| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Review
Volume 17, Number 10, October 2025, pages 537-549
Non-Suicidal Self-Injury: Pain Addiction Mechanisms, Neurophysiological Signatures, and Therapeutic Advances
Yuan Yuan Zenga , Saboor Saeedb, c, d, l
, Shao Hua Hub, c, d, e, f, g, h, i, j, k, l
aSchool of Clinical Medicine, Hangzhou Medical College, Hangzhou, China
bDepartment of Psychiatry, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
cNanhu Brain-Computer Interface Institute, Hangzhou, China
dSchool of Medicine, Zhejiang University, Hangzhou, China
eSchool of Mental Health, Wenzhou Medical University, Wenzhou,China
fZhejiang Key Laboratory of Precision Psychiatry, Hangzhou, China
gBrain Research Institute of Zhejiang University, Hangzhou, China
hZhejiang Engineering Center for Mathematical Mental Health, Hangzhou, China
iDepartment of Biochemistry, Zhejiang University School of Medicine, Hangzhou, China
jMOE Frontier Science Center for Brain Science and Brain-Machine Integration, Zhejiang University, Hangzhou, China
kDepartment of Psychology and Behavioural Sciences, Zhejiang University, Hangzhou, China
lCorresponding Authors: Saboor Saeed and Shao Hua Hu, Department of Psychiatry, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Manuscript submitted July 28, 2025, accepted October 10, 2025, published online October 29, 2025
Short title: Pain Addiction, Neurophysiology, and Treatment in NSSI
doi: https://doi.org/10.14740/jocmr6332
- Abstract
- Introduction
- The Epidemiology of NSSI
- Exploring the Pathogenesis of NSSI
- Neurobiological and Neuroelectrophysiological Features of NSSI
- Therapeutic Interventions for NSSI
- Conclusions
- References
| Abstract | ▴Top |
The aim of this study was to review the neurobiological mechanisms, epidemiology, and therapeutic interventions for non-suicidal self-injury (NSSI), emphasizing the pain addiction model and electroencephalographic biomarkers as frameworks for precision intervention. A narrative review of the literature was conducted using PubMed, Web of Science, CNKI, and Wanfang Data up to October 2025. Search strategy employed the terms “non-suicidal self-injury,” “pain addiction,” “electroencephalography,” “endogenous opioid system,” and “HPA axis.” Selection criteria prioritized original human studies, high-quality systematic reviews, and mechanistic investigations. Pain addiction and electroencephalography (EEG) were selected as focal variables based on their explanatory power: pain addiction elucidates NSSI perpetuation through endogenous opioid-mediated reward sensitization and dopaminergic reinforcement, while event-related potentials (ERPs) provide temporal precision in mapping cognitive-affective dysregulation underlying emotional impulsivity and regulatory deficits. Global adolescent NSSI prevalence averages 17.2%, with Chinese rates reaching 24.7% and trends toward earlier onset. Neurobiological substrates include fronto-limbic dysregulation, hypoactive hypothalamic-pituitary-adrenal (HPA) axis function with blunted cortisol reactivity, and endogenous opioid system alterations producing widespread hypoalgesia. EEG/ERP studies demonstrate increased N2 amplitude with decreased P3 amplitude and prolonged latency during negative stimuli processing, reflecting impaired conflict monitoring and attentional resource allocation. Dialectical behavior therapy shows established efficacy, while repetitive transcranial magnetic stimulation and opioid antagonists demonstrate therapeutic potential. NSSI emerges from neurobiological vulnerability within pain-reward-emotion circuits interacting with psychosocial factors. The pain addiction framework and EEG signatures provide translatable targets for biomarker development and personalized intervention. Future research requires multimodal neuroimaging, longitudinal designs, and genetic integration to establish predictive algorithms and precision therapeutics.
Keywords: Non-suicidal self-injury; Addiction; Endocrine hormone; Pain
| Introduction | ▴Top |
Non-suicidal self-injury (NSSI) refers to a series of deliberate, repeated, and direct behaviors where individuals intentionally harm their own body tissues without suicidal intent, causing mild to moderate physical damage that is socially unacceptable [1-4]. Common methods of NSSI include cutting, hitting, scraping, burning, stabbing, and biting - with cutting (using knives) being the most prevalent practice [1-4]. The functions of NSSI extend beyond pain modulation into multiple dimensions. In terms of emotion regulation, NSSI often functions to rapidly alleviate intense negative emotions. Its neurobiological basis may lie in dysfunctional connectivity within the prefrontal-limbic system (particularly between the dorsolateral prefrontal cortex (PFC) and amygdala), which self-injury may temporarily modulate [5]. Interpersonally, NSSI can function as a non-verbal communication strategy to express distress or influence others; research indicates that interpersonal dysfunction is a core factor in the initiation and maintenance of NSSI [6]. Furthermore, while some individuals with NSSI exhibit heightened pain tolerance, this often stems from “distress overtolerance” developed through chronic suffering and is closely linked to self-punishment motives rather than representing an adaptive mechanism [7].
Given this complex psychosocial background, NSSI constitutes a severe public health challenge. NSSI is recognized as an independent clinical diagnosis in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [4, 8] and can severely harm an individual’s physical and mental health. Furthermore, when its prevalence rises within a population, NSSI may also impair social functioning - for example, by straining healthcare resources and reducing workforce productivity, thereby undermining overall societal efficiency. Epidemiological studies indicate a rising annual incidence of NSSI among adolescents globally in recent years [9]. The World Health Organization identifies NSSI as one of the top five health threats to adolescents.
However, addressing this challenge effectively hinges on a deeper understanding of the mechanisms driving the behavior. While existing research has outlined the psychosocial profile of NSSI, the neurobiological foundations explaining its progression from a coping strategy to a persistent and addictive behavioral pattern remain insufficiently explained. Bridging this knowledge gap from behavioral phenomenon to addictive mechanism is the central aim of this review. Specifically, the “pain addiction” hypothesis serves as a key framework, linking the repetitiveness of NSSI to the rewarding and regulatory roles of the endogenous opioid system (EOS) [1, 10, 11]. Concurrently, electroencephalographic characteristics, particularly event-related potentials (ERPs), provide a dynamic window for investigating NSSI-related deficits in impulse control, emotion regulation, and reward processing at a millisecond temporal resolution [12-14]. Integrating the “pain addiction” hypothesis with objective evidence from electroencephalography (EEG) characteristics offers a novel and powerful perspective for systematically elucidating the neuropsychological mechanisms of NSSI.
Therefore, this review aims to systematically summarize the epidemiological patterns and clinical personality foundations of NSSI, with a focused effort on integrating multi-level evidence surrounding the “pain addiction” framework - spanning neuroendocrine, neurochemical, and neural circuitry mechanisms. It will delve into how EEG/ERP features serve as dynamic biomarkers of cognitive-affective dysfunction in NSSI. Building upon this neuroscientific framework, the review will evaluate current effective psychological and physical interventions and finally propose directions for future research to advance precise diagnosis, treatment, and mechanistic understanding of NSSI.
| The Epidemiology of NSSI | ▴Top |
Incidence of NSSI
NSSI is a global public health issue that spans developmental stages. While adolescents represent a high-risk group, its prevalence in the adult population is also significant. A systematic review [15] synthesizing multiple studies indicates that the global average incidence rate of NSSI among adolescents is approximately 17.2% (Fig. 1). Specific data show detection rates of 17.5% (Fig. 1) in the United States, and a joint survey across 11 European countries indicates that 27.6% (Fig. 1) of adolescents have experienced NSSI. In developing countries, the overall incidence of NSSI ranges from approximately 11.5% to 33.8% [16]. Within this global context, the average incidence rate of NSSI among Chinese adolescents is about 24.7% [9, 17] (Fig. 1).
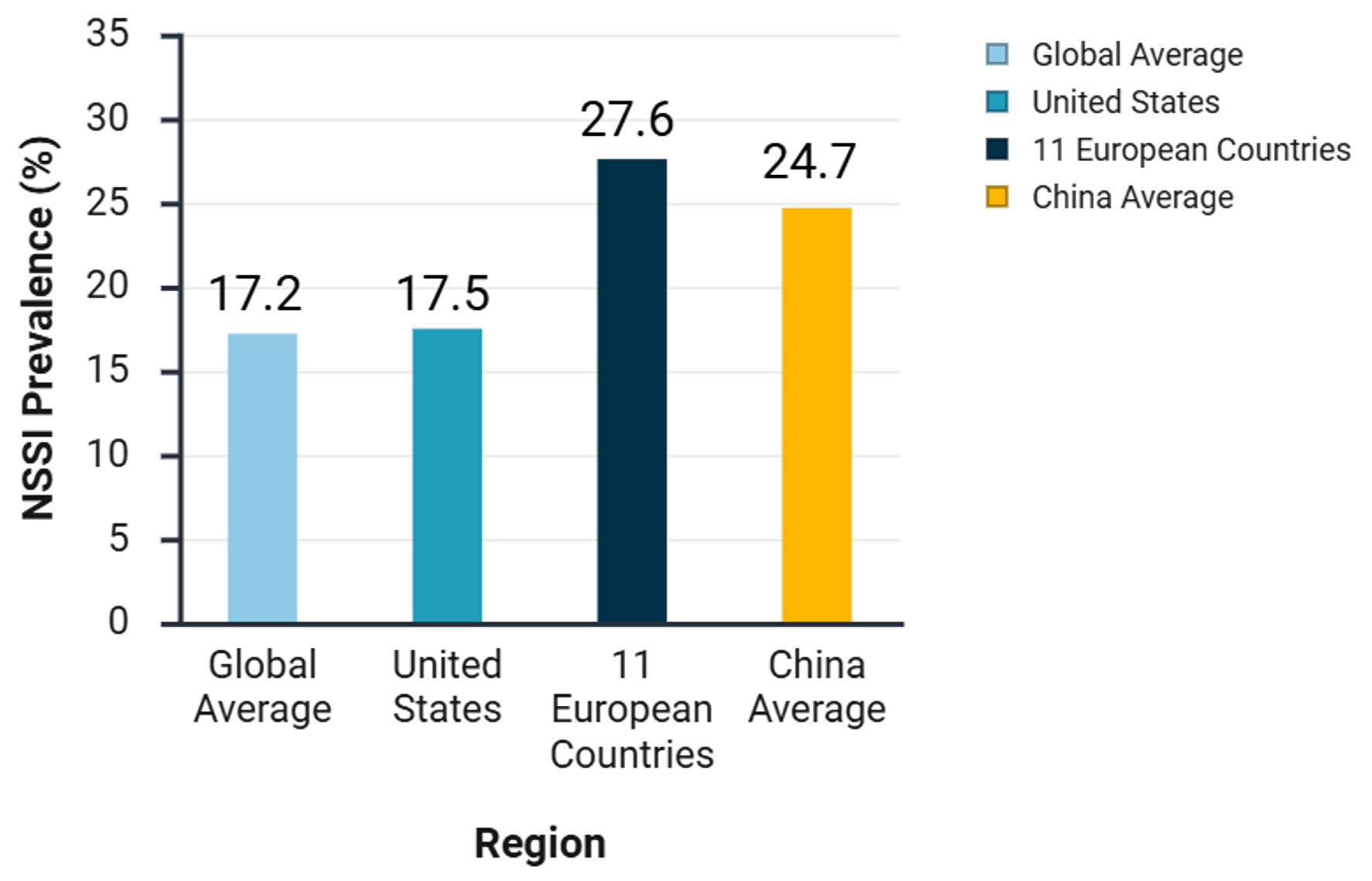 Click for large image | Figure 1. Prevalence of non-suicidal self-injury (NSSI) among adolescents in different regions. |
It is crucial to emphasize that NSSI is not confined to adolescence. Epidemiological research confirms that NSSI behavior is also significant in adult populations. A meta-analysis based on community adult samples found the lifetime prevalence of NSSI to range between 6.0% and 7.8% [18]. In clinical settings, for instance, among adult patients meeting the diagnostic criteria for borderline personality disorder (BPD), the occurrence of NSSI is extremely common, with reported rates reaching as high as 70% to 80% [19]. These data clearly indicate that NSSI is a clinical concern that requires attention across the entire lifespan.
Overall, the global lifetime prevalence of NSSI in non-clinical samples is approximately 13% to 24% [10]. When disaggregated by group, the point prevalence in adolescent non-clinical samples is about 4% [20], while this figure rises sharply to approximately 50% in inpatient adolescent samples [20]. Research on psychiatric samples shows that up to 60% of adolescents have experienced at least one NSSI episode [21], and around 50% engage in repetitive NSSI [21]. Across the lifespan, about 5% to 17% of people engage in NSSI at some point, and these behaviors often persist for several years [22]. The public health impact is severe. A 2017 World Health Organization survey results revealed that self-injurious behavior was already the third leading cause of accidental death among adolescents aged 10 to 19; simultaneously, it is the second leading cause of accidental death among female adolescents and the fifth among male adolescents globally [23].
Epidemiological characteristics of NSSI incidence
The majority of epidemiological investigations indicate that the onset of NSSI frequently occurs during adolescence, with globally high and progressively increasing rates showing a trend toward younger ages [9]. However, NSSI behavior demonstrates both persistence and late onset patterns. Longitudinal studies reveal that a significant proportion of adolescent-onset NSSI continues into adulthood; and additionally, some individuals first engage in NSSI during adulthood. Studies show that an initial onset age under 12 years is a risk factor for more frequent and severe NSSI in the future [9]. A systematic review of longitudinal studies on NSSI found that its prevalence peaks during mid-adolescence (approximately 15 - 16 years) and declines in late adolescence (around 18 years) [21, 24]. For NSSI that persists into adulthood, it is often associated with more severe psychopathology and higher levels of psychological distress [25].
Regarding gender differences, extensive research demonstrates higher NSSI incidence, earlier onset ages, and greater frequency in females compared to males [9, 24]. This pattern of gender disparity is also prevalent in adult samples, with a meta-analysis confirming a significant female-to-male ratio in NSSI risk [26]. In cognitive aspects, some studies indicate that high intelligence quotient (IQ) may be a potential risk factor for NSSI behavior in adolescents [24].
Concerning environmental risk factors, multiple studies confirm that family, school, and social environments significantly influence NSSI behaviors across all age groups [3, 24, 27]. Individuals from incomplete family structures or exposed to adverse parenting styles are at a significantly increased risk. Parental mental states and personality traits also constitute risk factors for adolescent NSSI [3, 28]. Furthermore, childhood trauma, particularly emotional abuse and emotional neglect, is one of the strongest predictors of NSSI [3, 24, 28], with one study reporting that 72% of NSSI patients had experienced emotional abuse [28]. School bullying is another crucial risk factor contributing to adolescent NSSI [3, 28]. For adults, in addition to the long-term effects of early trauma, current stressors such as workplace pressure, intimate relationship breakdowns, and social isolation become significant triggers or maintaining factors for NSSI behavior [29].
Methods and motivations of NSSI
Existing studies have identified 12 forms of NSSI behaviors, including cutting, burning, scratching, head banging, hitting, among others. According to research by Saraff et al [30], approximately 42.3% of individuals engaging in NSSI reported using one method of self-harm, while 28.8% used two methods and 13.5% used three. The distribution of specific self-harm methods was as follows: the most common form was cutting with a sharp object (71.2%), followed by severe scratching (32.7%). Other methods, in descending order of prevalence, included hitting oneself (26.9%), interfering with wound healing (19.2%), biting (15.4%), burning (13.5%), rubbing skin on rough surfaces (13.5%), carving on the body (9.6%), piercing with needles (7.7%), swallowing dangerous objects (7.7%), hair pulling (5.8%), and pinching (3.8%) (Fig. 2).
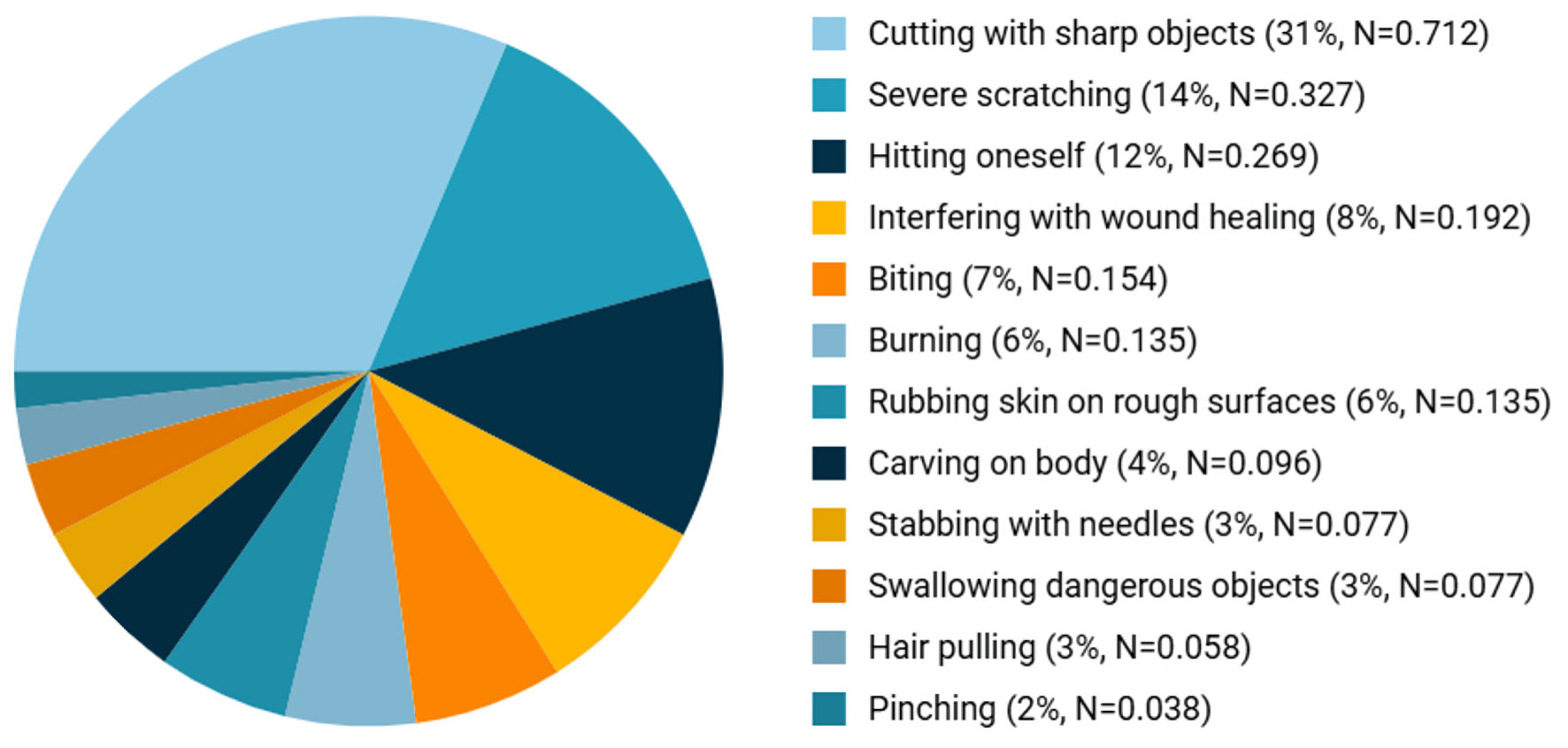 Click for large image | Figure 2. Prevalence distribution of different NSSI methods. NSSI: non-suicidal self-injury. |
Regarding gender differences in NSSI behaviors, it is generally recognized that distinct patterns exist between males and females. Studies conducted among university students indicate that females tend to initiate NSSI at an earlier age and are more likely to use cutting and scratching methods, whereas males show a greater tendency toward burning [3].
The primary motivation for engaging in NSSI is to relieve and manage negative emotions (92.7%), followed by the desire to control or influence others (60.6%) [30].
Personality profiles in clinical populations with NSSI
Individuals with NSSI commonly exhibit significant emotional dysregulation and are prone to persistent negative thought patterns. This combination, frequently exacerbated by interpersonal difficulties, a lack of social support, and deficient problem-solving skills, leads to the use of self-injury as a coping mechanism for intense negative affect. Thus, their behavioral profile is often marked by impulsivity, affective instability, and extreme coping strategies [2, 4, 23].
| Exploring the Pathogenesis of NSSI | ▴Top |
The psychological mechanisms of NSSI behavior addiction
Understanding why NSSI can transition from an episodic behavior to a persistent, addiction-like pattern requires explanatory models that address its complex development and maintenance. Currently, psychologists have proposed several key frameworks, including the experiential avoidance model, the four-function model, and the interaction of person-affect-cognition-execution (I-PACE) model, to explain the underlying mechanisms of NSSI addiction. Additionally, concepts from related fields, such as the “sensitization hypothesis” and “catharsis effect” from suicide research, are often explored for their potential relevance [1].
The experiential avoidance model [31] provides a foundational explanation by focusing on the role of negative reinforcement in escaping aversive emotional states. It posits that NSSI addiction stems from self-administered negative reinforcement, whereby individuals engage in self-harm to escape aversive emotional reactions triggered by external stimuli. The repetition of self-injurious behavior is thus directly reinforced by the subsequent reduction of negative emotional experiences [1]. A critical consideration of this model is its primary focus on negative reinforcement; while powerful, this narrow scope may not fully account for the diversity of motivations, such as seeking sensation or social outcomes, that can also contribute to addictive patterns.
Expanding on the reinforcement concept, the four-function model [32] offers a more nuanced framework by categorizing the motivations that maintain NSSI. It proposes that the behavior is reinforced through either social (interpersonal) or automatic (intrapersonal) events, each operating via negative or positive reinforcement. Specifically, social negative reinforcement involves using self-injury to escape interpersonal demands; social positive reinforcement aims to gain attention or support; automatic negative reinforcement is used to reduce stress and negative emotions; and automatic positive reinforcement seeks to generate desired physiological sensations [1]. Consequently, individuals driven by these combined reinforcement mechanisms may escalate to more frequent and severe self-harm. However, it is crucial to avoid overgeneralization; while these reinforcement processes are central to the behavior’s maintenance, equating them directly with a clinical “addiction” requires caution, as not all reinforced behaviors necessarily meet the full threshold for an addictive disorder.
The I-PACE model [33] conceptualizes NSSI addiction as a behavioral outcome resulting from the complex interaction between a vulnerable individual’s predispositions, affective and cognitive responses to stimuli, and their executive functions (EFs). This model integrates multiple domains, suggesting that for predisposed individuals, core characteristics like emotion dysregulation (affect) and behavioral impulsivity (execution) interact in a feedback cycle that can facilitate a loss of behavioral control, mirroring addictive processes [1]. The I-PACE model’s strength lies in its comprehensive, dynamic framework, but as a relatively new model, its proposed pathways require further empirical validation, particularly in adolescent NSSI populations.
Furthermore, theories from suicide research, namely the “sensitization hypothesis” and “catharsis effect,” are sometimes applied to NSSI addiction by analogy. The sensitization hypothesis suggests that individuals with a history of self-injury become increasingly sensitized to related thoughts and behaviors, lowering the threshold for recurrence upon exposure to triggers [34]. The catharsis effect proposes that if self-injury provides interpersonal support or emotional release, the resultant relief reinforces repetition and intensification, creating a relapse risk similar to substance addiction [24, 35]. It is important to note, however, that these concepts have primarily been supported by research on suicidal behavior; their specific applicability and validity in explaining NSSI addiction in adolescents remain areas for future investigation [1].
In summary, while each model offers a valuable perspective - from negative reinforcement and multifaceted functions to integrated person-environment interactions - they also possess limitations. The field would benefit from research that integrates these views to better delineate the precise pathway from behavioral reinforcement to a clinical addictive state, while remaining cautious about direct overgeneralizations.
Neurobiological mechanisms of NSSI
Fronto-limbic dysregulation and hypothalamic-pituitary-adrenal (HPA) axis dysfunction
The model of cognitive control of emotion (MCCE) posits that the fronto-limbic neural network serves as the key neural circuitry for emotional cognition and regulation [36]. Within this system, subcortical limbic structures (such as the amygdala) generate instinctive emotions, while the PFC is responsible for higher-order cognitive control. Research indicates that both acute and chronic stress can significantly impair the structure and function of the PFC, while simultaneously enhancing amygdala reactivity, by activating specific neurochemical signaling pathways [37]. This attenuation of top-down control from the PFC is a crucial upstream mechanism leading to dysregulation of the downstream stress response system - the HPA axis.
In individuals with NSSI, HPA axis dysfunction often manifests as a “low-activity” state. Chronic psychological stress can drive the HPA axis from an initial compensatory hyperactive state towards eventual functional exhaustion, characterized by reduced basal cortisol levels and blunted stress reactivity [38]. This transition from high to low activity is closely associated with altered glucocorticoid receptor sensitivity, impaired hippocampal function, and a consequent weakening of the negative feedback inhibition on the HPA axis [38]. Neuroinflammation acts as a critical bridge linking chronic stress to HPA axis dysregulation. Studies confirm that chronic stress can activate the NLRP3 inflammasome in microglial cells, promoting the release of pro-inflammatory cytokines, which in turn exacerbate HPA axis dysregulation and associated brain dysfunction [39].
Persistent HPA axis dysregulation exerts multifaceted adverse effects on the brain. Elevated cortisol levels are not only neurotoxic, directly inhibiting hippocampal neuronal function [40], but may also impair astrocyte function, leading to decreased glymphatic system clearance efficiency. This results in the accumulation of toxic waste products in the brain and alterations in white matter structure [41]. These cumulative neurobiological impairments - including PFC hypoactivity, blunted HPA axis reactivity, and neuroinflammation - significantly increase an individual’s vulnerability to stress-related psychological disorders and conditions associated with immunosuppression [28, 36].
Therefore, the observed phenomena of lower cortisol levels and poorer HPA axis reactivity and adaptability in adolescent NSSI patients [28, 40] reflect a coherent pathological progression from neural circuit dysfunction to neuroendocrine dysregulation. Longitudinal studies confirm that adolescents exhibiting such neuroendocrine characteristics, when facing emotional distress and due to an inefficient stress response system, are more likely to develop NSSI behavior as a maladaptive, externalized coping strategy [42].
Pain addiction
1) Pain
Pain is a complex and subjective experience that functions as an early warning mechanism to help individuals avoid physical harm [12]. However, NSSI presents a paradox - individuals actively seek out painful sensations [12]. The key to understanding this paradox lies in examining individual characteristics of pain perception, particularly two core dimensions: pain threshold and pain tolerance [43]. The former refers to the minimum stimulus intensity required to elicit a perception of pain [43], while the latter is the maximum intensity of pain an individual can endure [43]. A substantial body of empirical research consistently finds that, compared to healthy individuals, those who engage in NSSI show significant alterations in both dimensions, manifesting as a general reduction in pain sensitivity. This provides a direct physiological basis for understanding how self-injurious behavior can be carried out [44].
This altered pain sensitivity is considered one of the core factors in the onset and maintenance of NSSI behavior [12]. Evidence supporting this view shows that individuals with NSSI generally exhibit higher pain thresholds and greater pain tolerance than the average population [45]. More importantly, this characteristic of pain sensitivity is not merely a consequence of the behavior; it may also play a role as a precursor risk marker. A longitudinal study confirmed that a higher baseline pain threshold effectively predicts more frequent NSSI behaviors in adolescents 1 year later, establishing a sequential and predictive relationship between altered pain perception and NSSI [11]. The blunting of pain perception weakens the body’s natural barrier against self-harm, a mechanism that also partly explains why reduced pain sensitivity is associated with a higher risk of suicide death [12]. Further in-depth analysis reveals that the alteration in pain perception among individuals with NSSI follows a specific pattern: they show reduced responsiveness to sharp, physical pain stimuli but heightened sensitivity to diffuse, psychosocially sourced “emotional pain.” This dissociation between physical and psychological pain perception creates a powerful behavioral motivation - to generate controllable, perceptible physical pain as a means of diverting or substituting for unbearable and uncontrollable emotional pain [46].
Why, then, do individuals with NSSI exhibit such characteristic changes in pain regulation? Current theories primarily offer explanations at the neurochemical and neural circuit levels [17]. At the neurochemical level, the predominant hypothesis suggests that NSSI behavior can trigger the release of endogenous opioids (such as endorphins), thereby producing analgesic effects during the act [17]. Recent neurobiological research has significantly deepened this understanding, clearly identifying the EOS, particularly the µ-opioid receptor, as a molecular hub integrating pain modulation and negative emotion regulation. An elegant animal model study, using single-neuron-level circuit tracing and manipulation techniques, revealed that specific activation of µ-opioid receptors on GABAergic neurons in the central amygdala efficiently blocks the transmission of pain signals via a neural circuit termed the “CEA-PB disinhibition” pathway. This provides a precise neural circuit mechanism explaining the endogenous analgesia observed in NSSI [47]. At the neural circuit level, it is widely believed that brain regions involved in pain recognition, emotional evaluation, and behavioral control (such as the anterior cingulate cortex, insula, and PFC) exhibit functional or structural abnormalities [17]. Dysregulation in these regions may collectively weaken the protective negative feedback typically triggered by pain signals, allowing self-injury to persist despite insufficient physiological warning [17].
2) Addiction
a) The EOS hypothesis and supporting evidence
The EOS, a key neurochemical system involved in pain and emotion regulation, is recognized as a critical mediator of emotional regulation in self-injury [36]. This system consists of endogenous opioid peptides (including endorphins, enkephalins, dynorphins, and endomorphins) and their receptors (µ, δ, and κ receptors), which exert physiological effects through specific peptide-receptor interactions [36]. Research on patients with NSSI indicates that their elevated pain threshold and tolerance are closely associated with the release of endogenous opioid peptides in the cerebrospinal fluid [28]. Specifically, levels of β-endorphin and enkephalin are significantly lower in the cerebrospinal fluid of NSSI patients at rest compared to healthy controls but increase markedly following self-injury [28]. This dynamic alteration suggests a causal relationship between NSSI behavior and the release of endogenous opioid peptides, providing experimental support for the opioid system hypothesis [28, 36].
b) The addiction mechanism: from receptor sensitization to behavioral tolerance
The opioid system hypothesis elucidates the neurobiological mechanism underlying NSSI addiction. It posits that low resting-state levels of β-endorphin and enkephalin in NSSI patients lead to compensatory sensitization (receptor hypersensitivity) of µ and δ receptors [36]. When tissue damage from self-injury triggers the release of β-endorphin and enkephalin, these opioids bind to the hypersensitive µ and δ receptors, producing intense euphoria and satisfaction [36]. Notably, the activation of µ and δ receptors is intrinsically associated with reward, hedonic properties, and analgesia, a mechanism that helps alleviate negative affect and enhance pleasurable sensations [1, 22, 36]. With repeated NSSI acts, opioid tolerance develops [1, 10], necessitating more severe or frequent self-injury to achieve equivalent emotional regulation, thereby forming a vicious cycle of “tolerance-behavioral escalation” that ultimately transforms NSSI into an addictive behavior [1, 10].
c) Involvement of the neural reward circuitry
From a behavioral perspective, addictive behavior is defined as inappropriate, recurrent pleasurable-yet-harmful actions driven by irresistible cravings and poorly controlled impulses [9, 22]. Because NSSI provides immediate emotional relief, the behavior becomes reinforced through repetition, forming a typical reward behavior [9, 22]. Neuroimaging studies indicate that adolescent NSSI is closely linked to functional alterations in the neural reward circuitry [1, 17, 48]. This circuit involves multiple brain regions, including the frontal lobes, ventral tegmental area (VTA), nucleus accumbens (NAc), striatum, orbitofrontal cortex (OFC), and cingulate gyrus [1, 17, 48]. Dopamine, serving as the key reward neurotransmitter, acts through four major pathways (mesolimbic, mesocortical, nigrostriatal, and tuberoinfundibular pathways) originating primarily from the VTA and substantia nigra [1, 17, 48]. Importantly, a recent study first demonstrated enhanced reward-related brain activity (e.g., in the bilateral putamen) in adolescents with NSSI ideation, indicating that heightened reward circuit activity, coupled with prefrontal cortical limitations, increases vulnerability to NSSI [1, 17, 48].
d) Opioid-dopamine system interactions
The EOS plays a critical role in the development and maintenance of addictive behavior [1, 17, 48]. Clinical research shows that pain stimuli activate not only traditional pain-processing regions but also EOS-related areas (e.g., thalamus, caudate nucleus, globus pallidus) in adolescents with NSSI compared to controls [1, 17, 48]. Crucially, pain-triggered opioid receptor activation within the reward circuit elevates dopamine levels, generating pleasure and reducing suffering [1, 17, 48]. Prolonged pleasure induction leads to dopamine system desensitization, compelling individuals to repeat or intensify self-injury to maintain dopamine levels, thus establishing an addictive cycle [1, 17, 48]. Abnormal EOS function causing reward system hyperactivation sustains recurrent NSSI [1, 17, 48].
e) Psychological reinforcement mechanisms and clinical assessment
Research indicates that adolescents with NSSI exhibit impaired emotional regulation and increased susceptibility to self-injury-related stimuli [1, 49]. As long as self-injury immediately reduces negative affect, the tendency to engage in this behavior strengthens during subsequent distress [10]. Regarding psychological mechanisms, NSSI is maintained primarily through automatic negative reinforcement (e.g., reduction of anger, tension) and automatic positive reinforcement (e.g., inducing pleasure or feeling “alive”), with automatic negative reinforcement being the most commonly reported function [21].
In summary, the aforementioned psychosocial risk factors - such as family conflict, childhood trauma, and school bullying - are not isolated events. Rather, they contribute to the pathogenesis of NSSI by inducing core neurobiological alterations, including prefrontal-limbic system dysregulation, a hypoactive HPA axis state, and EOS dysfunction. These alterations collectively form the key pathological foundation for the transition of NSSI from an emotion-regulating behavior to an addictive pattern.
| Neurobiological and Neuroelectrophysiological Features of NSSI | ▴Top |
NSSI involves co-occurring abnormalities in the brain’s emotion, pain, and reward systems. Neuroimaging studies have revealed alterations in brain structure and functional connectivity underlying these abnormalities, while EEG and ERPs, with their high temporal resolution, provide dynamic electrophysiological evidence for these dysfunctions.
Impaired emotion regulation: neural basis and electrophysiological correlates
Emotion dysregulation is considered a core driver of NSSI. Its neural basis involves a key network where the amygdala serves as a central node for emotional responses, and the PFC (particularly the dorsal anterior cingulate and orbitofrontal gyri) is responsible for top-down regulation [4, 28, 50]. Studies have found that adolescents with NSSI commonly exhibit weakened functional connectivity in the amygdala-prefrontal pathway, alongside aberrant hyperconnectivity between the amygdala and the supplementary motor area [36, 50]. This aberrant connectivity signifies an imbalance between emotional impulse and behavioral control. For instance, when viewing NSSI-related images, patients show reduced amygdala activation coupled with enhanced activity in the cingulate gyrus and orbitofrontal gyrus, suggesting disrupted emotion evaluation and regulation processes [28].
EFs, mediated by the PFC and particularly inhibitory control, are crucial cognitive components for effective emotion regulation. Significant EFs deficits have been identified in adolescents with NSSI, with the low-frequency self-injury group showing impaired inhibitory control and the high-frequency group exhibiting working memory impairments [12, 14, 51]. ERP technology can precisely capture transient abnormalities in these cognitive processes. The N2 component (a negative wave around 200 ms post-stimulus) reflects conflict monitoring and inhibitory control processes originating from the anterior cingulate cortex and inferior frontal gyrus. Studies reveal that the NSSI group exhibits significantly higher N2 amplitudes compared to healthy controls [12, 14], indicating the need to mobilize more cognitive resources to inhibit impulses when processing negative stimuli, which reflects inefficient inhibitory control. The subsequent P3 component (a positive wave peaking around 300 ms) is associated with attention allocation and higher-order cognitive evaluation, reflecting inhibitory control processing originating from the anterior cingulate and orbitofrontal cortex [13]. Research finds that the NSSI group demonstrates significantly increased P3 latency and significantly reduced P3 amplitude [12, 14, 51]. These abnormalities in both N2 and P3 collectively demonstrate deficits in both the early monitoring and late evaluation stages of emotional stimulus processing in NSSI individuals, providing robust neuroelectrophysiological evidence for their emotion regulation difficulties.
Neurofunctional reorganization of pain perception and processing
NSSI behavior involves self-inflicted pain stimuli, yet patients often display reduced pain sensitivity. This paradox is underpinned by profound changes in the brain’s pain processing pathways. Neuroimaging studies suggest that during self-injury, the insula, which is responsible for the affective component and subjective perception of pain, shows functional dysregulation. Concurrently, the dorsolateral PFC (dlPFC) becomes overactivated and, together with abnormal amygdala-prefrontal-thalamic connectivity patterns, creates a “top-down” inhibitory effect that suppresses insular processing of nociceptive signals [12, 17]. This mechanism potentially blocks normal pain signal transmission, thereby disrupting pain processing, increasing pain tolerance, and reducing pain sensitivity in patients [12, 17].
Evidence from brain structure further supports this functional abnormality. A previous study using voxel-based morphometry (VBM) revealed significant reductions in gray matter volume (GMV) in the right anterior insula, bilateral secondary somatosensory cortex, and left inferior frontal gyrus in patients with NSSI [45]. These brain regions, particularly the insula and the secondary somatosensory cortex, are core areas for pain perception and integration. This finding indicates that structural abnormalities in these key regions may constitute a neurobiological substrate, altering patients’ perception and regulation of painful stimuli and thereby predisposing them to rely on intense stimulation for emotion regulation. However, it must be emphasized that these structural differences should be interpreted as a risk factor rather than a deterministic cause. The eventual manifestation of NSSI behavior likely stems from a complex interplay between this aberrant pain-emotion regulation circuitry and other factors involving cognitive control, emotional processing, and the environment.
Reward circuit dysfunction and addictive reinforcement of behavior
NSSI behavior exhibits addictive characteristics, maintained by abnormal responses in the brain’s reward system. Following self-injury, patients experience transient emotional relief through automatic negative reinforcement (rapid alleviation of negative affect) and positive reinforcement (experience of pleasure or satisfaction). This powerful reward effect leads to repetition and habituation of the behavior [12]. Research indicates that the neural reward circuit associated with NSSI shows altered brain activity and connectivity early in the course of the behavior among adolescents, and this abnormality may continuously reinforce self-injurious behavior [12].
ERP studies offer valuable insights into the neurophysiological activity during reward processing. Feedback-related negativity (FRN), also termed reward positivity (RewP), is an objective electrophysiological index for assessing the initial reactivity to reward outcomes, while the P3 component reflects later attention allocation and motivational salience. Studies have found that compared to controls, the NSSI group demonstrates a larger neural differentiation between responses to rewards and losses, exhibiting a more positive RewP to rewards and a more negative RewP to losses [12]. This heightened reward sensitivity suggests that dysfunctional reward processing is a core neurobiological driver underlying the maintenance and reinforcement of NSSI behavior.
| Therapeutic Interventions for NSSI | ▴Top |
Psychotherapy
Dialectical behavior therapy (DBT)
DBT for adolescents is an evidence-based, multicomponent intervention designed to reduce NSSI and suicidal behaviors in youth through systematic training in emotion regulation, distress tolerance, interpersonal effectiveness, and mindfulness [8]. The treatment follows a structured stage-based model, initially focusing on behavioral stabilization and elimination of life-threatening behaviors, then addressing comorbid conditions to improve overall functioning [8] (Table 1).
 Click to view | Table 1. NSSI Treatments Comparison |
Linehan et al (2015) confirmed sustained therapeutic effects up to 12 months post-treatment [52]. Neuroimaging studies further validate DBT’s mechanisms, showing enhanced PFC activation and reduced amygdala reactivity on functional magnetic resonance imaging (fMRI), indicating normalized emotion regulation circuitry [4, 24].
The intervention employs five core strategies (dialectical, core, communication, case management, and structural) across four delivery modalities (individual therapy, skills groups, phone coaching, therapist consultation teams) [8]. DBT significantly improves emotion regulation, enhances social adaptation, and reduces treatment dropout rates by more than 50% compared to conventional approaches [4, 24] (Table 1).
Acceptance and commitment therapy (ACT)
ACT is an innovative cognitive-behavioral intervention that enhances psychological flexibility through six core processes: self-acceptance, present-moment awareness, value clarification, cognitive defusion, committed action, and mindful engagement. This approach empowers individuals to embrace distressing internal experiences, reconnect with personal values, disentangle from negative thoughts and emotions, and cultivate present-focused living. By teaching patients to accept psychological discomfort without avoidance, ACT disrupts depressive thought patterns, reduces self-injury ideation, and improves life quality. It enables individuals to respond adaptively to negative feelings/thoughts rather than relying on self-harm, thereby fostering positive behavioral changes, enhancing self-awareness, and effectively ameliorating NSSI [53, 54] (Table 1).
Physical interventions
Repetitive transcranial magnetic stimulation (rTMS)
rTMS is a noninvasive neuromodulation technique that alters brain function through electromagnetic induction: pulsed currents in scalp-positioned coils generate magnetic fields penetrating skull barriers, inducing electrical currents in targeted cortical regions. This neuronal depolarization modulates cortical excitability, neuroplasticity, and neurotransmitter dynamics (e.g., dopamine release) [28, 36, 55]. rTMS reduces adolescent NSSI by normalizing hypo-/hyper-activated neural circuits [28], improving neurocognitive markers predictive of self-injurious behaviors. Its advantages include noninvasiveness, outpatient applicability, minimal side effects, and significant reductions in self-injury frequency/severity within 2 weeks post-treatment. Combined with pharmacotherapy, rTMS demonstrates superior efficacy in lowering NSSI risk without adverse reactions like skin irritation or headaches [56] (Table 1).
Pharmacotherapy
Opioid receptor antagonists naltrexone (NTX) and buprenorphine (BUP) have shown potential in the treatment of NSSI and may be particularly beneficial for patients with strong impulsivity or emotional dysregulation [1]. NTX acts by blocking µ, δ, and κ opioid receptors, thereby attenuating the role of the opioid system in the pain-reward circuit. Studies suggest that low-dose NTX may reduce self-injurious behaviors in specific NSSI patient populations, but its overall efficacy still requires confirmation through more large-scale studies [1]. Clinically, it is important to note that NTX may cause adverse effects such as nausea and headache and may precipitate withdrawal symptoms in opioid-dependent individuals; therefore, a thorough assessment is necessary before initiating treatment [57]. BUP, as a partial µ-opioid receptor agonist and κ-opioid receptor antagonist, has demonstrated certain tolerability and safety in low-dose regimens for improving comorbid anxiety, depression, and self-injurious behaviors [2]. A preliminary controlled trial confirmed that ultra-low-dose sublingual BUP may help reduce suicidal ideation, but its role in the treatment of primary NSSI is not yet fully established [58]. It is crucial to be aware that the combination of BUP and benzodiazepines carries a risk of synergistic respiratory depression and should be strictly avoided in clinical practice [59] (Table 1).
Lithium is considered to potentially have a specific “anti-suicidal effect” in NSSI patients comorbid with bipolar disorder, and its mechanism may be partially mediated by the EOS [1]. However, it must be strongly emphasized that the use of lithium in adolescent NSSI patients requires extreme caution and should be strictly limited to specific circumstances. Lithium is by no means a first-line or conventional medication for NSSI; its use should be restricted to complex cases comorbid with bipolar disorder where other interventions have been ineffective. Evidence from large systematic reviews indicates that lithium has a clear preventive effect on suicidal behavior in patients with mood disorders [60]. This is primarily due to its narrow therapeutic window (effective concentration 0.4 - 0.8 mmol/L, with toxicity occurring at > 1.2 mmol/L) and significant safety risks, including renal impairment, hypothyroidism, tremor, cognitive slowing, and weight gain [61]. Clinical guidelines (such as NICE) mandate that before initiating lithium therapy in adolescents, a comprehensive physical assessment (including renal function, thyroid function, and electrocardiogram) must be conducted. During treatment, strict monitoring of serum lithium levels, regular renal and thyroid function tests are essential, and it is imperative to ensure that patients and their guardians provide fully informed consent after understanding the potential risks and therapeutic benefits [62]. Recommending lithium for adolescent NSSI patients without a clear diagnosis of bipolar disorder is inappropriate (Table 1).
Atypical antipsychotics such as aripiprazole and ziprasidone have shown moderate efficacy in reducing self-injurious behaviors in some cases and may be suitable for NSSI patients with psychotic symptoms, severe agitation, or borderline personality traits [63]. Aripiprazole stabilizes the dopamine-serotonin system by partially agonizing D2, D3, D4, and 5-HT1A receptors while antagonizing 5-HT2A receptors. Ziprasidone has similar D2/5-HT2A receptor antagonism and carries a relatively lower risk of weight gain; its additional norepinephrine and serotonin reuptake inhibition may provide benefits for anxiety and mild depression [2]. It is important to note that atypical antipsychotics are associated with significant risks of metabolic abnormalities, including weight gain, and disturbances in glucose and lipid metabolism. Strict monitoring is necessary during long-term use in children and adolescents [64] (Table 1).
Antidepressants such as fluoxetine and venlafaxine have also been reported to reduce self-injurious behaviors in NSSI patients comorbid with depressive disorders [63]. Fluoxetine, a widely used selective serotonin reuptake inhibitor (SSRI), generally has relatively good tolerability due to its low affinity for adrenergic, histaminergic, and cholinergic receptors. Venlafaxine, a serotonin-norepinephrine reuptake inhibitor (SNRI), may offer unique advantages in treating patients with comorbid depression and significant somatic pain symptoms [2]. It is crucial to emphasize that SSRIs/SNRIs carry a black box warning for use in children and adolescents, particularly during the initial treatment phase, due to the potential increased risk of anxiety, agitation, and even suicidal ideation. Therefore, the starting dose should be low, and close clinical monitoring is required [65] (Table 1).
Neurofeedback combined with sertraline has been investigated as a treatment approach for NSSI [66]. Sertraline, an SSRI antidepressant, often has limited efficacy as monotherapy for the core symptoms of NSSI, with low remission rates. Neurofeedback technology aims to enhance cognitive control function and alleviate depressive symptoms, potentially producing synergistic effects when combined with sertraline. Some studies have explored the combination of sertraline with low-dose olanzapine [67, 68]. Olanzapine exerts mood-stabilizing effects by antagonizing various receptors including 5-HT2A, D1, D2, and D4. Current evidence suggests that combination therapy may produce synergistic receptor blockade effects, potentially improving treatment outcomes in some refractory NSSI patients. However, this combination strategy, particularly when involving olanzapine, significantly increases the risk of metabolic side effects (such as weight gain, abnormalities in blood glucose and lipids) and must be used cautiously with careful monitoring after weighing the risks and benefits [64] (Table 1).
| Conclusions | ▴Top |
This review systematically explores the neurobiological mechanisms of “pain addiction” and the associated electroencephalographic characteristics in NSSI. Through an integrative analysis of existing evidence, we derive the following core conclusions: the persistence of NSSI behavior hinges critically on the development of an addiction-like pattern [1, 10]. The core neurochemical basis of this process is dysfunction of the EOS. Research indicates that individuals with NSSI have low resting-state levels of β-endorphin and enkephalin, leading to compensatory sensitization of µ- and δ-opioid receptors. The release of endogenous opioids triggered by self-injury binds to these sensitized receptors, producing intense euphoria and satisfaction, thereby driving behavioral repetition through positive reinforcement mechanisms [11, 28, 36]. With repeated behavior, opioid tolerance develops, compelling individuals to seek equivalent emotion-regulating effects through more frequent or severe self-injury, ultimately forming a vicious cycle of “tolerance-behavioral escalation” that transforms NSSI into a difficult-to-control addictive behavior [1, 10].
At the neural circuit level, this addictive pattern is supported by abnormal activity in the reward system and emotion regulation network. Painful stimuli not only activate traditional pain-processing brain regions but, more crucially, activate areas related to the opioid system and reward (e.g., thalamus, caudate nucleus). Through opioid-dopamine system interactions, this increases dopamine levels in areas such as the nucleus accumbens, generating pleasure and reducing suffering [1, 17, 48]. Simultaneously, functional dysregulation of the prefrontal-limbic system (e.g., insufficient prefrontal regulation and amygdala over-reactivity), leading to emotion dysregulation, is a key prerequisite prompting individuals to rely on this extreme method for rapid relief from negative emotions [5, 28, 36].
EEG and ERP studies provide high temporal resolution electrophysiological evidence for this. Individuals with NSSI show increased N2 amplitude and reduced P3 amplitude/prolonged P3 latency when processing negative stimuli [12, 14]. These features indicate efficiency deficits in both early conflict monitoring and inhibitory control (N2) and later-stage higher-order cognitive evaluation and attentional allocation (P3) [13, 14]. Furthermore, during reward processing, they demonstrate greater neural differentiation between responses to rewards and losses (e.g., RewP), revealing abnormal reward sensitivity [12]. These EEG/ERP characteristics collectively constitute dynamic biomarkers of cognitive-affective dysfunction in NSSI, providing objective neuroelectrophysiological evidence for its impulsivity, emotion dysregulation, and addictive properties.
Based on the aforementioned mechanisms, targeted interventions show promising potential. These include enhancing prefrontal regulatory function through DBT [8, 12], blocking the addictive circuit using opioid receptor antagonists (e.g., NTX) [1, 57], and modulating abnormal neural activity using rTMS [28].
In summary, NSSI can be conceptualized as an addictive behavioral disorder driven by the complex interplay of the EOS, the dopaminergic reward circuit, and the prefrontal-limbic emotion regulation circuitry. The neurochemical model of “pain addiction”, combined with specific EEG/ERP features, provides a powerful integrative framework for understanding its intractability and relapse tendency. Future research should focus on longitudinally tracking the evolution of these neurobiological markers and exploring precision intervention strategies targeting these mechanisms, with the ultimate goal of improving clinical outcomes for individuals with NSSI.
Acknowledgments
The study was supported by funding from Fundamental Research Funds for the Central Universities (226-2022-00193, 226-2022-00002), the Leading Talent of Scientific and Technological Innovation - “Ten Thousand Talents Program” of Zhejiang Province (No. 2021R52016), the National Natural Science Foundation of China (32222023 and 32100598), the Natural Science Foundation of Zhejiang Province (LR22C070002), and A Project Supported by Scientific Research Fund of Zhejiang Provincial Education Department (Y202353713).
Financial Disclosure
None of the authors received any payment from a pharmaceutical company or other agency for writing this article.
Conflict of Interest
None to declare.
Author Contributions
YYZ drafted the initial manuscript and write the final manuscript. YYZ and SS revised the manuscript. YYZ and SS helped in data collection and study design. SHH supervised the manuscript preparation and provided critical feedback on errors and revisions.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Lei JL, Hu L, Qiu CJ. Research progress on the addictive attributes of non-suicidal self-injury behavior in adolescents. Chinese Journal of Nervous and Mental Diseases. 2023;49(09):559-564.
- Zeng QL, Wang GM. Research progress on the clinical efficacy of psychotropic drugs in the treatment of non-suicidal self-injury. Injury Medicine (Electronic Edition). 2021;10(04):47-53.
- Lin MJ, Li P, Lu QH. Research status of non-suicidal self-injury in adolescents. Journal of Psychiatry. 2018;31(01):67-70.
- Li HZ, Kuang L. Research progress on the efficacy of dialectical behavior therapy for non-suicidal self-injury: Evidence from brain functional imaging. Sichuan Mental Health. 2021;34(03):280-284.
- Wu D, Wang S, Wang H, Zhang J, Tian Y. Neural mechanisms and neuromodulation therapies for non-suicidal self-injury. Mol Psychiatry. 2025;30(11):5513-5522.
doi pubmed - Fox KR, Millner AJ, Mukerji CE, Nock MK. Examining the role of sex in self-injurious thoughts and behaviors. Clin Psychol Rev. 2018;66:3-11.
doi pubmed - Chung H, Kim G, Kim DI, Hur JW. The double-edged sword of distress tolerance: Exploring the role of distress overtolerance in nonsuicidal self-injury. Compr Psychiatry. 2025;141:152610.
doi pubmed - Cui JL, Shu YP, Zhou C, et al. Research progress on the application of dialectical behavior therapy for non-suicidal self-injury in adolescents. Journal of Psychiatry. 2021;34(06):561-564.
- Sun Q, Li YL, Ye LX, et al. Research progress on risk factors and pathogenesis of non-suicidal self-injury in adolescents. New Medicine. 2023;54(01):17-21.
- Liu RT. Characterizing the course of non-suicidal self-injury: a cognitive neuroscience perspective. Neurosci Biobehav Rev. 2017;80:159-165.
doi pubmed - Bresin K, Gordon KH. Endogenous opioids and nonsuicidal self-injury: a mechanism of affect regulation. Neurosci Biobehav Rev. 2013;37(3):374-383.
doi pubmed - Yao KK, Si XY, Ye LX. Research progress on electrophysiology related to neurocognitive function in adolescents with non-suicidal self-injury. Chinese Journal of Contemporary Pediatrics. 2023;25(06):653-657.
- Wei X, Lu XY, Yang P. Intervention effect of personalized intervention strategies adjusted based on P300 event-related potential on non-suicidal self-injury behavior in adolescents. Psychology Monthly. 2025;20(08):75-77.
- Zhao L, Zhou D, Ma L, Hu J, Chen R, He X, Peng X, et al. Changes in emotion-related EEG components and brain lateralization response to negative emotions in adolescents with nonsuicidal self-injury: An ERP study. Behav Brain Res. 2023;445:114324.
doi pubmed - Li ZY, Wang GM, Ban C, et al. Research progress on the current situation and risk factors of non-suicidal self-injury in adolescents. Journal of International Psychiatry. 2021;48(04):586-589.
- Chen X, Chen H, Liu J, Tang H, Zhou J, Liu P, Tian Y, et al. Functional connectivity alterations in reward-related circuits associated with non-suicidal self-injury behaviors in drug-naive adolescents with depression. J Psychiatr Res. 2023;163:270-277.
doi pubmed - Huan ZS, He XB, Du N, et al. Research progress on the pathogenesis and treatment of non-suicidal self-injury in adolescents. Chinese Journal of School Health. 2023;44(04):636-640.
- Lim KS, Wong CH, McIntyre RS, Wang J, Zhang Z, Tran BX, Tan W, et al. Global lifetime and 12-month prevalence of suicidal behavior, deliberate self-harm and non-suicidal self-injury in children and adolescents between 1989 and 2018: a meta-analysis. Int J Environ Res Public Health. 2019;16(22).
doi pubmed - Mendez-Miller M, Naccarato J, Radico JA. Borderline Personality Disorder. Am Fam Physician. 2022;105(2):156-161.
pubmed - Murner-Lavanchy I, Koenig J, Lerch S, van der Venne P, Hoper S, Resch F, Kaess M. Neurocognitive functioning in adolescents with non-suicidal self-injury. J Affect Disord. 2022;311:55-62.
doi pubmed - Brown RC, Plener PL. Non-suicidal self-injury in adolescence. Curr Psychiatry Rep. 2017;19(3):20.
doi pubmed - Chen YL, Yan WS. Neurobiological mechanisms and research progress of non-suicidal self-injury behavior. China Journal of Health Psychology. 2022;30(04):635-640.
- Zhang C, Liao XL, Ou JJ, et al. Research progress on dialectical behavior therapy for non-suicidal self-injury behavior. Journal of Xinxiang Medical University. 2022;39(12):1187-1191.
- Wang Y, He WZ, Liang LJ, et al. Application of dialectical behavior therapy in non-suicidal self-injury behavior among adolescents. Sichuan Mental Health. 2021;34(01):92-96.
- Fox KR, Franklin JC, Ribeiro JD, Kleiman EM, Bentley KH, Nock MK. Meta-analysis of risk factors for nonsuicidal self-injury. Clin Psychol Rev. 2015;42:156-167.
doi pubmed - Bresin K, Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: A meta-analysis. Clin Psychol Rev. 2015;38:55-64.
doi pubmed - Wang MJ, Zuo Y, Tang YX, et al. Relationship between non-suicidal self-injury behavior and anger emotion, family dynamics in adolescents: Based on latent profile analysis. Journal of Psychiatry. 2025;(01):41-46.
- Kang PL, Sun N, Liu PH, et al. Research progress on repetitive transcranial magnetic stimulation for non-suicidal self-injury behavior in adolescent depression. Journal of Bio-Education. 2022;10(06):493-497
- Liu RT, Cheek SM, Nestor BA. Non-suicidal self-injury and life stress: A systematic meta-analysis and theoretical elaboration. Clin Psychol Rev. 2016;47:1-14.
doi pubmed - Saraff PD, Pepper CM. Functions, lifetime frequency, and variety of methods of non-suicidal self-injury among college students. Psychiatry Res. 2014;219(2):298-304.
doi pubmed - Chapman AL, Gratz KL, Brown MZ. Solving the puzzle of deliberate self-harm: the experiential avoidance model. Behav Res Ther. 2006;44(3):371-394.
doi pubmed - Nock MK, Prinstein MJ. A functional approach to the assessment of self-mutilative behavior. J Consult Clin Psychol. 2004;72(5):885-890.
doi pubmed - Liu J, Gao Y, Liang C, Liu X. The potential addictive mechanism involved in repetitive nonsuicidal self-injury: The roles of emotion dysregulation and impulsivity in adolescents. J Behav Addict. 2022;11(4):953-962.
doi pubmed - Pettit JW, Joiner TE, Jr., Rudd MD. Kindling and behavioral sensitization: are they relevant to recurrent suicide attempts? J Affect Disord. 2004;83(2-3):249-252.
doi pubmed - Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162(8):1414-1422.
doi pubmed - Gao JB. Research progress on repetitive transcranial magnetic stimulation for non-suicidal self-injury in adolescent depression. China Journal of Health Psychology. 2020;28(11):1738-1744.
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10(6):410-422.
doi pubmed - Zorn JV, Schur RR, Boks MP, Kahn RS, Joels M, Vinkers CH. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology. 2017;77:25-36.
doi pubmed - Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105-114.
doi pubmed - Bai YJ, Guo Q, Hu H, et al. Research progress on HPA axis dysfunction and non-suicidal self-injury in adolescent depression. Journal of Neuroscience and Mental Health. 2024;24(12):858-862.
- Chen S, Xu Z, Guo Z, Lin S, Zhang H, Liang Q, Chen Q, et al. Glymphatic dysfunction associated with cortisol dysregulation in major depressive disorder. Transl Psychiatry. 2025;15(1):265.
doi pubmed - Zhou J, Hu S, Wu H, Wang S, Gong J. From thoughts to actions: a longitudinal examination of NSSI risk factors in adolescence. Psychol Res Behav Manag. 2025;18:1915-1930.
doi pubmed - Law KC, Khazem LR, Jin HM, Anestis MD. Non-suicidal self-injury and frequency of suicide attempts: The role of pain persistence. J Affect Disord. 2017;209:254-261.
doi pubmed - Kao HT, Murner-Lavanchy I, von Stosch E, Josi J, Berger T, Koenig J, Kaess M. Pain sensitivity as a state marker and predictor for adolescent non-suicidal self-injury. Psychol Med. 2024;54(9):2291-2298.
doi pubmed - Liu Q, Chen LJ, Wang MY, et al. Research progress of neuroimaging in adolescent non-suicidal self-injury behavior. Chinese Journal of CT and MRI. 2025;23(06):197-200.
- Kirtley OJ, O'Carroll RE, O'Connor RC. Pain and self-harm: a systematic review. J Affect Disord. 2016;203:347-363.
doi pubmed - Dou YN, Liu Y, Ding WQ, Li Q, Zhou H, Li L, Zhao MT, et al. Single-neuron projectome-guided analysis reveals the neural circuit mechanism underlying endogenous opioid antinociception. Natl Sci Rev. 2024;11(7):nwae195.
doi pubmed - Li Y, Kuang L. Neurobiological mechanisms of non-suicidal self-injury in adolescents. Sichuan Mental Health. 2019;32(04):371-374.
- Yan R, Huang Y, Shi J, Zou H, Wang X, Xia Y, Zhao S, et al. Alterations of regional spontaneous neuronal activity and corresponding brain circuits related to non-suicidal self-injury in young adults with major depressive disorder. J Affect Disord. 2022;305:8-18.
doi pubmed - Westlund Schreiner M, Klimes-Dougan B, Mueller BA, Eberly LE, Reigstad KM, Carstedt PA, Thomas KM, et al. Multi-modal neuroimaging of adolescents with non-suicidal self-injury: Amygdala functional connectivity. J Affect Disord. 2017;221:47-55.
doi pubmed - Yu CF, Deng YT, Li MJ, et al. Cognitive neuroscience and genetic mechanisms of non-suicidal self-injury [J]. Journal of South China Normal University (Social Science Edition). 2021;(02):137-145.
- Linehan MM, Korslund KE, Harned MS, Gallop RJ, Lungu A, Neacsiu AD, McDavid J, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder: a randomized clinical trial and component analysis. JAMA Psychiatry. 2015;72(5):475-482.
doi pubmed - Li P, Zhou LJ, Tang QJ, et al. Intervention effect of acceptance and commitment therapy on adolescent depression with non-suicidal self-injury behavior. China Modern Doctor. 2025;63(13):15-18.
- Ren R, Yu YY, Wang XF, et al. Effect of acceptance and commitment therapy combined with cognitive behavioral therapy on mood state in adolescents with non-suicidal self-injury. Journal of International Psychiatry. 2025;52(03):798-801.
- Liu ZW, Zhang YL, Wang J, et al. Research progress on neuromodulation techniques for the treatment of non-suicidal self-injury. Journal of International Psychiatry. 2023;50(03):395-397.
- van Hoorn AC. Could affect regulation via vagal nerve self- stimulation be a maintaining factor in non-suicidal self-harm? Med Hypotheses. 2020;136:109498.
doi pubmed - Srivastava AB, Gold MS. Naltrexone: a history and future directions. Cerebrum. 2018;2018:cer-13-18.
pubmed - Blasco-Fontecilla H, Fernandez-Fernandez R, Colino L, Fajardo L, Perteguer-Barrio R, de Leon J. The addictive model of self-harming (Non-suicidal and Suicidal) behavior. Front Psychiatry. 2016;7:8.
doi pubmed - Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, Ferri M, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550.
doi pubmed - Cipriani A, Pretty H, Hawton K, Geddes JR. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):1805-1819.
doi pubmed - McKnight RF, Adida M, Budge K, Stockton S, Goodwin GM, Geddes JR. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
doi pubmed - National Institute for Health and Care Excellence. Bipolar disorder: assessment and management. NICE guideline CG18. 2025.
- Novak MA, Meyer JS. A rhesus monkey model of non-suicidal self-injury. Front Behav Neurosci. 2021;15:674127.
doi pubmed - De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114-126.
doi pubmed - Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, Ren L, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683-1696.
doi pubmed - Qi H, Su P, Sui CP, et al. Clinical observation and adverse reaction analysis of electroencephalographic biofeedback combined with sertraline in the treatment of non-suicidal self-injury behavior in adolescent depression. Sichuan Medical Journal. 2025;46(06):617-622.
- Chen LX, Zhang LY, Dai LL, et al. Efficacy evaluation of low-dose olanzapine combined with sertraline in adolescent depressive patients with non-suicidal self-injury behavior. Journal of Clinical Psychiatry. 2024;34(05):341-343.
- Ma N, Liu ZH, Li JF, et al. Effect of low-dose olanzapine combined with sertraline in adolescent depressive patients with non-suicidal self-injury. Chinese Journal of Drug Abuse Prevention and Treatment. 2024;30(08):1389-1392.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.