| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 9, October 2025, pages 507-517
Evaluating the Effectiveness of Triple Therapy in Chronic Obstructive Pulmonary Disease Patients: An Asian Population-Based Survey
Chih-Yuan Sunga, Chuan-Wei Shena, Ye Gua, Rewadee Jenraumjitb, c, Chung-Yu Chena, d, e, f, j, Kuang-Ming Liaog, j, Fu-Shih Chenh, i
aSchool of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan, Republic of China
bDepartment of Pharmaceutical Care, Faculty of Pharmacy, Chiang Mai University, Chiang Mai, Thailand
cMultidisciplinary and Interdisciplinary School, Chiang Mai University, Chiang Mai, Thailand
dDepartment of Pharmacy, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan, Republic of China
eDepartment of Medical Research, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan, Republic of China
fCenter for Big Data Research, Kaohsiung Medical University, Kaohsiung, Taiwan, Republic of China
gDepartment of Internal Medicine, Chi Mei Medical Center, Chiali, Taiwan, Republic of China
hGraduate School of Pharmaceutical Sciences, Nihon Pharmaceutical University, Ina, Saitama 362-0806, Japan
iFaculty of Pharmaceutical Sciences, Nihon Pharmaceutical University, Ina, Saitama 362-0806, Japan
jCorresponding Authors: Kuang-Ming Liao, Department of Internal Medicine, Chi Mei Medical Center, Chiali, Taiwan, Republic of China; Chung-Yu Chen, School of Pharmacy, Kaohsiung Medical University, Kaohsiung 80708, Taiwan, Republic of China
Manuscript submitted July 20, 2025, accepted September 23, 2025, published online October 10, 2025
Short title: Effectiveness of Triple Therapy in COPD
doi: https://doi.org/10.14740/jocmr6329
| Abstract | ▴Top |
Background: The optimal treatment strategy for chronic obstructive pulmonary disease (COPD) remains debated, especially when choosing between triple therapy and long-acting beta agonist (LABA) + long-acting muscarinic antagonist (LAMA). This study aimed to develop a model that simulates real-world prescription patterns and compares the effectiveness of these two treatment options.
Methods: This population-based cohort study used Taiwan’s National Health Insurance Research Database to follow COPD patients who had been on LABA plus inhaled corticosteroids (ICSs) for more than 28 days. These patients were followed until they either upgraded to triple therapy or switched to LABA plus LAMA. The study enrolled patients from 2013 to 2021. Cox proportional hazard models were used to evaluate the risk of seven individual outcomes, including mortality, COPD exacerbations, acute respiratory failure, pneumonia, and respiratory-related admissions, adjusting for fixed and time-dependent variables.
Results: Among the 20,997 included patients (mean (standard deviation (SD)) age: 66.06 (11.54) years; 12,977 males (61.80%)), 16,792 (79.97%) were in the triple therapy group, and 4,205 (20.03%) were in LABA plus LAMA group. The triple therapy group showed significantly higher relative risks in several outcomes: composite outcome (adjusted hazard ratio (aHR): 1.162; 95% confidence interval (CI): 1.098 - 1.230; P < 0.0001), acute respiratory failure (aHR: 1.315; 95% CI: 1.047 - 1.653; P = 0.0186), severe acute exacerbation (aHR: 1.346; 95% CI: 1.078 - 1.682; P = 0.0088), pneumonia (aHR: 1.221; 95% CI: 1.109 - 1.344; P < 0.0001) and respiratory-related admission (aHR: 1.264; 95% CI: 1.157 - 1.382; P < 0.0001), indicating superior effectiveness of LABA plus LAMA in these indicators.
Conclusions: In conclusion, in COPD patients, the combination of LABA plus LAMA can significantly improve many major symptoms and reduce the frequency of exacerbations.
Keywords: Chronic obstructive pulmonary disease; Triple therapy; Effectiveness
| Introduction | ▴Top |
Chronic obstructive pulmonary disease (COPD) is a prevalent respiratory condition characterized by airflow limitation, often linked to smoking and environmental factors [1]. Notably, in 2019, COPD ranked as the third leading cause of death worldwide, accounting for 6% of the total number of lives lost [2]. Patients with COPD commonly experience some typical symptoms, such as respiratory distress, continuous cough with sputum production, and restricted activities of daily living [3]. The objective of treatments was to relieve symptoms and improve lung function [4, 5]. Different inhaler combinations are used to manage COPD symptoms [6]; however, the effectiveness and safety of triple therapy, including long-acting beta agonist (LABA), long-acting muscarinic antagonist (LAMA), and inhaled corticosteroids (ICSs), remain a concern [7]. The GOLD guidelines suggest that patients aiming to alleviate major symptoms should use LABA plus LAMA, while those aiming to prevent further exacerbations should use triple therapy [4, 5]. However, these two goals often coexisted in the patients’ treatment process.
When a COPD patient’s treatment needs escalation, healthcare providers often face difficulties in determining whether to add ICS. Many previous randomized control trials (RCTs) have demonstrated that triple therapy reduces the risk of COPD exacerbation [8-11]. However, a real-world study investigating the same topic stated that triple therapy did not perform better than dual therapy in preventing all-cause mortality, pneumonia, and severe COPD exacerbations [12]. Another significant issue of prior research was the inability to accurately simulate the prescription patterns of COPD patients. During the maintenance period of COPD, a patient’s inhaler combination might be escalated or de-escalated due to various factors or symptom control conditions [13]. The conventional Cox proportional hazards model only allocated treatment at the beginning of the study, failing to account for COPD patients’ dynamic prescription patterns [14]. This highlighted the ongoing need to explore optimal COPD management strategies with more appropriate modeling approaches.
To address these issues, this study proposed establishing a Cox model using the counting process [15-17]. The counting process is a statistical method used in survival analysis to manage time-dependent variables, which allows for accurately capturing the evolution of a patient’s prescription [18, 19]. This method enables a precise comparative effectiveness assessment and allows for the inclusion of time-dependent variables.
| Materials and Methods | ▴Top |
Data source
Taiwan’s National Health Insurance system covers 99.99% of Taiwan population [20, 21]. We utilized the National Health Insurance Research Database (NHIRD) from January 1, 2012, to December 31, 2022. This period included 1 year before the cohort entry date of January 1, 2013, to assess exclusion criteria and baseline characteristics, and 1 year after the study end date of October 31, 2021, to ensure a minimum duration for assessing exposure.
Study design and study population
This is a retrospective, national-databased and population-based cohort study. Patients who had at least one inpatient or outpatient diagnosis of COPD (International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes: 490, 491, 492, 496; International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes: J40, J41, J43, J44) and who used LABA plus ICS for more than 28 days between January 1, 2013, and December 31, 2021, were included in the study cohort. The cohort entry date was defined as the first date of the LABA plus ICS prescription, followed by an escalation to triple therapy or a subsequent switch to LABA plus LAMA.
Patients were excluded if they met any of the following criteria: 1) Age was not between 40 and 90 years; 2) They had used triple therapy or LABA plus LAMA before the cohort entry date; 3) They had missing or incomplete demographic data (e.g., gender or age); 4) They had never used LABA plus ICS in their lifetime; 5) They had prescriptions that did not switch to LABA plus LAMA or escalate to triple therapy for at least 28 days (i.e., window period < 28 days); 6) They were diagnosed with lung cancer within 1 year prior to the index date (ICD-9-CM: 162; ICD-10-CM: C33, C34, C78.00, C78.01, C78.02, C7A.090, Z85.118); 7) They underwent lung transplantation within 1 year prior to the index date (ICD-9-CM: 996.84, V42.6; ICD-10-CM: T86.81, Z94.2, Z94.3, Z4824, Z48280); 8) They died within 28 days after the index date.
Case and reference definition
Thereafter, patients who upgraded to triple therapy or switched to LABA plus LAMA for more than 28 days would be targeted study population. The index date was defined as the first date of escalating to triple therapy or switching to LABA plus LAMA. Patients who upgraded their treatment combinations to triple therapy would be intervention group, and those who switched to LABA plus LAMA would be reference group.
Study outcomes
The composite outcome included all-cause mortality, respiratory-related mortality, moderate and severe COPD exacerbations, acute respiratory failure, pneumonia, and respiratory-related admissions. COPD exacerbation was the primary outcome examined in this study and was classified into two categories: moderate and severe. Moderate exacerbations were defined as outpatient visits for COPD with prescriptions for 3 to 14 days of oral antibiotics or corticosteroids. Severe exacerbations were defined as hospital admissions or emergency department visits for COPD requiring oral or parenteral antibiotics or corticosteroids. Additionally, patients whose inhaled medication regimens were escalated to higher-level combinations were also classified as having severe exacerbations. Secondary outcomes included acute respiratory failure and pneumonia. Acute respiratory failure was identified using ICD-9-CM codes 518.81 - 518.84 and ICD-10-CM code J96. Pneumonia was identified using ICD-9-CM codes 4800 - 4870 and ICD-10-CM codes J12 - J18, A481, A3701, A3711, A3781, A3791, A221, B250, B440, and B7781. Respiratory-related admission was defined as any inpatient hospitalization with a diagnosis of COPD or other COPD-related respiratory diseases and syndromes.
Statistical analysis
Descriptive statistics were used to summarize baseline characteristics. Categorical variables were expressed as percentages and compared using the Chi-square test. Continuous variables were presented as means with standard deviations (SDs) and medians with interquartile ranges (IQRs), analyzed using Student’s t-test or analysis of variance (ANOVA), as appropriate. For non-normally distributed data, nonparametric tests such as the Mann-Whitney U test and Kruskal-Wallis H test were employed.
To assess outcome risks, hazard ratios (HRs) were estimated using Cox proportional hazards models with time-dependent covariates. The multivariable models were adjusted for baseline characteristics, comorbidities, and concomitant medications. The counting process approach was applied to account for time-varying drug combinations and covariates. This approach enabled dynamic modeling of time-to-event data, accommodating censoring and changes in treatment over time. Patients were followed from the cohort entry date. Whenever a patient experienced an outcome or switched treatment regimens, the time frame was partitioned. Each resulting time window was assigned corresponding covariate values, allowing for estimation of time-varying effects.
To validate the findings, a conventional Cox proportional hazards model was performed as a sensitivity analysis. An additional sensitivity analysis excluded patients with asthma-COPD overlap. Subgroup analyses were conducted by stratifying patients based on age (< 65 vs. ≥ 65 years), gender, disease duration, and disease severity to evaluate potential effect modifications on treatment outcomes.
Ethics statement
This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-E(II)-20230260) and conducted in accordance with the principles of the Declaration of Helsinki.
| Results | ▴Top |
Patients characteristics
A total of 539,545 patients were identified in the NHIRD from 2013 to 2021. Eligible patients were aged between 40 and 90 years, had at least one inpatient or outpatient diagnosis of COPD, and had used long-acting bronchodilators or ICS for more than 28 days. After applying exclusion criteria - including incomplete data, history of lung cancer or lung transplantation, no prior use of LABA + ICS, no escalation to either LABA + LAMA or triple therapy, and death within 28 days after the index date - a final study cohort of 20,997 patients was included. Of these, 4,205 patients escalated to LABA + LAMA and 16,792 patients escalated to triple therapy (Fig. 1).
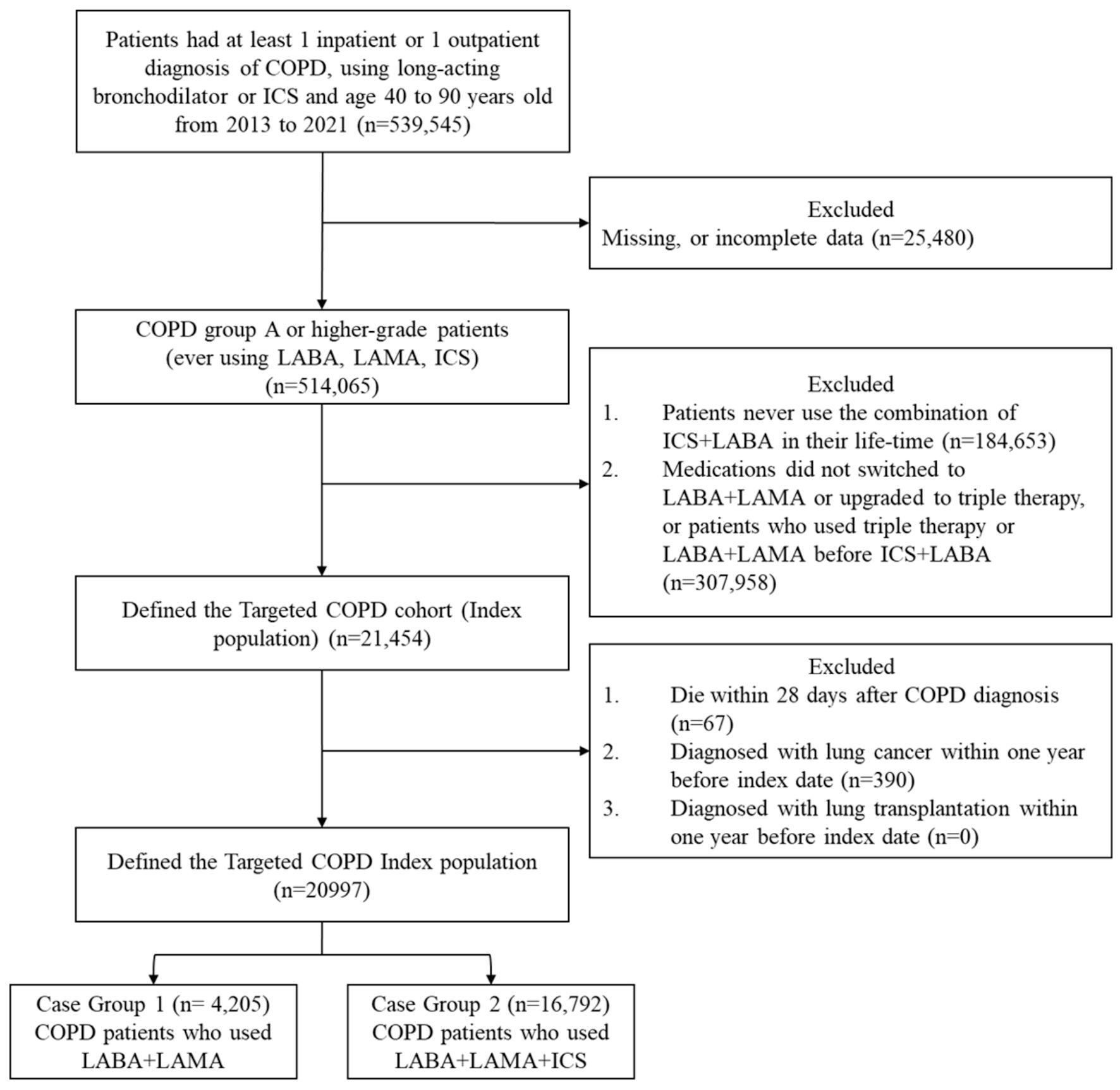 Click for large image | Figure 1. Study flowchart for the composite outcome. COPD: chronic obstructive pulmonary disease; LABA: long-acting beta2-agonist; LAMA: long-acting muscarinic antagonist; ICSs: inhaled corticosteroids. |
The mean age was 66.06 years (SD: 11.54), with patients in the triple therapy group being older on average. There were 12,977 male patients (61.80%) and 8,020 female patients (38.20%), with a higher proportion of males in the LABA + LAMA group. Within 1 year prior to the index date, 16,538 patients (78.78%) had no COPD exacerbation, 2,017 (9.61%) had one exacerbation, and 2,442 (11.61%) had two or more exacerbations. The median disease duration (from cohort entry to index date) was 203.1 days (SD: 275.3). Baseline characteristics are detailed in Table 1.
 Click to view | Table 1. Baseline Characteristics of Study Population |
Outcomes in counting Cox model
As shown in Table 2, patients receiving triple therapy had a significantly higher risk of composite outcomes compared to those on LABA + LAMA (primary outcome 1: aHR = 1.162, 95% CI: 1.098 - 1.230; primary outcome 2: aHR = 1.171, 95% CI: 1.105 - 1.241; both P < 0.0001). Although crude analyses suggested lower risks of all-cause and respiratory-related mortality with triple therapy, these differences were not significant after adjustment (all-cause mortality: aHR = 1.074, P = 0.0694; respiratory-related mortality: aHR = 1.089, P = 0.0762).
 Click to view | Table 2. The Study Outcomes of Counting Cox Model |
Triple therapy was associated with higher risks of severe exacerbations (aHR = 1.346, P = 0.0088), acute respiratory failure (aHR = 1.315, P = 0.0186), pneumonia (aHR = 1.221, P < 0.0001), and respiratory-related admissions (aHR = 1.264, P < 0.0001), compared to LABA + LAMA.
Sensitivity analysis
Table 3 shows results from the conventional Cox model. For both primary outcomes, there were no significant differences between triple therapy and LABA + LAMA (aHR = 1.004 and 1.013). However, triple therapy was associated with higher risks of all-cause mortality (aHR = 1.110) and respiratory-related mortality (aHR = 1.179). It also showed increased risk for moderate exacerbation (aHR = 1.134) but a lower risk for severe exacerbation (HR = 0.324). Findings for acute respiratory failure and respiratory-related admissions were consistent with the counting process Cox model.
 Click to view | Table 3. The Study Outcomes of Conventional Cox Model |
In the sensitivity analysis excluding asthma-COPD overlap syndrome (ACO) patients (Table 4), results remained consistent. Triple therapy showed significantly higher risks for both primary outcome 1 (aHR = 1.174) and primary outcome 2 (aHR = 1.187; both P < 0.0001).
 Click to view | Table 4. Sensitivity Analysis of Population Excluding Asthma-COPD Overlap Patients |
Subgroup analysis
In our subgroup analyses (Supplementary Material 1, jocmr.elmerjournals.com), male patients, those over 65 years old, and those with a longer disease duration had a higher risk of the composite outcome with triple therapy. Regarding disease severity, patients with no exacerbations or more than two COPD exacerbations within 1 year before index date had a lower risk with LABA plus LAMA therapy.
| Discussion | ▴Top |
This retrospective cohort study of 20,997 patients compared triple therapy with LABA + LAMA using a counting-process Cox model. LABA + LAMA was linked to lower short-term risks of acute respiratory failure, severe exacerbations, and pneumonia, consistent with guidelines recommending this combination for managing symptoms and short-term events in COPD [4, 5]. The superior outcomes of LABA + LAMA may be attributed to glucocorticoid receptor (GR) downregulation caused by long-term ICS use [22]. Initially, ICSs effectively bind to GRs, which then modulate gene expression to reduce inflammation [23, 24]. However, prolonged ICS exposure may reduce both the number and sensitivity of GRs through negative feedback, thereby diminishing therapeutic effectiveness [24-26]. This process, known as receptor downregulation, may explain the reduced efficacy of triple therapy over time [27]. Our subgroup analysis further showed that longer disease duration favors LABA + LAMA, offering useful guidance for optimizing COPD treatment.
Previous RCTs have consistently shown greater effectiveness of triple therapy over LABA + LAMA. The ETHOS trial, for example, found that triple therapy (budesonide, glycopyrrolate, and formoterol) significantly reduced moderate or severe exacerbations compared to LABA + LAMA (glycopyrrolate and formoterol) at various doses [28]. Similarly, the IMPACT and TRIBUTE trials also showed that triple therapy was more effective in reducing COPD exacerbations than LABA plus LAMA [8, 9]. However, in our study, LABA + LAMA was linked to lower risks for most outcomes. This contrast may be due to study design differences. While RCTs use an intention-to-treat approach with a fixed 52-week follow-up, our counting process model tracked treatment changes over a 10-year period (2012 - 2022), offering a more realistic and dynamic assessment. By incorporating time-dependent variables, our model better reflects real-world treatment patterns, helping explain the differing results.
The counting Cox model effectively reflected treatment changes over time and better assessed the comparative effectiveness of drug combinations than the conventional Cox model. It also incorporated time-dependent factors such as co-medications and age. Sensitivity and subgroup analyses confirmed the model’s robustness. In contrast, both RCTs and conventional models may be limited by low medication possession ratios (MPRs); in this study, the average MPR was only 33%, well below the 80% threshold, potentially impacting treatment consistency and outcome accuracy [29]. The reason of low MPR might attributed to employment (could not use inhaler while working), smoker, incorrect technique and belief in the necessity of medication [30, 31]. By using the counting Cox model, we could track patients’ adherence simultaneously [19]. If a patient did not have a sufficient degree of adherence, they would have a large proportion of time segments marked as not using any inhaler [16]. Therefore, when analyzing the comparative effectiveness of COPD treatments, counting Cox model was more appropriate.
In sensitivity analysis, the conventional Cox model resembled an intention-to-treat (ITT) setting in RCTs, keeping patients in their initial groups despite treatment changes [32, 33]. Conversely, the counting process model was like an as-treated setting, splitting patients’ treatment journeys into time segments and attributing events to respective treatment groups [34]. Due to the short duration of inhaler effectiveness in COPD, poor symptom control increases the risk of adverse outcomes. The difference in severe exacerbation results between models may be due to two factors. First, the conventional Cox model included treatment escalation as a severe event, potentially affecting classification. Second, patients who stopped inhaler use for over 28 days were censored, possibly before experiencing severe exacerbations, leading to underestimated event rates.
Our analysis had some limitations. Some important information could not be accessed in NHIRD, such as eosinophil levels and lung function indices [35]. With the role of eosinophils becoming more important, considering eosinophil levels could have better evaluated the comparative effectiveness of triple therapy [36-38]. Time segmentation was based on pharmaceutical categories rather than specific drug ingredients to maintain clarity. While ingredient-based classification might offer more detail, it could also introduce complexity due to repeated or overlapping prescriptions. To keep the analysis straightforward, we used three broad treatment categories. Additionally, the study cohort was limited to patients initially on LABA + ICS, a regimen no longer recommended in the 2023 GOLD guidelines [4, 39, 40]. Nevertheless, the combination of LABA plus ICS accounted for a high proportion of total prescriptions in Taiwan [41-43]. Our study also has some limitations, including the lack of data on body mass index and incomplete smoking status information. Both of these factors are known to significantly influence outcomes and mortality in COPD patients, and their absence may affect the overall results and conclusions of the study.
Conclusions
This study applied a Cox model with a counting process to NHIRD data to compare triple therapy and LABA + LAMA in COPD patients. LABA + LAMA was linked to lower risks for short-term outcomes, while triple therapy was more effective for all-cause and respiratory-related mortality, in line with GOLD guidelines. Male patients, those aged 65 or older, and those with longer disease duration were more likely to use LABA + LAMA, possibly due to GR downregulation from long-term ICS use. These findings provide useful insights for optimizing treatment and inhaler selection.
| Supplementary Material | ▴Top |
Suppl 1. The stratified study outcomes of counting Cox model.
Acknowledgments
This study was based in part on data from the NHIRD provided by the Bureau of National Health Insurance (BNHI) of the Ministry of Health and Welfare. We thank the Center for Medical Informatics and Statistics of Kaohsiung Medical University for providing administrative and funding support.
Financial Disclosure
This study was supported by a grant from the Chi-Mei Medical Center and Kaohsiung Medical University Research Foundation (112CM-KMU-03).
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Chih-Yuan Sung: Lead in data curation, analysis, investigation, visualization, and drafting. Chuan-Wei Shen, Ye Gu, Rewadee Jenraumjit, Fu-Shih Chen: Supporting roles in data curation, methodology, and drafting. Chung-Yu Chen: Lead in funding, project administration, software, validation; equal contribution in methodology and supervision; manuscript review and editing. Kuang-Ming Liao: Lead in resources; equal contribution in data curation, project administration, methodology, supervision, and editing.
Data Availability
Data are available from the NHIRD published by Taiwan National Health Insurance (NHI) Bureau. Due to legal restrictions imposed by the government of Taiwan in relation to the “Personal Information Protection Act”, data cannot be made publicly available. Requests for data can be sent as a formal proposal to the NHIRD (http://nhird.nhri.org.tw).
| References | ▴Top |
- Devine JF. Chronic obstructive pulmonary disease: an overview. Am Health Drug Benefits. 2008;1(7):34-42.
pubmed - Organization WH. The top 10 causes of deaths. Accessed February 1, 2024. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- Vogelmeier CF, Roman-Rodriguez M, Singh D, Han MK, Rodriguez-Roisin R, Ferguson GT. Goals of COPD treatment: Focus on symptoms and exacerbations. Respir Med. 2020;166:105938.
doi pubmed - Agusti A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P, Bourbeau J, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J. 2023;61(4).
doi pubmed - Patel N. An update on COPD prevention, diagnosis, and management: The 2024 GOLD Report. Nurse Pract. 2024;49(6):29-36.
doi pubmed - Jenkins C. Drugs for chronic obstructive pulmonary disease. Aust Prescr. 2017;40(1):15-19.
doi pubmed - Mammen MJ, Lloyd DR, Kumar S, Ahmed AS, Pai V, Kunadharaju R, Gupta S, et al. Triple therapy versus dual or monotherapy with long-acting bronchodilators for chronic obstructive pulmonary disease. A systematic review and meta-analysis. Ann Am Thorac Soc. 2020;17(10):1308-1318.
doi pubmed - Papi A, Vestbo J, Fabbri L, Corradi M, Prunier H, Cohuet G, Guasconi A, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391(10125):1076-1084.
doi pubmed - Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, Dransfield MT, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671-1680.
doi pubmed - Ferguson GT, Rabe KF, Martinez FJ, Fabbri LM, Wang C, Ichinose M, Bourne E, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747-758.
doi pubmed - Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, Balter M, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146(8):545-555.
doi pubmed - Suissa S, Dell'Aniello S, Ernst P. Triple inhaler versus dual bronchodilator therapy in COPD: real-world effectiveness on mortality. COPD. 2022;19(1):1-9.
doi pubmed - Barnes PJ, Burney PG, Silverman EK, Celli BR, Vestbo J, Wedzicha JA, Wouters EF. Chronic obstructive pulmonary disease. Nat Rev Dis Primers. 2015;1:15076.
doi pubmed - Abd ElHafeez S, D'Arrigo G, Leonardis D, Fusaro M, Tripepi G, Roumeliotis S. Methods to analyze time-to-event data: the cox regression analysis. Oxid Med Cell Longev. 2021;2021:1302811.
doi pubmed - Olofsson P. Counting process: theory. Wiley StatsRef: Statistics Reference Online.
- Andersen PK, Gill RD. Cox's regression model for counting processes: a large sample study. Ann Stat. 1982;10(4):1100-1120.
doi - Odd A. Nonparametric inference for a family of counting processes. Ann Stat. 1978;6(4):701-726.
doi - Jayaraman R. On minimizing expected warranty costs in 1-dimension and 2-dimensions with different repair options. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=c8be78cdd5823809077ac3db9c1fe6122eb5c5e9.
- Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis-Oudshoorn CGM. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med. 2018;6(7):121.
doi pubmed - Lin LY, Warren-Gash C, Smeeth L, Chen PC. Data resource profile: the National Health Insurance Research Database (NHIRD). Epidemiol Health. 2018;40:e2018062.
doi pubmed - Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, Lai EC. Taiwan's National Health Insurance Research Database: past and future. Clin Epidemiol. 2019;11:349-358.
doi pubmed - Silva CM, Powell-Oliver FE, Jewell CM, Sar M, Allgood VE, Cidlowski JA. Regulation of the human glucocorticoid receptor by long-term and chronic treatment with glucocorticoid. Steroids. 1994;59(7):436-442.
doi pubmed - Witchel SF, DeFranco DB. Mechanisms of disease: regulation of glucocorticoid and receptor levels—impact on the metabolic syndrome. Nat Clin Pract Endocrinol Metab. 2006;2(11):621-631.
doi pubmed - Wilkinson L, Verhoog NJD, Louw A. Disease- and treatment-associated acquired glucocorticoid resistance. Endocr Connect. 2018;7(12):R328-R349.
doi pubmed - Eickelberg O, Roth M, Lorx R, Bruce V, Rudiger J, Johnson M, Block LH. Ligand-independent activation of the glucocorticoid receptor by beta2-adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. J Biol Chem. 1999;274(2):1005-1010.
doi pubmed - Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373(9678):1905-1917.
doi pubmed - Foster JM, Aucott L, van der Werf RH, van der Meijden MJ, Schraa G, Postma DS, van der Molen T. Higher patient perceived side effects related to higher daily doses of inhaled corticosteroids in the community: a cross-sectional analysis. Respir Med. 2006;100(8):1318-1336.
doi pubmed - Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, Trivedi R, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35-48.
doi pubmed - Rolnick SJ, Pawloski PA, Hedblom BD, Asche SE, Bruzek RJ. Patient characteristics associated with medication adherence. Clin Med Res. 2013;11(2):54-65.
doi pubmed - Amini S, Ghasemi A, Solduzian M, Rahimi B, Heidari K, Hadjibabaie M, Kargar M. Is inhaler technique associated with quality of life in patients with chronic obstructive pulmonary disease? Curr Ther Res Clin Exp. 2020;93:100608.
doi pubmed - Hometowska H, Swiatoniowska-Lonc N, Klekowski J, Chabowski M, Jankowska-Polanska B. Treatment adherence in patients with obstructive pulmonary diseases. Int J Environ Res Public Health. 2022;19(18).
doi pubmed - Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res. 2011;2(3):109-112.
doi pubmed - McCoy CE. Understanding the intention-to-treat principle in randomized controlled trials. West J Emerg Med. 2017;18(6):1075-1078.
doi pubmed - Smith VA, Coffman CJ, Hudgens MG. Interpreting the results of intention-to-treat, per-protocol, and as-treated analyses of clinical trials. JAMA. 2021;326(5):433-434.
doi pubmed - Kahnert K, Jorres RA, Behr J, Welte T. The diagnosis and treatment of COPD and its comorbidities. Dtsch Arztebl Int. 2023;120(25):434-444.
doi pubmed - Brusselle G, Pavord ID, Landis S, Pascoe S, Lettis S, Morjaria N, Barnes N, et al. Blood eosinophil levels as a biomarker in COPD. Respir Med. 2018;138:21-31.
doi pubmed - Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R, investigators E. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697-1700.
doi pubmed - Sivapalan P, Bikov A, Jensen JU. Using Blood Eosinophil Count as a Biomarker to Guide Corticosteroid Treatment for Chronic Obstructive Pulmonary Disease. Diagnostics (Basel). 2021;11(2).
doi pubmed - Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT, Thach C, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222-2234.
doi pubmed - Suissa S, Dell'Aniello S, Ernst P. Comparative effectiveness and safety of LABA-LAMA vs LABA-ICS treatment of COPD in real-world clinical practice. Chest. 2019;155(6):1158-1165.
doi pubmed - Wang MT, Lai JH, Huang YL, Liou JT, Cheng SH, Lin CW, Pan HY, et al. Comparative effectiveness and safety of different types of inhaled long-acting beta(2)-agonist plus inhaled long-acting muscarinic antagonist vs inhaled long-acting beta(2)-agonist plus inhaled corticosteroid fixed-dose combinations in COPD a propensity score-inverse probability of treatment weighting cohort study. Chest. 2021;160(4):1255-1270.
doi pubmed - Qiu K, Chen C. Evaluating the epidemiology of osteoporosis in chronic obstructive pulmonary disease and association with inhaled corticosteroid use in Taiwan. Master’s Program, School of Pharmacy, Master Program in Clinical Pharmacy. 2020.
- Liao PA, Pan SW, Chen CY, Deng CY, Dong YH. Prescription patterns of new use of fixed-dose combination inhalers in patients with chronic obstructive pulmonary disease: long-acting beta2 agonists plus long-acting muscarinic antagonists versus long-acting beta2 agonists plus inhaled corticosteroids. Int J Chron Obstruct Pulmon Dis. 2023;18:553-563.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.