| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://jocmr.elmerjournals.com |
Original Article
Volume 17, Number 9, October 2025, pages 490-498
Association of Sex Difference With Thiamine Responsive Disorders in Internal Medicine: A Pilot Case-Control Study
Takahiro Hadanoa, h , Yoshifumi Sugiyamab, c, Sadako Matsuid, Kei Fujimotoe, Takae Ebiharaf, Hiroshi Yoshidaa, f, g, h
aSection of Internal Medicine of Metabolism and Nutrition, The Jikei University Graduate School of Medicine, Tokyo 105-8461, Japan
bDivision of Clinical Epidemiology, Research Center for Medical Sciences, The Jikei University School of Medicine, Tokyo 105-8461, Japan
cDivision of Community Health and Primary Care, Center for Medical Education, The Jikei University School of Medicine, Tokyo 105-8461, Japan
dDepartment of Nutritional Science, Faculty of Food and Nutritional Sciences, Japan Women’s University, Tokyo 112-8681, Japan
eDivision of Diabetes, Metabolism and Endocrinology, Department of Internal Medicine, The Jikei University Daisan Hospital, Tokyo 201-8601, Japan
fDepartment of General Medicine, The Jikei University Kashiwa Hospital, Chiba 277-8567, Japan
gDepartment of Laboratory Medicine, The Jikei University Kashiwa Hospital, Chiba 277-8567, Japan
hCorresponding Author: Takahiro Hadano, Section of Internal Medicine of Metabolism and Nutrition, The Jikei University Graduate School of Medicine, 3-25-8, Nishishimbashi, Minato, Tokyo 105-8461, Japan; Hiroshi Yoshida, Department of Laboratory Medicine, The Jikei University Kashiwa Hospital, 163-1, Kashiwashita, Kashiwa, Chiba 277-8567, Japan
Manuscript submitted July 30, 2025, accepted September 22, 2025, published online October 10, 2025
Short title: Sex Associated With TRDs in Internal Medicine
doi: https://doi.org/10.14740/jocmr6337
| Abstract | ▴Top |
Background: Thiamine responsive disorder (TRD), a clinical condition which improves with the administration of vitamin B1, has been proposed in pediatrics to detect the atypical and diverse symptoms of vitamin B1 deficiency which has been widely overlooked. Previous studies were not conducted in internal medicine and in developed countries. Therefore, the aim of this study was to determine the characteristics of Japanese TRD patients in internal medicine.
Methods: This case-control study was conducted at Department of General Medicine in the Jikei University Kashiwa Hospital from January 2018 to December 2022. Participants were tested for whole blood total vitamin B1, serum albumin levels, and total lymphocyte count; started newly on a vitamin B1 preparation for treating patients’ signs and symptoms; and re-visited the hospital to evaluate drug effectiveness. Signs and symptoms were chosen whether they had been suspected to be a vitamin B1 deficiency by the treating physicians. All enrolled patients were assessed recovery status by the clinician at that time, and one of the authors assigned them to a TRD or not using a two-point scale (getting better vs. no change or getting worse) based on all the information available from electronic medical records.
Results: A total of 35 patients were enrolled (median age 48 years; 31.4% male). A univariate logistic regression analysis showed that sex was associated with TRDs (female: unadjusted odds ratio (OR) = 0.08, 95% confidence interval (CI): 0.0043 - 0.55, P = 0.007), which was the same as the result of a multivariate logistic regression analysis with forced entry method (female: adjusted OR = 0.03, 95% CI: 0.0009 - 0.32, P = 0.002). In addition, there was an atypical symptom of vitamin B1 deficiency such as orthostatic dysregulation (OD): lightheadedness and syncope in the TRDs albeit being nonsignificant.
Conclusions: Japanese TRD patients in internal medicine were associated with sex, and had a symptom of OD. Further well-designed, large-scale studies are needed to explore the features of TRDs in internal medicine.
Keywords: Vitamin B1 deficiency; Thiamine responsive disorders; Orthostatic dysregulation
| Introduction | ▴Top |
Vitamin B1 (thiamine) is essential for energy generation, widely distributed in the body, and metabolized mainly in nervous tissues and the cerebellum; therefore, its deficiency causes peripheral and central nervous system disorders such as “beriberi” and “Wernicke’s encephalopathy” or “Wernicke-Korsakoff syndrome” [1-3]. In 1884, Kanehiro Takaki observed an association between beriberi and inadequate dietary intake, such as a diet with an imbalance between protein and carbohydrates, which led to the identification of vitamin B1 by Umetaro Suzuki in 1910 [4-8]. Actually, animals including human, which are unable to synthesize vitamin B1 in the bodies, need the intake of vitamin B1 from the diet [9]. Therefore, malnutrition can give rise to vitamin B1 deficiency which has been assumed to be overcome in developed countries in the modern era of adequate nutrition.
However, this assumption has led to today’s vitamin B1 deficiency cases being overlooked [10, 11]. For example, there is an increased risk of vitamin B1 deficiency in elderly individuals in nursing homes, critically ill patients in the intensive care unit, and patients with refeeding syndromes that require hospitalization [12-15]. In addition, there are nonspecific symptoms such as malaise, anorexia, and irritability in early stages of vitamin B1 deficiency, and it can be easily misdiagnosed with other diseases [1, 16].
These days, the concept of thiamine responsive disorders (TRDs) has been proposed in the field of pediatrics, in response to recognition of the atypical and diverse symptoms of vitamin B1 deficiency [17, 18]. TRDs are clinical conditions which improve with the administration of vitamin B1. This term depends on the patient having signs and symptoms attributed to the thiamine deficiency, and thus, in the absence of specific diagnostic tests now, the diagnosis of TRDs is based on a clinician’s interpretation of the constellation of symptoms with which the patient presents, the perceived risk of that patient having an underlying vitamin B1 insufficient diet, and the response to initial treatment [18]. To solve this ambiguous definition, a large prospective cohort study in Lao People’s Democratic Republic, which is a low and middle-income country and has a public health concern about thiamine deficiency, was conducted from June 2019 to December 2020 [19-23]. It showed a predictive model for TRDs among infants and young children 21 days to under 18 months, and the low-resource model of clinical decisions was composed only of signs and symptoms without biochemical laboratory data [19]. The biomarkers of vitamin B1 require a costly and time-consuming method because they are obtained from the erythrocytes, which contain 75-80% of blood vitamin B1; thus, this predictive model without such biomarkers is easy to use [24-27].
While this prior study suggests that TRD patients under 18 months in a low and middle-income country could be predicted by their signs and symptoms, and other information of anthropometry and life-style, no such studies, to the best of our knowledge, have investigated the characteristics of TRD patients in the field of internal medicine in a high-income country like Japan [28].
Therefore, the aim of the present study was to determine the characteristics of Japanese TRD patients in internal medicine, and to gain insights into detecting TRD patients who are overlooked.
| Materials and Methods | ▴Top |
Study design
We used a case-control design, and compared with TRDs and non-TRDs as a reference group.
Setting
This study recruited patients who visited the Internal Medicine Outpatient Clinic of General Medicine in the Jikei University Kashiwa Hospital, Chiba, Japan from January 2018 to December 2022.
Participants
Inclusion criteria were as follows: patients who tested for whole blood total vitamin B1 levels as well as serum albumin levels (Alb) and total lymphocyte count which were used for nutritional assessment as below; started newly on a vitamin B1 preparation for treating patients’ signs and symptoms; and re-visited the hospital to evaluate drug effectiveness. In addition, we excluded the patients whose whole blood total vitamin B1 concentrations had been over 219 nmol/L (the upper limit of the reference range at our hospital; converted to the International System of Units by the conversion factor = 3.324) to eliminate potential prior prescription of a vitamin B1 preparation; who had taken vitamin B1 not as a treatment for the signs and symptoms but as a nutritional supplement to evaluate which signs and symptoms had been getting better with vitamin B1; who were not applicable to being treated with a minimum of 50 mg oral vitamin B1 daily for at least 3 days, which were reasonable dosages following previous studies; who had refused to participate in this study [19-21, 29-34].
All enrolled patients were treated with any other treatments which were considered necessary by the treating physicians. Assessment of recovery was conducted after the third day from the initiation of vitamin B1 supplementation by the clinician at that time, and then, one of the authors assigned each patient to a TRD as the case or a non-TRD group as the control, using a two-point scale (getting better vs. no change or getting worse) based on all the information available from electronic medical records [19].
The study was approved by the Ethics Committee of the Jikei University School of Medicine (approval number 34-396) and performed in accordance with the principles laid down in the Declaration of Helsinki. A waiver for informed consent was approved with opt-out consent in view of the retrospective observational nature of the research.
Data collection
Patient data were selected in terms of the risk factor of vitamin B1 deficiency such as poor dietary intake, alcoholism, and chronic illness [1]. That is, patient characteristics (sex, age, body mass index (BMI), diet, alcohol consumption, specific comorbidity, and medication), biochemical laboratory data (whole blood total vitamin B1 concentration, white blood cell count (WBC), red blood cell count (RBC), hemoglobin (Hb), mean corpuscular volume (MCV), platelet count (Plt), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (γGT), Alb, blood urea nitrogen (BUN), creatinine (Cre), estimated glomerular filtration rate (eGFR), Prognostic Nutritional Index (PNI), and modified Controlling Nutritional Status (CONUT) score), and signs and symptoms which were suspected to be a vitamin B1 deficiency by the treating physicians, were collected from electronic medical records. The last two items in the biochemical laboratory data are nutritional indicators, which were calculated as follows: PNI = 10 × Alb (g/dL) + 0.005 × total lymphocyte count (/µL); and the modified CONUT score was calculated by adding the Alb, total lymphocyte count, and Hb scores [35, 36]. Missing blood data were collected at the time of another visit at the hospital outpatient clinic within about a month. Furthermore, similar signs and symptoms were combined into one category as follows: malaise and unidentified complaint; early satiety, loss of appetite, nausea and dyspepsia; lightheadedness and syncope (orthostatic dysregulation (OD)); double and blurred vision, fatigability, palpitation, diaphoresis and shortness of breath on exertion.
Study size
The number of cases in the medical area around the Jikei University Kashiwa Hospital during the study period consequently determined the sample size.
Statistical analysis
Continuous variables (i.e., age, BMI, whole blood total vitamin B1 concentration, WBC and RBC counts, Hb, MCV, Plt count, AST, ALT, γGT, Alb, BUN, Cre, eGFR, and PNI) were shown as the median (interquartile range) and were compared between the two groups using Wilcoxon rank-sum test. Categorical variables (i.e., sex, diet, alcohol consumption, specific comorbidities, medication, modified CONUT score, and signs and symptoms) were summarized as the frequency (percentage) and were compared between the groups using Pearson’s Chi-square test or Fisher’s exact test.
A logistic regression model was prepared to estimate the risk of TRDs associated with potential predictors, including sociodemographic and clinical variables such as age, alcohol-related factors, nutritional factors, and sex which were based on existing knowledge of risk factors for vitamin B1 deficiency and a possible risk factor of TRDs [1, 37, 38]. The variable of alcohol-related factors used a combination of AST, ALT, γGT, and MCV (all of them were within normal limits or not), which are common alcohol-related blood biomarkers, because there were many missing values or incorrect answers found in the routine medical interview about alcohol consumption [39]. In addition, the variable of nutritional factors used a combination of PNI and modified CONUT score (all of them were within normal limits or not; PNI ≥ 41 was defined as normal), because there were also many missing values or incorrect answers found in the routine medical interview about diet contents. Subsequently, we carried out a univariate logistic regression analysis, and then, performed a multivariate logistic regression analysis with forced entry method. Furthermore, variance inflation factor (VIF) was used to check for multicollinearity.
All statistical analyses were performed using JMP Student Edition version 18.2.2 (SAS Institute Inc., Cary, NC, USA), and a two-sided P value < 0.05 was considered statistically significant.
| Results | ▴Top |
A total of 40 patients, whose whole blood total vitamin B1, serum albumin levels, and total lymphocyte count were available from electronic medical records and were newly treated with vitamin B1, were identified as potentially eligible for participation, of whom 35 met the eligibility criteria in the study (Fig. 1). Their baseline characteristics and clinical data are shown in Table 1. There existed specific comorbidity (gastrointestinal tract diseases) and medication (diuretics), whereas did not exist patients with a diagnosis of chronic heart failure or pulmonary edema requiring long-term treatment with diuretics, hyperemesis gravidarum, human immunodeficiency virus infection or acquired immunodeficiency syndrome, diabetes, end-stage chronic kidney disease, or active cancer, all of which are risk factors for vitamin B1 deficiency, in this study [1, 40-43]. In addition, BMI, diet, and alcohol consumption were not included in statistical analyses because they had more than 10% missing values (Table 1) [44].
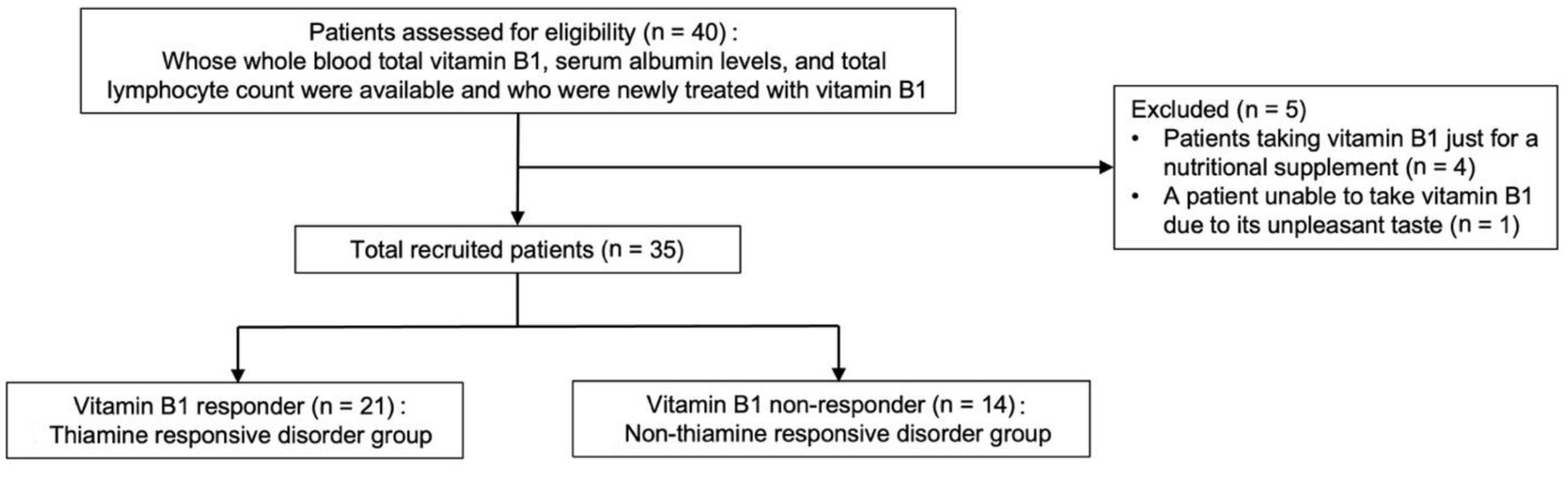 Click for large image | Figure 1. Flowchart depicting study participant enrollment. |
 Click to view | Table 1. Demographic and Clinical Characteristics (n = 35) |
Sixty percent of the patients were in the TRD group and 40% were in the non-TRD group (Table 1). Compared with the non-TRD group, the TRD group contained a higher proportion of male (47.6% vs. 7.1%; P = 0.023, Table 1), and had a lower median WBC count (5,300/µL (4,100 - 6,300) vs. 6,400/µL (4,950 - 8,050); P = 0.035; Table 1) and higher median monocyte subset (6.1% (4.9 - 8.0) vs. 4.7% (3.7 - 5.8); P = 0.008, Table 1), MCV (92.6 fL (88.0 - 97.8) vs. 88.7 fL (85.7 - 91.1); P = 0.031, Table 1), and AST (19 U/L (16.5 - 60.5) vs. 16 U/L (14.5 - 23.0); P = 0.028, Table 1) values. There was no significant difference in the median whole blood total vitamin B1 concentration (93.1 nmol/L (74.8 - 106.4) vs. 88.1 nmol/L (82.3 - 111.4); P = 0.589, Table 1) between the TRD and non-TRD groups (i.e., reference range in Japanese adults: 89 - 262 nmol/L (male) and 80 - 235 nmol/L (female)) [45]. In addition, the majority of signs and symptoms were recorded as “malaise and unidentified complaint,” “early satiety, loss of appetite, nausea and dyspepsia,” or “OD: lightheadedness and syncope,” but without any statistically significant differences (Table 2).
 Click to view | Table 2. Signs and Symptoms Which Were Suspected to Be a Vitamin B1 Deficiency by the Treating Physicians |
Then, we carried out a univariate logistic regression analysis and it indicated that sex was associated with TRDs (female: unadjusted odds ratio (OR) = 0.08, 95% confidence interval (CI): 0.0043 - 0.55, P = 0.007; Table 3), which was the same as the result of a multivariate logistic regression analysis with forced entry method (female: adjusted OR = 0.03, 95% CI: 0.0009 - 0.32, P = 0.002; Table 3) that adjusted for sex, age, alcohol-related factors, and nutritional factors described above. The maximum VIF of them was 1.18, which means that there was no collinearity in the model.
 Click to view | Table 3. Risk Factors for TRDs |
| Discussion | ▴Top |
In the present study, the proportion of male and monocyte subset, MCV, and AST levels were significantly higher, whereas WBC count was significantly lower in the TRD group than those in the non-TRD group. In addition, we found atypical signs and symptoms of vitamin B1 deficiency such as OD (lightheadedness and syncope) in the present study.
Our result indicates that male may be more responsive to thiamine than female. Though, according to a widely used reference for dietary intake, men require a higher amount of vitamin B1 than women, there is no sex-related difference in vitamin intake when adjusted for total energy intake [46, 47]. However, it has been reported that men are more responsive to thiamine diphosphate replenishment than women, which could be consistent with our result [37, 38]. These findings indicate that male is more likely to be deficient in vitamin B1. In addition, there may be a relationship between alcohol and TRDs. For example, MCV and AST levels are common alcohol-related blood biomarkers, and a previous study suggests that alcohol may be associated with monocyte dysfunction [39, 48]. Needless to say, it is known that vitamin B1 deficiency is related to alcoholism, and it seems to be that alcohol consumption is also related to TRDs [1].
As for low WBC count in TRDs, vitamin B1 deficiency can relate to low leukocyte counts. For example, as shown in a previous research, leukocyte counts declined in mice with vitamin B1 deficiency [49]. In addition, another study shows that additional vitamin B1 inhibits neutrophil extracellular traps (NETs), which means that vitamin B1 deficiency may overlook an excessive apoptosis of neutrophils, regarded as NETosis [50]. In spite of these results, it is not well known whether vitamin B1 decreases WBC, and so further researches are needed.
Furthermore, our result shows unique signs and symptoms such as OD, which is not typical in vitamin B1 deficiency. For example, as shown in basic research, it was reported that vitamin B1 deficiency induces hypoxia-inducible factor 1, which in turn triggers release of vasodilating factors [51, 52]. In clinical research, to our best knowledge, only one cohort study on the relationship between OD and vitamin B1 deficiency has been published despite long history of vitamin B1 deficiency, and only one patient, who has a low whole blood total vitamin B1 level, responds to oral vitamin B1 [53]. These findings imply that we should focus on the relationship between OD and TRDs without whole blood total vitamin B1 levels. But it remains to be cleared in further studies.
The strengths of the present study were as follows. First, a range of age of study subjects was relatively broad. Participants of the present study were aged 15 to 89 years, which have not been investigated on TRDs yet. In addition, this range of age was similar to important references (16 - 70 years old) in the previous important studies relevant to sex differences as mentioned in Discussion [37, 38]. Furthermore, we included common age of patients with orthostatic intolerance (16 - 45 years old), has almost the same meaning as OD, led to the awareness of OD as a possible main symptom of TRDs [54]. Second, the present study was conducted in current Japan, a developed country in an era of excessive eating, which would be a unique point because previous studies on TRDs were conducted in low- and middle-income countries [19-23, 28].
The present study had several limitations. First, it had a retrospective pilot design, which meant that an appropriate sample size could not be calculated. Second, the criteria for determining TRDs were vague. For example, the period required for evaluation of specific vitamin B preparations was not standardized, as well as a kind of vitamin B1 preparations. Finally, there was a considerable amount of missing data. In particular, data on history of alcohol consumption were many missing values, so we could not adjust directly for confounders. In addition, selection bias could influence this study because the setting was a university hospital which usually provides critical of tertiary medical care, and severe patients with vitamin B1 deficiency could be included as participants. To prevent this bias, we did not include patients who visited only the emergency room (ER) but included patients triaged as having mild symptoms in ER, and then mainly examined in the internal medicine outpatient clinic. Furthermore, information bias could also affect this study because only one of the authors assigned each patient to TRDs or non-TRDs (diagnostic bias). This bias was minimized to compare author’s decision with clinical decision by another attending physician at that time.
Conclusions
TRDs in patients aged 15 years and above were associated with sex, and have a symptom of OD, which is an atypical symptom of vitamin B1 deficiency. Although the present study offered insight on the current status of TRDs in the field of internal medicine in developed countries, further well-designed, large-scale studies are needed to explore the characteristics of patients with TRDs in more detail.
Acknowledgments
We would like to thank all the participants in this study, and Dr. Nobuyuki Furutani.
Financial Disclosure
This research was funded in part by a Grant-in-Aid for Scientific Research from the Japan Ministry of Education, Culture, Sports, Science, and Technology (Hiroshi Yoshida: Grant No. 24K11253) and in part by a grant from the Labor and Welfare Sciences Research Grant for Research on Rare and Intractable Diseases DECEMBER 2023:881-891 (Hiroshi Yoshida: Grant No. 24FC1012).
Conflict of Interest
HY received honoraria for speaking activities from Kowa and Novartis company, a manuscript fee from Kowa company, and advisory role fee from Denka company. KF received honoraria from Eli Lilly Japan and Sumitomo Pharma and subsidies or donations from Nippon Boehringer Ingelheim and Mitsubishi Chemical Group. TH, YS, SM, and TE declare no conflict of interest.
Informed Consent
A waiver for informed consent was approved with opt-out consent in view of the retrospective observational nature of the research.
Author Contributions
TH and HY contributed to this study’s concept and design. TH collected information from medical records and analyzed the data. All authors interpreted the data. TH was mainly in charge of the manuscript preparation, and YS, SM, KF, TE, and HY supervised the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
Alb: albumin; ALT: alanine aminotransferase; AST: asparate aminotransferase; BMI: body mass index; BUN: blood urea nitrogen; CI: confidence interval; CONUT: Controlling Nutritional Status; Cre: creatinine; eGFR: estimated glomerular filtration rate; ER: emergency room; γGT: gamma-glutamyl transpeptidase; Hb: hemoglobin; MCV: mean corpuscular volume; NETs: neutrophil extracellular traps; OD: orthostatic dysregulation; OR: odds ratio; Plt: platelet count; PNI: Prognostic Nutritional Index; RBC: red blood cell count; TRD: thiamine responsive disorder; VIF: variance inflation factor; WBC: white blood cell count
| References | ▴Top |
- Suter PM. Vitamin and trace mineral deficiency and excess. In: Longo D, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J, Holland S, et al. Harrison's principles of internal medicine, 22nd Edition. McGraw Hill; 2026.
- Gangolf M, Czerniecki J, Radermecker M, Detry O, Nisolle M, Jouan C, Martin D, et al. Thiamine status in humans and content of phosphorylated thiamine derivatives in biopsies and cultured cells. PLoS One. 2010;5(10):e13616.
doi pubmed - Rindi G, Patrini C, Comincioli V, Reggiani C. Thiamine content and turnover rates of some rat nervous regions, using labeled thiamine as a tracer. Brain Res. 1980;181(2):369-380.
doi pubmed - Takaki K. On the cause and prevention of kakke. Sei-I-Kwai. 1885;33:29-37 (in Japanese).
- Takaki K. On the preservation of health amongst the personnel of the Japanese Navy and Army. Lecture I, delivered on May 7th. Lancet. 1906;1:1369-1374.
- Takaki K. On the preservation of health amongst the personnel of the Japanese Navy and Army. Lecture II, delivered on May 9th. Lancet. 1906;1:1451-1455.
- Takaki K. On the preservation of health amongst the personnel of the Japanese Navy and Army. Lecture III, delivered on May 11th. Lancet.1906;1:1520-1523.
- Suzuki U. Uber Oryzanin: ein Bestandteil der Reiskleie und seine physiologische Bedeudung. Biochem. Z. 1912;43:89-153 (in German).
- Palmieri F, Monne M, Fiermonte G, Palmieri L. Mitochondrial transport and metabolism of the vitamin B-derived cofactors thiamine pyrophosphate, coenzyme A, FAD and NAD(+) , and related diseases: A review. IUBMB Life. 2022;74(7):592-617.
doi pubmed - Gomes F, Bergeron G, Bourassa MW, Fischer PR. Thiamine deficiency unrelated to alcohol consumption in high-income countries: a literature review. Ann N Y Acad Sci. 2021;1498(1):46-56.
doi pubmed - Marrs C, Lonsdale D. Hiding in plain sight: modern thiamine deficiency. Cells. 2021;10(10):2595.
doi pubmed - Ito Y, Yamanaka K, Susaki H, Igata A. A cross-investigation between thiamin deficiency and the physical condition of elderly people who require nursing care. J Nutr Sci Vitaminol (Tokyo). 2012;58(3):210-216.
doi pubmed - Attaluri P, Castillo A, Edriss H, Nugent K. Thiamine deficiency: an important consideration in critically ill patients. Am J Med Sci. 2018;356(4):382-390.
doi pubmed - da Silva JSV, Seres DS, Sabino K, Adams SC, Berdahl GJ, Citty SW, Cober MP, et al. ASPEN consensus recommendations for refeeding syndrome. Nutr Clin Pract. 2020;35(2):178-195.
doi pubmed - Erratum: Joshua S. V. da Silva. ASPEN consensus recommendations for refeeding syndrome. Nutr Clin Pract. 2020;35(2):178-195. Nutr Clin Pract. 2020;35(3):584-585.
doi pubmed - Fattal-Valevski A. Thiamine (Vitamin B1). Journal of Evidence-Based Complementary & Alternative Medicine. 2011;16(1):12-20.
- Whitfield KC, Bourassa MW, Adamolekun B, Bergeron G, Bettendorff L, Brown KH, Cox L, et al. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann N Y Acad Sci. 2018;1430(1):3-43.
doi pubmed - Smith TJ, Johnson CR, Koshy R, Hess SY, Qureshi UA, Mynak ML, Fischer PR. Thiamine deficiency disorders: a clinical perspective. Ann N Y Acad Sci. 2021;1498(1):9-28.
doi pubmed - Smith TJ, Arnold CD, Fischer PR, Trehan I, Hiffler L, Sitthideth D, Stein-Wexler R, et al. A predictive model for thiamine responsive disorders among infants and young children: results from a prospective cohort study in Lao People's democratic republic. J Pediatr. 2024;268:113961.
doi pubmed - Cardiel Nunez K, Hess SY, Arnold CD, Smith TJ, Trehan I, Hiffler L, Sitthideth D, et al. Relationship between lactate and thiamine-responsive disorders in hospitalised infants and children in Lao PDR: secondary analysis of a prospective cohort study. Paediatr Int Child Health. 2024;44(3-4):95-104.
doi pubmed - Hess SY, Smith TJ, Fischer PR, Trehan I, Hiffler L, Arnold CD, Sitthideth D, et al. Establishing a case definition of thiamine responsive disorders among infants and young children in Lao PDR: protocol for a prospective cohort study. BMJ Open. 2020;10(2):e036539.
doi pubmed - Johnson CR, Fischer PR, Thacher TD, Topazian MD, Bourassa MW, Combs GF, Jr. Thiamin deficiency in low- and middle-income countries: Disorders, prevalences, previous interventions and current recommendations. Nutr Health. 2019;25(2):127-151.
doi pubmed - World Bank Open Data. Lao PDR. https://data.worldbank.org/country/lao-pdr (Accessed July 29, 2025).
- Talwar D, Davidson H, Cooney J, St JRD. Vitamin B(1) status assessed by direct measurement of thiamin pyrophosphate in erythrocytes or whole blood by HPLC: comparison with erythrocyte transketolase activation assay. Clin Chem. 2000;46(5):704-710.
pubmed - Burch HB, Bessey OA, Love RH, Lowry OH. The determination of thiamine and thiamine phosphates in small quantities of blood and blood cells. J Biol Chem. 1952;198(1):477-490.
pubmed - Schrijver J, Speek AJ, Klosse JA, van Rijn HJ, Schreurs WH. A reliable semiautomated method for the determination of total thiamine in whole blood by the thiochrome method with high-performance liquid chromatography. Ann Clin Biochem. 1982;19(Pt 1):52-56.
doi pubmed - Herve C, Beyne P, Delacoux E. Determination of thiamine and its phosphate esters in human erythrocytes by high-performance liquid chromatography with isocratic elution. J Chromatogr B Biomed Appl. 1994;653(2):217-220.
doi pubmed - World Bank Open Data. Japan. https://data.worldbank.org/country/japan (Accessed July 29, 2025).
- Miyagawa H, Gondo K, Nakaura H, Kato M, Hashizume N. Method for determining total vitamin B1 in whole blood by LC/MS/MS. Int J Anal Bio-Sci. 2013;36(4):327-330 (in Japanese).
pubmed - Coats D, Shelton-Dodge K, Ou K, Khun V, Seab S, Sok K, Prou C, et al. Thiamine deficiency in Cambodian infants with and without beriberi. J Pediatr. 2012;161(5):843-847.
doi pubmed - Frank LL. Thiamin in clinical practice. JPEN J Parenter Enteral Nutr. 2015;39(5):503-520.
doi pubmed - Crook MA, Sriram K. Thiamine deficiency: the importance of recognition and prompt management. Nutrition. 2014;30(7-8):953-954.
doi pubmed - McCandless DW. Thiamine deficiency and associated clinical disorders. New Jersey: Humana Press, 2009.
- Park SW, Yi YY, Han JW, Kim HD, Lee JS, Kang HC. Wernicke's encephalopathy in a child with high dose thiamine therapy. Korean J Pediatr. 2014;57(11):496-499.
doi pubmed - Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85(9):1001-1005.
pubmed - Takahashi S, Takahashi H, Ide K, Takeuchi C, Kuribayashi K, Niimi M, Matsushima R, et al. Validity of the modified CONUT method to screen undernourished inpatients. J Jpn Soc Parenter Enteral Nutr. 2016;31(3):827-834 (in Japanese).
- Artiukhov AV, Solovjeva ON, Balashova NV, Sidorova OP, Graf AV, Bunik VI. Pharmacological doses of thiamine benefit patients with the Charcot-Marie-Tooth neuropathy by changing thiamine diphosphate levels and affecting regulation of thiamine-dependent enzymes. Biochemistry (Mosc). 2024;89(7):1161-1182.
doi pubmed - Artiukhov AV, Solovjeva ON, Balashova NV, Sidorova OP, Graf AV, Bunik VI. Erratum to: pharmacological doses of thiamine benefit patients with the Charcot-Marie-Tooth neuropathy by changing thiamine diphosphate levels and affecting regulation of thiamine-dependent enzymes. Biochemistry (Mosc). 2024;89(8):1531-1532.
doi pubmed - Harris JC, Leggio L, Farokhnia M. Blood biomarkers of alcohol use: a scoping review. Curr Addict Rep. 2021;8(4):500-508.
doi pubmed - Lu’o’ng Kvq, Nguyen LT. The role of thiamine in HIV infection. Int J Infect Dis. 2013;17(4):e221-227.
doi pubmed - Mouly S, Khuong MA, Cabie A, Saimot AG, Coulad JP. Beri-Beri and thiamine deficiency in HIV infection. AIDS. 1996;10(8):931-932.
doi pubmed - Liu J, Qin L, Zheng J, Tong L, Lu W, Lu C, Sun J, et al. Research progress on the relationship between vitamins and diabetes: systematic review. Int J Mol Sci. 2023;24(22):16371.
doi pubmed - Saka Y, Naruse T, Kato A, Tawada N, Noda Y, Mimura T, Watanabe Y. Thiamine status in end-stage chronic kidney disease patients: a single-center study. Int Urol Nephrol. 2018;50(10):1913-1918.
doi pubmed - Bennett DA. How can I deal with missing data in my study? Aust N Z J Public Health. 2001;25(5):464-469.
pubmed - Ihara H, Hirano A, Wang L, Okada M, Hashizume N. Reference values for whole blood thiamine and thiamine phosphate esters in Japanese adults. Int J Anal Bio-Sci. 2005;28(3):241-246.
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary reference intakes for Thiamin, Riboflavin, Niacin, Vitamin B(6), Folate, Vitamin B(12), Pantothenic Acid, Biotin, and Choline. Washington (DC), National Academies Press (US). 1998.
doi pubmed - Mataix J, Aranda P, Sanchez C, Montellano MA, Planells E, Llopis J. Assessment of thiamin (vitamin B1) and riboflavin (vitamin B2) status in an adult Mediterranean population. Br J Nutr. 2003;90(3):661-666.
doi pubmed - Voss JK, Li Z, Weinman SA. Elevated blood monocyte counts in alcohol-associated hepatitis. JGH Open. 2022;6(2):148-151.
doi pubmed - Sharma A, Bist R. Alteration in MDA, GSH level and hematological changes due to thiamine deficiency in Mus musculus. Interdiscip Toxicol. 2018;11(4):321-325.
doi pubmed - Riyapa D, Rinchai D, Muangsombut V, Wuttinontananchai C, Toufiq M, Chaussabel D, Ato M, et al. Transketolase and vitamin B1 influence on ROS-dependent neutrophil extracellular traps (NETs) formation. PLoS One. 2019;14(8):e0221016.
doi pubmed - Sweet RL, Zastre JA. HIF1-alpha-mediated gene expression induced by vitamin B1 deficiency. Int J Vitam Nutr Res. 2013;83(3):188-197.
doi pubmed - Olson N, van der Vliet A. Interactions between nitric oxide and hypoxia-inducible factor signaling pathways in inflammatory disease. Nitric Oxide. 2011;25(2):125-137.
doi pubmed - Blitshteyn S. Vitamin B1 deficiency in patients with postural tachycardia syndrome (POTS). Neurol Res. 2017;39(8):685-688.
doi pubmed - Winker R, Barth A, Dorner W, Mayr O, Pilger A, Ivancsits S, Ponocny I, et al. Diagnostic management of orthostatic intolerance in the workplace. Int Arch Occup Environ Health. 2003;76(2):143-150.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.