COVID-19, the cohort was divided into two groups. The clinical and demographic characteristics of the study groups are presented in Table 1.
 Click to view | Table 1. Main Demographic and Clinical Characteristics of Patients with COVID-19 |
Comparative analysis of COVID-19 severity groups revealed that patients with severe disease were significantly older (P = 0.003) and had higher RR (P < 0.001), body weight (P = 0.007), BMI (P = 0.006), and PFT volume (P = 0.003). This group also demonstrated higher comorbidity rates, with statistically significant greater prevalence of AH (P = 0.001) and type 2 diabetes mellitus type (T2DM) (P = 0.035).
Patients with severe COVID-19 had significantly higher levels of key systemic inflammation markers: CRP, ferritin, and D-dimer, along with significantly lower serum albumin levels.
Chest CT scans at the admission revealed that the severe COVID-19 group had a statistically higher frequency of more advanced pulmonary injury: CT1 was present in 14.29% compared to 36.81% in the moderate group, CT2 in 47.37% and 51.53%, CT3 in 34.59% and 7.98%, and CT4 in 3.75% and 3.68%, respectively (χ2 = 27.574, P < 0.001).
Correlation analysis was performed to identify the relationship between PFT volume and markers of systemic inflammation and metabolic disorders (Fig. 1). Significant positive correlations were obtained between PFT volume and age, markers of inflammation (CRP, D-dimer, ferritin), and metabolic parameters (BMI and blood glucose); a negative correlation was observed between PFT volume and SpO2.
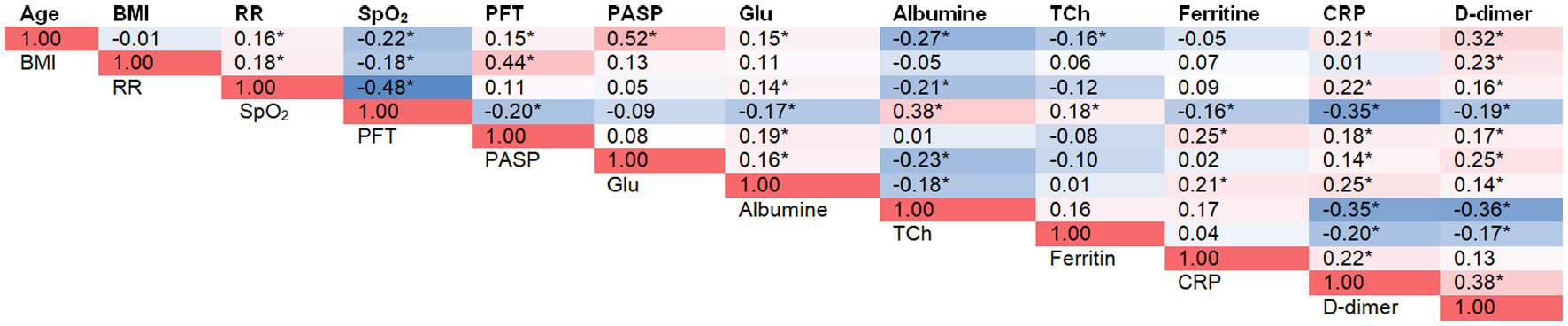 Click for large image | Figure 1. Associations of Visceral Adipose Tissue Volume With Disease Severity Markers in COVID-19 Patients. *P < 0.05. A heatmap color scale was used to visually represent correlation magnitudes. BMI: body mass index; COVID-19: coronavirus disease 2019; CRP: C-reactive protein; DBP: diastolic blood pressure; Glu: glucose; HR: heart rate; PASP: pulmonary artery systolic pressure; PFT: pericardial fat tissue; RR: respiratory rate; SBP: systolic blood pressure; SpO2: oxygen saturation; TCh: total cholesterol. |
Based on our results, an attempt to assess the predictive significance of PFT volume determined at the time of patient admission in relation to the severity of COVID-19 was made. We constructed four sequential logistic regression models to evaluate their predictive significance regarding COVID-19 severity. It should be noted that the models included factors that significantly correlated with markers of COVID-19 severity, both according to our results and literature data [19]. Model 1 included only PFT volume, model 2 combined it with age, model 3 included additional factors such as AH, blood glucose, and total cholesterol, while model 4 incorporated albumin levels, CRP, and D-dimer. The significant association of PFT volume with COVID-19 severity was demonstrated in the single-factor model, as well as in all multifactor models. The association between PFT volume and the severity of COVID-19 remained after adjusting for age, levels of glucose, total cholesterol, albumin, CRP, D-dimer, serum ferritin, and the presence of AH (Table 2). These data indicate the independent predictive significance of PFT volume. The AUC for model 4 was 0.859 (Fig. 2).
 Click to view | Table 2. PFT Volume as a Predictor of COVID-19 Disease Severity |
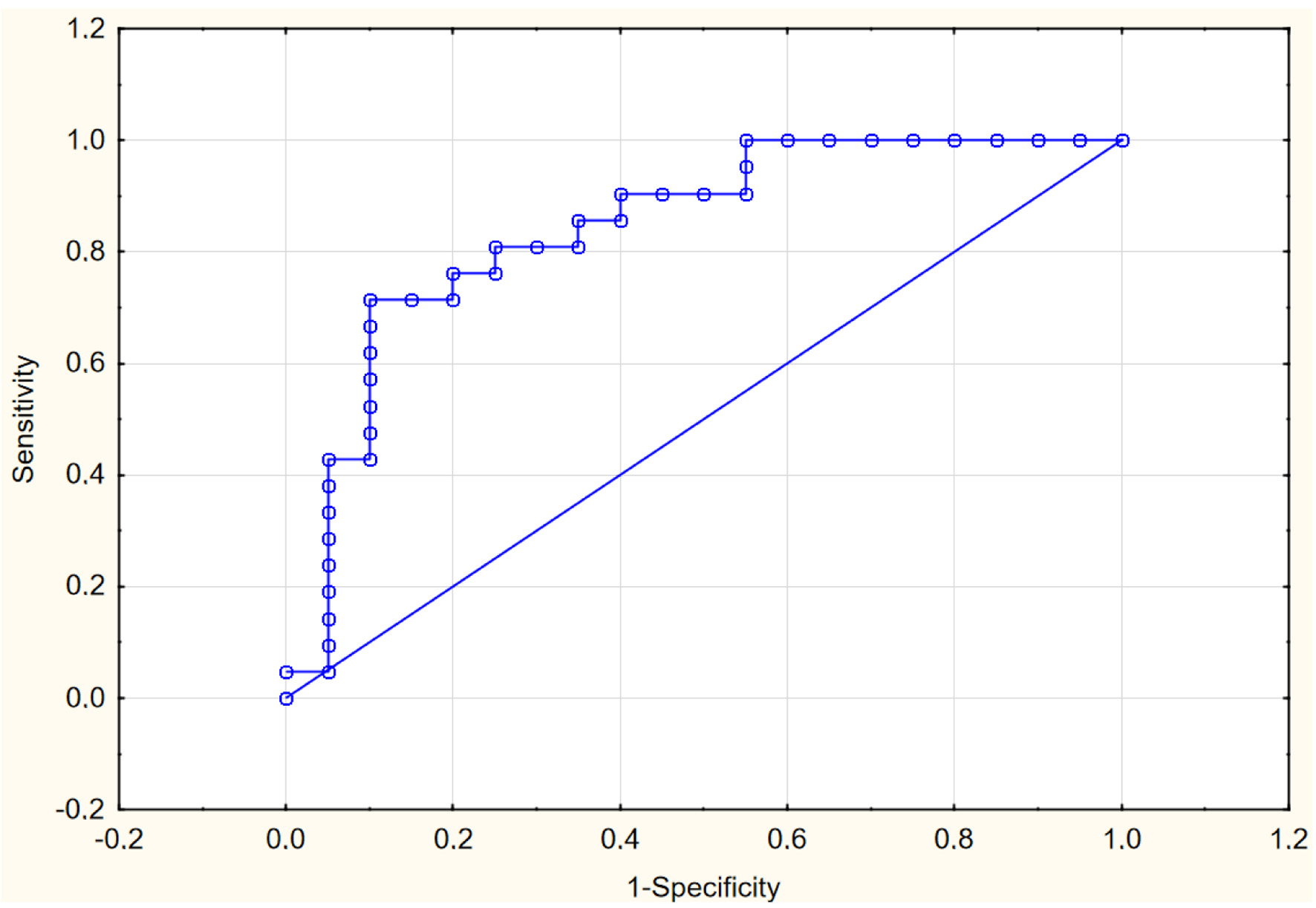 Click for large image | Figure 2. ROC curve for predictive model 4. ROC: receiver operating characteristic. |
We analyzed the sensitivity and specificity of PFT volume as a predictor of COVID-19 severity, and an ROC analysis was conducted, calculating the threshold value of PFT volume. The threshold was determined to be 3.60 cm3 (sensitivity 63.2%, specificity 68.4%, AUC = 0.680 (0.509 -0.851), P < 0.001). Exceeding this value, regardless of age, presence of AH, levels of glucose, total cholesterol, albumin, CRP, ferritin, and D-dimer in the blood, may be associated with an increased risk of more severe COVID-19 progression (odds ratio (OR) 2.68 (1.06 - 6.73), P = 0.037).
| Discussion | ▴Top |
As of today, broad evidence of the relation between obesity and the adverse prognosis of COVID-19 has been accumulated [4, 5]. Ectopic fat deposits are considered to be more strongly associated with cardiometabolic disorders compared to subcutaneous fat and BMI [8, 20]. Moreover, cardiometabolic disorders are also a risk factor for infection and severe COVID-19 [21]. In our study, we examined the correlation between the volume of PFT and the severity of the COVID-19 and evaluated the possibility of using this parameter as a potential predictor of the severe COVID-19 in the Russian population. Since all patients with acute COVID-19 undergone chest CT during admission to the hospital for assessment of pulmonary injury, a PFT volume estimate may be obtainable in routine clinical practice.
The main criteria for COVID-19 severity are the SpO2 and CRP levels [17, 18]. A decrease in SpO2 reflects the severity of respiratory failure, while CRP levels reflect the intensity of the inflammation. Our results are consistent with previous studies demonstrating associations between age, presence of T2DM, high BMI, and kidney dysfunction with advanced COVID-19 [22-25]. Patients with severe COVID-19 were significantly older and had higher BMI, volume of PFT, creatinine levels, and a greater prevalence of T2DM.
Obesity and insulin resistance are characterized by the presence of metainflammation and a tendency for hypercoagulation, which might cause more severe inflammatory response to SARS-CoV-2. Additionally, obesity is associated with altered cellular immunity, impaired immune responses to viral infections and may be one more factor that promotes progression of the disease [26].
Excessive EFT and PFT are risk factors for the development of CVDs [27-29]. As has been shown, SARS-CoV-2 may infect adipocytes, with further active and/or prolonged replication, inflammation, and the severe course of the disease in individuals with obesity [26]. The main finding of our study is the identified association between PFT volume and a severe course of COVID-19. In our analysis of 296 patients, we showed that a greater PFT volume was associated with lower SpO2, higher CRP and ferritin levels, and consequently, with a more severe course of the disease.
A few previous studies have also demonstrated an association between ectopic fat deposits and both the severity and the prognosis of COVID-19 across different populations.
In a study of 364 patients, Su et al demonstrated a significant correlation between the volume of EFT and inflammatory and myocardial injury markers, including IL-6, troponin I, and CRP [14]. Similar data were obtained in a small sample of 76 patients in Turkey. An EFT thickness of ≥ 4.85 mm was associated with elevated troponin I levels (65 % sensitivity and 39 % specificity) [30]. In an Iranian study, involving 80 patients, Abrishami et al demonstrated that the activity of inflammation in COVID-19 is more in individuals with greater EFT volume. Thus, significant correlations were identified: positive correlations between EFT volume and such inflammatory markers as neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio, and a negative correlation with the lymphocyte-to-neutrophil ratio, which is considered a potential predictor of survival in COVID-19 (sensitivity of 79.7% and specificity of 64.3%) [31]. Three more studies have investigated the association of EFT with the course and prognosis of COVID-19. In a Russian study by Druzhilova et al patients with COVID-19 and increased EFT thickness had more extensive pulmonary injury, higher levels of CRP, as well as hospitalization rate and the need for invasive ventilation [32]. Of note, this study included a small sample of patients with moderate and severe COVID-19 (50 and 12 individuals, respectively). EFT thickness was measured by echocardiography, which does not allow for a comprehensive evaluation of adipose tissue around the heart. The association between EFT volume and COVID-19 prognosis was also demonstrated in France by Bihan et al in a study including 100 hospitalized patients [33]. Admission to the intensive care unit (ICU) or death was associated with higher EFT volume, independent of all other comorbidities. Liu et al conducted a meta-analysis of 2,482 patients from 13 studies performed up to January 2022 in Europe (France, Italy, and Spain), the USA, and Asia (China, Iran, and Turkey). The study confirmed that individuals with larger EFT volume (assessed by chest CT) more frequently experienced severe COVID-19, and required hospitalization and invasive mechanical ventilation [34].
Of interest the study from the Netherlands by Aydeniz et al reported opposite findings in a small group of patients with severe COVID-19. The authors found no association between EFT and the severity of multiorgan failure assessed by the sequential organ failure assessment (SOFA) score. Moreover, PFT appeared to have “protective” effects regarding the severity markers of COVID-19 [35]. However, these unexpected outcomes might be explained by the small sample size (N = 63), which included patients with severe and critical course of disease, a high mortality rate (over 30%) and a high rate of mechanical ventilation (over 80%). In addition, these patients received high doses of systemic glucocorticoids and biological immunomodulatory treatment, which may have influenced the density and functional activity of ectopic fat tissue. Similar findings were reported in a small combined study of 72 patients by Iacobellis et al, conducted in the USA and Italy. The authors observed a reduction in EFT inflammation in COVID-19 patients receiving dexamethasone therapy [36].
Our study revealed significant associations between PFT volume and serum levels of CRP, D-dimer, and ferritin, suggesting that patients with ectopic obesity experience more active systemic inflammation during acute COVID-19. Existing literature has shown that individuals with ectopic obesity exhibit chronic, low-grade inflammation characterized by increased production of pro-inflammatory cytokines and chemokines (such as IL-6, TNF-α, MCP-1, and CRP) [6, 7, 37]. Additionally, it has been demonstrated that SARS-CoV-2 virus may persist in adipocytes of ectopic fat deposits and contribute to pathogenic inflammation. Thus, adipose tissue might serve as a potential reservoir for SARS-CoV-2, sustaining systemic and regional inflammation and potentially contributing to the severe clinical course of the disease in individuals with ectopic obesity [26].
Based on our correlation matrix and ROC analysis, we identified a clinically significant threshold value of PFT volume of 3.60 cm3 (sensitivity 63.2%, specificity 68.4%, AUC = 0.680 (0.509 - 0.851), P < 0.001). Exceeding this threshold may independently increase the risk of a severe course of acute COVID-19, regardless of age, history of AH, levels of glucose, total cholesterol, albumin, CRP, and D-dimer in the blood.
The limitations of our study include the lack of patients with multisystem failure and those requiring admission to the ICU.
Our findings suggest that ectopic PFT may have the impact on the inflammatory response in acute COVID-19 and may serve as one of the predictors of severity of COVID-19 in hospitalized patients without critical condition.
Acknowledgments
None to declare.
Financial Disclosure
The work was financed by the Priority 2030 program of the Ministry of Science and Higher Education of Russia, project “The Digital Cardiology with Artificial Intelligence”.
Conflict of Interest
The authors confirm that there is no potential conflict of interest to disclose in relation to this article.
Informed Consent
Completed written informed consent was obtained.
Author Contributions
Conceptualization: AB, AT, and VP; methodology: AB, YR, KO, TI, IM, and LV; software: LV, ND, KU, and OB; validation: AB, TI, and IM; formal analysis: KO, KU, and OB; data collection and analysis: LV, MK, and ZN; interpretation of data: AB, AT, YR, KO, TI, IM, ND, and LV; data curation: AB, AT, and VP; writing - original draft preparation: TI, IM, and LV; writing - review and editing: AB, AT, YR, KO, ND, KU, and OB; writing - literacy search: MK and ZN; supervision: VP; project administration: VP. All authors approved the final version of the manuscript for submission.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
AUC: area under the curve; AH: arterial hypertension; BMI: body mass index; CI: confidential interval; CRP: C-reactive protein; CT: computed tomography; CVDs: cardiovascular diseases; DBP: diastolic blood pressure; DM: diabetes mellitus; EF: ejection fraction; EFT: epicardial fat tissue; GFR: glomerular filtration rate; Glu: glucose; ICU: intensive care unit; IL-6: interleukin-6; MCP-1: monocyte chemoattractant protein-1; OR: odds ratio; PASP: pulmonary artery systolic pressure; PCR: polymerase chain reaction; PFT: pericardial fat tissue; PO: pericardial obesity; ROC: receiver operating characteristic; RR: respiratory rate; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; SBP: systolic blood pressure; SpO2: oxygen saturation; T2DM: type 2 diabetes mellitus; TCh: total cholesterol; TNF-α: tumor necrosis factor-α; VAT: visceral adipose tissue
| References | ▴Top |
- Obesity and overweight. 2025. Accessed: June 23, 2025. [Online]. Available: https://www.who.int/ru/news-room/fact-sheets/detail/obesity-and-overweight.
- NCD Risk Factor Collaboration (NCD-RisC). Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature. 2019;569(7755):260-264.
doi pubmed - Pugliese G, Liccardi A, Graziadio C, Barrea L, Muscogiuri G, Colao A. Obesity and infectious diseases: pathophysiology and epidemiology of a double pandemic condition. Int J Obes (Lond). 2022;46(3):449-465.
doi pubmed - Seidu S, Gillies C, Zaccardi F, Kunutsor SK, Hartmann-Boyce J, Yates T, Singh AK, et al. The impact of obesity on severe disease and mortality in people with SARS-CoV-2: A systematic review and meta-analysis. Endocrinol Diabetes Metab. 2021;4(1):e00176.
doi pubmed - Singh R, Rathore SS, Khan H, Karale S, Chawla Y, Iqbal K, Bhurwal A, et al. Association of obesity with COVID-19 severity and mortality: an updated systemic review, meta-analysis, and meta-regression. Front Endocrinol (Lausanne). 2022;13:780872.
doi pubmed - Chiappetta S, Sharma AM, Bottino V, Stier C. COVID-19 and the role of chronic inflammation in patients with obesity. Int J Obes (Lond). 2020;44(8):1790-1792.
doi pubmed - Muscogiuri G, Bettini S, Boschetti M, Barrea L, Savastano S, Colao A, Obesity Programs of nutrition ER, et al. Low-grade inflammation, CoVID-19, and obesity: clinical aspect and molecular insights in childhood and adulthood. Int J Obes (Lond). 2022;46(7):1254-1261.
doi pubmed - Neeland IJ, Ross R, Despres JP, Matsuzawa Y, Yamashita S, Shai I, Seidell J, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715-725.
doi pubmed - Bragina A, Rodionova Y, Druzhinina N, Suvorov A, Osadchiy K, Ishina T, Vasilchenko M, et al. Relationship between perivascular adipose tissue and cardiovascular risk factors: a systematic review and meta-analysis. Metab Syndr Relat Disord. 2024;22(1):1-14.
doi pubmed - Bragina AE, Tarzimanova AI, Osadchiy KK, Rodionova YN, Bayutina DA, Bragina GI, Djafarova ZB, et al. Relationship of pericardial fat tissue with cardiovascular risk factors in patients without cardiovascular diseases. Metab Syndr Relat Disord. 2021;19(9):524-530.
doi pubmed - Baichoo R, Fu J, Xiong X, Zhou Q, Guan X, Xu X. The role of cardiac adipose tissue in coronary artery disease: a narrative review of cardiac imaging studies. Quant Imaging Med Surg. 2024;14(12):9685-9697.
doi pubmed - Al-Makhamreh HK, Toubasi AA, Al-Harasis LM, Albustanji FH, Al-Sayegh TN, Al-Harasis SM. Pericardial fat and cardiovascular diseases: A systematic review and meta-analysis. J Evid Based Med. 2023;16(2):178-185.
doi pubmed - Masson W, Lavalle-Cobo A, Barbagelata L, Lobo M, Nogueira JP. Relationship between epicardial adipose tissue, systemic inflammatory diseases, and subclinical atheromatosis: A systematic review. Reumatol Clin (Engl Ed). 2023;19(7):363-373.
doi pubmed - Su T, Zhong B, Tang C, Qiao S, Feng Y, Peng H, Gu X. Correlation between epicardial adipose tissue and myocardial injury in patients with COVID-19. Front Physiol. 2024;15:1368542.
doi pubmed - Mehta R, Bello-Chavolla OY, Mancillas-Adame L, Rodriguez-Flores M, Pedraza NR, Encinas BR, Carrion CIP, et al. Epicardial adipose tissue thickness is associated with increased COVID-19 severity and mortality. Int J Obes (Lond). 2022;46(4):866-873.
doi pubmed - WHO COVID-19 dashboard. June 2025. Accessed: June 23, 2025. [Online]. Available: https://data.who.int/dashboards/covid19/deaths?n=o.
- Ministry of Health of the Russian Federation. Temporary guidelines: Prevention, diagnosis and treatment of new coronavirus infection (COVID-19). October 2023. Accessed: June 23, 2025. [Online]. Available: https://static-0.minzdrav.gov. ru/system/attachments/attaches/000/064/610/original/ ВМР_COVID-19_V18.pdf.
- NIH Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. n.d.). Accessed: June 23, 2025. [Online]. Available: https://www.ncbi.nlm.nih.gov/books/NBK570371/pdf/Bookshelf_NBK570371.pdf.
- Podzolkov VI, Bragina AE, Tarzimanova AI, Vasilyeva LV, Ogibenina ES, Bykova EE, Shvedov II, et al. Arterial hypertension and severe COVID-19 in hospitalized patients: data from a cohort study. Rational Pharmacotherapy in Cardiology. 2023;19(1):4-10.
- Matsuzawa Y. The role of fat topology in the risk of disease. Int J Obes (Lond). 2008;32(Suppl 7):S83-92.
doi pubmed - Kim CW, Aronow WS, Frishman WH. Coronavirus disease 2019 and cardiometabolic disease. Cardiol Rev. 2022;30(3):123-128.
doi pubmed - Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020;43(6):867-869.
doi pubmed - Yang J, Hu J, Zhu C. Obesity aggravates COVID-19: A systematic review and meta-analysis. J Med Virol. 2021;93(1):257-261.
doi pubmed - Chen R, Liang W, Jiang M, Guan W, Zhan C, Wang T, Tang C, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97-105.
doi pubmed - Gallo G, Calvez V, Savoia C. Hypertension and COVID-19: current evidence and perspectives. High Blood Press Cardiovasc Prev. 2022;29(2):115-123.
doi pubmed - Martinez-Colon GJ, Ratnasiri K, Chen HP, Jiang SZ, Zanley E, Rustagi A, Verma R, et al. SARS-CoV-2 infects human adipose tissue and elicits an inflammatory response consistent with severe COVID-19. BioRxiv. 2021.
- Mahabadi AA, Berg MH, Lehmann N, Kalsch H, Bauer M, Kara K, Dragano N, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;61(13):1388-1395.
doi pubmed - Kenchaiah S, Ding J, Carr JJ, Allison MA, Budoff MJ, Tracy RP, Burke GL, et al. Pericardial fat and the risk of heart failure. J Am Coll Cardiol. 2021;77(21):2638-2652.
doi pubmed - Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol. 2022;19(9):593-606.
doi pubmed - Ozer S, Bulut E, Ozyildiz AG, Peker M, Turan OE. Myocardial injury in COVID-19 patients is associated with the thickness of epicardial adipose tissue. Kardiologiia. 2021;61(8):48-53.
doi pubmed - Abrishami A, Eslami V, Arab-Ahmadi M, Alahyari S, Azhideh A, Sanei-Taheri M. Prognostic value of inflammatory biomarkers for predicting the extent of lung involvement and final clinical outcome in patients with COVID-19. J Res Med Sci. 2021;26:115.
doi pubmed - Druzhilov MA, Yu K. Epicardial adiposity as a predictor of COVID-19 severity in overweight and obese patients. Russian Journal of Cardiology. 2022;27(3):4850.
- Bihan H, Heidar R, Beloeuvre A, Allard L, Ouedraogo E, Tatulashvili S, Tandjaoui Y, et al. Epicardial adipose tissue and severe Coronavirus Disease 19. Cardiovasc Diabetol. 2021;20(1):147.
doi pubmed - Liu K, Wang X, Song G. Association of epicardial adipose tissue with the severity and adverse clinical outcomes of COVID-19: A meta-analysis. Int J Infect Dis. 2022;120:33-40.
doi pubmed - Aydeniz E, Weberndorfer V, Brandts L, Smulders MW, van Herpt TTW, Martens B, Vernooy K, et al. Pericardial fat is associated with less severe multiorgan failure over time in patients with coronavirus disease-19: the maastricht intensive care COVID cohort. J Thorac Imaging. 2024;39(3):W32-W39.
doi pubmed - Iacobellis G, Malavazos AE, Basilico S, Tresoldi S, Rinaldo RF, Dubini C, Capitanio G, et al. Epicardial fat inflammation response to COVID-19 therapies. Obesity (Silver Spring). 2021;29(9):1427-1433.
doi pubmed - Majidpoor J, Mortezaee K. Interleukin-6 in SARS-CoV-2 induced disease: Interactions and therapeutic applications. Biomed Pharmacother. 2022;145:112419.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.